INTRODUCTION
Characterized by high growth dynamics and plasticity, Zostera marina is the most widely distributed and abundant marine plant in the Northern Hemisphere (Lee et al. 2006). It is found along the Pacific coast of North America, from the temperate Alaska coastal waters to the hot waters in the Gulf of California (Riosmena-Rodríguez et al. 2013). Zostera marina grows in shallow bays, coastal lagoons, and estuaries, in subtidal and intertidal environments (Riosmena-Rodríguez et al. 2013). Its upper distribution limit is determined by shoot tolerance to desiccation from air exposure, and its lower distribution limit by light penetration (Cabello-Pasini et al. 2002). At low tide, intertidal Z. marina populations are exposed to extreme light intensity, air, water temperature variations, and other factors like grazing by waterfowl, all stressful conditions for shoot growth, vegetative biomass growth, and sexual reproduction. The subtidal environment is more stable (Lee et al. 2006); here stress is caused by light penetration as a function of depth, illumination, and water turbidity (Nielsen et al. 2002).
Sexual reproduction may play a crucial role in the maintenance of Z. marina meadows. Plants allocate more energy to sexual reproduction in stressful or disturbed environments (Cabaço and Santos 2012, Kaul 2016), and increased water temperatures provide the right conditions for vegetative biomass growth and other demographic characteristics (Cabello-Pasini et al. 2002). Increased water temperature probably promotes the development of enhanced sexual phenological characteristics; however, long periods of high temperature or exceptional events of extreme temperature decompensate the respiration/photosynthesis rate and carbohydrates are not passed on to physiological functions. This has strong negative effects on the number of leaves per shoot and reduces shoot biomass (Hammer et al. 2018).
San Quintín Bay (SQB) is relatively pristine, but some ecological processes there have an uncertain future, for example water turbidity, which increased by 2-fold over the last 2 decades (Cabello-Pasini et al. 2003). SQB represents the southern limit of the central portion of the Z. marina distribution range (Phillips et al. 1983). Here Z. marina beds grow in areas that leave them exposed during low tides. Ibarra-Obando et al. (1997) and Poumian-Tapia and Ibarra-Obando (1999) have described several structural and functional characteristics of intertidal Z. marina beds in SQB. Differences in environmental conditions lead to differences in biomass, morphological characteristics, and depth distribution. Echavarria-Heras et al. (2006) compared Z. marina leaf morphology and biomass before and after the 1986-1987 El Niño/Southern Oscillation (ENSO) event and found differences for most of the measured variables; these differences could be explained by the warm water associated with the ENSO event.
The vegetative and sexual biomass, shoot density, shoot length, and reproductive effort of Z. marina growing within a depth gradient ranging from the shallow subtidal to the intertidal areas of SQB have never been compared simultaneously, nor have they been assessed during an ENSO event. Information on Z. marina vegetative biomass and sexual phenology associated with reproductive effort is scarce and fragmented, and so is information on how this species is affected by unusual high-temperature events such as ENSO or within a depth gradient. We hypothesize that the water temperature increase that took place from 1996 to the 1997-1998 ENSO period resulted in reduced vegetative biomass and increased sexual phenology for Z. marina. The best environment for reproductive phenology is the intertidal zone, whereas the subtidal zone is best for developing vegetative characteristics.
With the advent of climate change, we need deeper knowledge on the behavior of Z. marina meadows. If extreme temperature events become more frequent and sea level changes the norm, we must be able to predict how the species will respond.
MATERIALS AND METHODS
SQB is a coastal lagoon (30º30′ N, 116º01′ W) on the Pacific coast of Baja California, Mexico. Its community ecology has been described by Poumian-Tapia and Ibarra-Obando (1999). In order to describe vegetative and reproductive phenology and the reproductive effort of Z. marina, we selected and sampled 4 locations monthly between June 1996 and November 1997. Locations were chosen to cover most of the bay area where Z. marina is present. At each location 6 sites were sampled, 3 in the subtidal environment (-1.90, -0.50, and -0.10 m with respect to the mean lower low water, MLLW) and 3 in the intertidal environment (+0.01, +0.10, and +0.20 m MLLW). At each site, a 45-50 m transect was established to randomly collect three 0.04 m2 samples (24 quadrats per location per month), but given the ease of sampling at -0.50 and +0.10, we duplicated transects and samples (96 quadrats per month).
Whole vegetative and reproductive shoots were collected within each quadrat, including rhizomes and roots. Collected material was sieved and rinsed with bay water to remove sediments. Shoots were placed in labeled plastic bags and transported to the lab in an ice chest. In the lab, both vegetative and reproductive shoots were rinsed with distilled water and treated separately. The material was immersed in 10% phosphoric acid for 5 min to separate epibionts and sediments. Dry weight was measured after drying the sample at 75 ºC for 24 h. Samples were separated into above- and below-ground vegetative biomass. Vegetative shoot density was calculated as the number of shoots per square meter. The date of first reproductive shoot apparition, date of reproductive shoot release, and number of seeds per shoot were recorded. Reproductive shoots were separated into leaves and reproductive structures. The reproductive effort (RE) of Z. marina is the ratio of the dry weight of reproductive shoots to the total above- and below-ground vegetative biomass in dry weight multiplied by 100, i.e., the proportion of total shoot biomass allocated to sexual reproduction. This method is common and reliable (Kaul 2016) for representing the proportion of plant energy invested in reproduction. Seeds are potential plants, so we used seed number and total biomass (vegetative and reproductive) to assess seed RE (REs), which represents the proportion of total biomass invested in the production of seeds (REs = seeds·m-2/total biomass g dry wet [DW]·m-2).
We used air temperature at noon and at 3:00 AM (local time) and air humidity records from the Molino Viejo Tourist Hotel weather station. We also measured water temperature and photon irradiance at -1.0 m with a Li-Cor Radiation Sensor (Lincoln, Nebraska, USA) using 19 s integration time; irradiance measurements at noon (local time) and all other measurements were taken until a consistent reading was obtained for 30 s. Intertidal exposure time was calculated from the Centro de Investigación Científica y de Educación Superior de Ensenada tide charts (CICESE 2019).
Above- and below-ground vegetative biomass, shoot density, and shoot length data showed a normal distribution, so we used parametric statistics for their analyses (Zar 2010). Between-year comparisons were done over the same months: June-November 1996 and June-November 1997. Given the broad classification of our experimental plots (depth gradient from the subtidal to the intertidal zones, and between years) and to test whether the complete sets of homologous variables were significantly different, we applied parametric and non-parametric statistics, using the normal approximation where appropriate. In all cases, α = 0.05. The performed statistical tests, both Student’s t test and the parametric correlations, are summarized in Table 1.
Table 1 Statistical tests performed on data for Zostera marina vegetative and reproductive biomass in San Quintín Bay, Baja California, Mexico. Sample size = n.
| Statistical test | Variables | n | Test value | P value |
| Student’s t | Annual vegetative biomass (1996 vs 1997) | 18 | 8.43 | <0.05 |
| Above and below ground vegetative biomass at the subtidal (1996 vs 1997) | 18 | 10.72 | <0.05 | |
| Vegetative shoot density intertidal vs subtidal | 18 | 7.56 | <0.05 | |
| Correlation (r) | Vegetative shoot length intertidal vs subtidal | 18 | 9.23 | <0.05 |
| Vegetative biomass vs depth | 6 | 0.74 | <0.05 | |
| Above ground vegetative biomass at the subtidal vs below ground | 18 | 0.84 | <0.05 | |
| June-Feb vegetative biomass at the subtidal vs temperature (1996) | 9 | 0.74 | <0.05 | |
| Feb-Nov vegetative biomass at the subtidal vs temperature (1997) | 10 | 0.78 | <0.05 | |
| Vegetative shoot density vs depth | 6 | -0.50 | <0.05 | |
| Vegetative shoot density vs vegetative biomass | 18 | 0.57 | <0.05 | |
| Vegetative shoot length vs depth | 6 | 0.94 | <0.05 | |
| Vegetative shoot length vs vegetative biomass | 18 | 0.72 | <0.05 | |
| Subtidal and intertidal reproductive biomass vs reproductive shoot density (1996 and1997) | 15 | 0.81 | <0.05 | |
| Reproductive shoot density vs depth | 6 | 0.69 | <0.05 | |
| Reproductive shoot density vs vegetative density | 15 | 0.59 | <0.05 | |
| Seeds/reproductive shoot vs reproductive biomass | 10 | 0.69 | >0.05 | |
| Reproductive effort vs vegetative density | 15 | 0.62 | <0.05 | |
| Reproductive effort vs reproductive biomass | 15 | 0.72 | <0.05 |
RESULTS
Low water temperatures were recorded during winter-spring, with the lowest (16.9 ºC) in January 1996. Temperature increased during summer-autumn. The highest water temperature (23.8 ºC) was recorded in August 1997. In 1997 water temperature was 1.5 ºC higher than in 1996, and it was higher than air temperature from January through April. During the same months, air temperature was 14.7 ºC higher in 1997 than in 1996. Mean air temperature was 5.3 ºC higher in 1997 than in 1996. In February 1997 mean air temperature was 13.2 ºC, and in August 1997 it reached 29.9 ºC. The largest water-air temperature difference was 15.0 ºC.
Photons showed no trend over time, nor were they correlated with other variables. They were higher in 1996 (508.67 ± 113.6 µE m-2·s-1) than in 1997 (372.3 ± 62.9 µE m-2·s-1). Salinity did not show any noticeable effects on the biological variables. Exposure time for intertidal Z. marina beds ranged from 3.0 to 4.5 h during sunlight hours at low tides, for 10-19 days per month. We did not find a significant statistical effect of exposure time on any Z. marina biological variables in the intertidal zone. The results of the performed statistical tests are shown in Table 1.
Annual mean vegetative biomass (g DW·m-2) was significantly higher in the subtidal environment than in the intertidal environment, and it gradually decreased from -1.90 to +0.20 m (Table 2). Vegetative biomass was higher by 68.3% in 1996 than in 1997 (Table 2). Above-ground biomass (leaves and sheaths) was higher than below-ground biomass (roots and rhizomes) at all subtidal sites year-round (Fig. 1). Subtidal below-ground biomass was lower than intertidal above-ground biomass from November through April. Above-ground vegetative biomass was significantly correlated with water temperature but not with below-ground vegetative biomass. Almost all biological parameters were higher in 1996 than in 1997, especially above-ground biomass in the subtidal environment. Vegetative biomass was associated with water temperature from June 1996 to February 1997 and from February to November 1997.
Table 2 Mean vegetative biomass values (g DW·m-2) for shoots (above-ground biomass) and rhizomes/roots (below-ground biomass) at each site sampled in the subtidal and intertidal environments. Mean values are also shown per year (1996, 1997) for above- and below-ground vegetative biomass and per row.
| Subtidal | Intertidal | |||||||
| -1.9 m | -0.50 m | -0.10 m | +0.01 m | +0.10 m | +0.20 m | Mean | ||
| Above | 303.3 | 227.5 | 230.8 | 152.9 | 113.9 | 74.2 | 183.8 | |
| Below | 99.6 | 71.8 | 68.4 | 57.2 | 42.8 | 35.1 | 62.5 | |
| 1996 | 232.5 | 201.9 | 172.4 | 119.7 | 93.9 | 70.2 | 148.4 | |
| 1997 | 171.1 | 102.3 | 125.9 | 89.9 | 63.7 | 41.1 | 99.0 | |
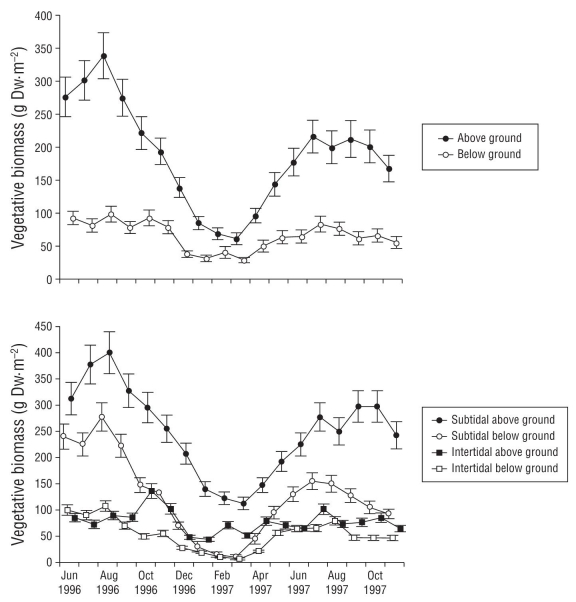
Figure 1 Annual above- and below-ground biomass trends (upper panel) and annual above- and below-ground vegetative biomass trends (lower panel) for Zostera marina in subtidal and intertidal environments in San Quintín Bay between June 1996 and October 1997. Bars in both panels are standard errors.
Vegetative shoot density was higher in the intertidal environment than in the subtidal environment (Fig. 2). The highest vegetative shoot density was found at +0.20 m, decreasing at greater depths, with the lowest density at -1.90 m. Shoot density was highest at +0.01 and +0.20 m. In the subtidal environment, at -0.20 m, the highest shoot density was 1,460 ± 286.5 shoots·m-2 and at the same depth the minimum was 472.1 ± 85.77 shoots·m-2. Vegetative shoot density was not statistically different between years and was negatively associated with depth. It was also positively associated with vegetative biomass and with above- and below-ground biomass at almost all sites, but not with below-ground biomass at -1.90 m.
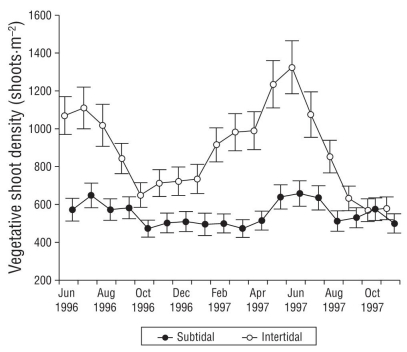
Figure 2 Annual trends of vegetative shoot density in subtidal and intertidal environments in San Quintín Bay between June 1996 and October 1997. Bars are standard errors.
Mean vegetative shoot length (Fig. 3) was higher by more than 50% in the subtidal environment (43.5 ± 19.6 cm) than in the intertidal environment (20.9 ±12.4 cm). Shoot length was highest (87.2 ± 38.7 cm) at -1.90 m, and it decreased at shallower sites, with the lowest (8.3 ± 2.7 cm) at +0.20 m. Vegetative shoot length in 1996 was statistically higher than in 1997, showed correlation with depth, and was significantly correlated with vegetative biomass.
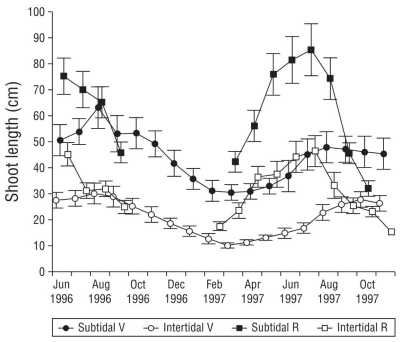
Figure 3 Annual trends of vegetative shoot length in subtidal and intertidal environments in San Quintín Bay between June 1996 and October 1997. Bars are standard errors. V, vegetative; R, reproductive.
Mean reproductive biomass was 9.2% of vegetative biomass (11.3 ± 3.2 g DW·m-2). Its proportion in 1997 was higher than in 1996 (Fig. 4). Mean reproductive biomass was higher in the intertidal zone than in the subtidal zone. During 1997, in both the subtidal and intertidal zones, reproductive biomass peaked in July and was correlated with reproductive shoot density. Reproductive shoot density was lower in the subtidal environment than in the intertidal environment (Fig. 5). Peak reproductive shoot density values were different through time. Reproductive shoot density was higher in 1997 than in 1996. In May we found 25.0 ± 15.2 reproductive shoots per square meter (RS·m-2) in the subtidal zone and in August 118.8 ± 24.4 RS·m-2 in the intertidal zone. The lowest density (16 ± 1.2 RS·m-2) was found at -1.90 m, and the highest density in the intertidal environment (46.6 ± 2.6 RS·m-2) was found at +0.20 m. Reproductive shoot density correlated with depth and vegetative density.
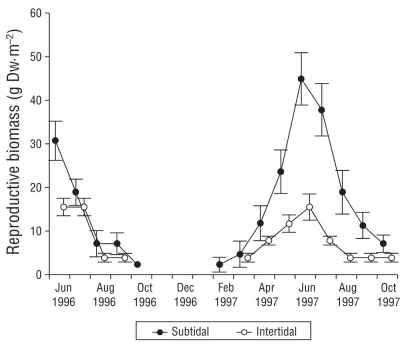
Figure 4 Reproductive Zostera marina biomass in subtidal and intertidal environments in San Quintín Bay between June 1996 and October 1997. Bars are standard errors.
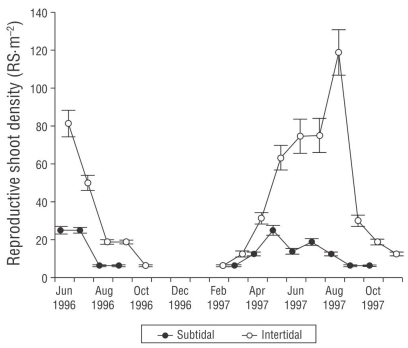
Figure 5 Reproductive Zostera marina shoot density in subtidal and intertidal environments in San Quintín Bay between June 1996 and October 1997. Bars are standard errors. RS = reproductive shoot.
Seed release took place from May to October in the subtidal environment and from May to November in the intertidal environment. Maximum mean values were 219.5 ± 45.8 seeds per reproductive shoot at -0.50 m, reaching 238.2 seeds per reproductive shoot in the subtidal environment, and 151.3 ± 21.5 seeds per reproductive shoot at +0.10 m, reaching 185.4 seeds per reproductive shoot in the intertidal environment, both in July. In 1997, the mean number of seeds per reproductive shoot in the subtidal and the intertidal environments was, respectively, 138 and 73 seeds·m-2 (Table 3), and it correlated with reproductive biomass.
Table 3 Structural features of Zostera marina reproductive shoots from both the subtidal and the intertidal environments in San Quintín Bay, Baja California, Mexico, for the years 1996 and 1997. Values show the mean (standard error) for the 4 sampled sites. SM = seeds·m-2; RE = reproductive effort (seeds g DW·m-2); SG = seeds per reproductive shoot; SW = shoot weight (g DW·shoot-1).
| Environment | SM | RE | SG | SW |
| Subtidal 1996 | 1,659 (125.0) | 3.4 (0.8) | 96 (8.6) | 0.71 (0.01) |
| Intertidal 1996 | 3,607 (295.4) | 10.4 (1.2) | 101 (11.6) | 0.42 (0.01) |
| Subtidal 1997 | 1,967 (23.4) | 6.2 (0.5) | 138 (11.5) | 0.62 (0.01) |
| Intertidal 1997 | 4,293 (489.9) | 25.5 (0.3) | 73 (6.6) | 0.38 (0.01) |
| Subtidal 1996 | 1,659 (125.0) | 3.4 (0.8) | 96 (8.6) | 0.71 (0.01) |
RE was higher in 1997 than in 1996. The June 1997 values were higher than those for June 1996 and they were higher in the intertidal environment. Mean RE using seed number was 22.6 ± 12.1 seeds g DW·m-2. REs was lower in 1996 than in 1997. In the subtidal environment mean REs decreased from May to October. In the intertidal environment REs was 61.5 ± 23.1 seeds g DW·m-2. REs started in May (36.8 ± 0.6 seeds g DW·m-2) and ended in November (3.4 ± 0.2 seeds g DW·m-2). We found maximum values for this variable in the intertidal environment in June, which coincided with maximum reproductive biomass (Fig. 3), one month earlier than the peak for vegetative biomass. In both years RE was lower at -1.90 m in the subtidal environment (6.2 ± 0.5 seeds g DW·m-2), while the highest in the intertidal environment was found at +0.10 m (25.5 ± 0.3 seeds g DW·m-2). Also RE values were higher at both depths and all sites in 1997 than in 1996 (Table 3). RE was positively correlated with vegetative density and reproductive biomass.
DISCUSSION
SQB is the southern range limit for Z. marina and other land and marine organisms in the Northern Hemisphere (Cabello-Pasini et al. 2002). It is located in a temperate zone limit and close to a desert; therefore, dramatic changes in air temperature are common. SQB was impacted by the 1997-1998 ENSO event, the most drastic ENSO event recorded in modern history (Kumar et al. 2001). We recorded a 1.5 ºC increase in sea surface temperature in 1997 in SQB with respect to 1996. This increase is higher than the 0.25 ºC increase measured during other ENSO events (Trenberth et al. 2002). This extraordinary increase was critical for Z. marina, as shown by the low above-ground biomass produced and by the below-ground biomass at a smaller scale. The same trend was reported by Johnson et al. (2003) for San Diego, California, USA, about 280 km due north, and by Shields et al. (2018) for Chesapeake Bay, Virginia. This decrease in biomass production associated with the increase in water temperature is evidence that Z. marina underwent continuous stress during the ENSO event. We confirmed our hypothesis that during our study period, the increase in surface water temperature caused some of the productivity and phenological differences between years. In June, at low tides, intertidal eelgrass beds in SQB were exposed to higher air temperatures (39.9 ºC) in 1997 than in 1996 (20.1 ºC). Minimum temperatures in November were 3.9 ºC and 12.8 ºC for 1996 and 1997, respectively. In SQB Cabello-Pasini et al. (2003) recorded 22.0 ºC as maximum mean air and water temperatures during 1999-2000, but we recorded 28.5 ºC for air temperature and 23.8 ºC for water temperature. Mean temperature was 2.4 ºC warmer in air than in water during 1997. Similar water temperature values were found by Hernández-Carmona et al. (2011) on the central Baja California coast, 300 km south of SQB.
The only variables that showed no differences between years were vegetative and reproductive densities. All the other variables measured for Z. marina showed significant differences as a function of depth, site, time, and year (Tables 2, 3). Differences were notable for vegetative above-ground biomass and very evident for reproductive phenology. Differences between subtidal and intertidal depths were evident, but they were more site-specific, especially in both subtidal and intertidal boundaries. The intertidal environment had lower vegetative and reproductive biomass, shorter vegetative and reproductive shoots, higher vegetative and reproductive shoot density, and a longer reproductive cycle.
Although RE decreased at the deepest site (-1.90 m), the best Z. marina stands were found between -0.50 and +0.10 m. This distribution of reproductive shoot density suggests that Z. marina is more parsimonious in the area that overlaps the subtidal and intertidal environments, and its distributional edges are well adapted to colonize new spaces. Both extremes in the depth range, -1.90 and +0.20 m, in SQB act as shoot expansion limits for Z. marina.
Edge theory states that organisms in edge populations face harsher conditions, whereas populations that survive and reproduce in the distributional boundary may disperse a greater number of seeds than those entering the boundary from outside (Cadenasso et al. 2003). In SQB the deepest edge acts as a control for the reproductive shoot, and the highest edge as a control for vegetative biomass. We found reduced RE in a small number of reproductive shoots at -1.90 m, the deepest site. We also recorded low vegetative biomass at +0.20 m (Table 2). Light is probably limiting the distribution of Z. marina at deeper sites (Cabello-Pasini et al. 2002, Bertelli and Unsworth 2018), and desiccation is limiting its expansion to elevations above the tideline with longer air exposure (Nielsen et al. 2002). Zostera marina confronts these conditions with high vegetative biomass and shoot density. Edge populations may experience greater variability in survival and reproduction because they experience both harsh and limiting factors more frequently (Sexton et al. 2009). Here, sexual reproduction plays an important role in the species’ adaptation to a constantly changing environment or in the invasion of new spaces. Given the unknown and largely unpredictable changes in the environment that will be produced by climate change, the chances of survival for Z. marina can change drastically at its southernmost range.
The responses of reproductive shoot density to light gradients found in our study are similar to those reported for perennial populations of Z. marina and other seagrass species (Henderson and Hacker 2015). Lee et al. (2005) suggested that increases in water temperature trigger RE. Qin et al. (2014) found that in response to subtidal disturbances and stress, Z. marina increased seed production and RE. This trend has been described for other seagrasses (Cabaço and Santos 2012) but was not the case for Z. marina at -1.90 m in SQB.
Vegetative and reproductive shoot lengths and densities were not significantly different between years. Vegetative and reproductive shoot lengths were not affected by temperature, but they were significantly associated with depth. Longer shoots were found at deeper sites, but the effect was negative on shoot density; Nielsen et al. (2002) and Henderson and Hacker (2015) reported similar results. The length of the reproductive cycle and the timing of reproductive shoots and their release vary with light or temperature (Johnson et al. 2003, Lee et al. 2005). We found differences in biomass but not in density between years. Similar patterns were described by Olesen and Sand-Jensen (1994) for eelgrass populations subject to severe biomass reductions, where biomass was predominantly allocated to extensive shoot recruitment.
Climate change is shifting the ranges of taxa, communities, and ecosystems, and the dynamics of populations that inhabit the latitudinal margins of the ranges will be critically important in determining species responses to climate change (Hampe and Petit 2005). SQB is a lagoon with low incident radiation and high turbidity (Nielsen et al. 2002, Cabello-Pasini et al. 2003), where the seasonal temperature trend has an important effect on biomass and growth (Ibarra-Obando et al. 1997, Poumian-Tapia and Ibarra-Obando 1999, Cabello-Pasini et al. 2003). We observed the same trend in our study, high biomass in summer and low in winter (Figs. 1-3). We found that above-ground vegetative biomass increased by more than 4-fold in 1996-1997 (187.5 ± 79.7 g DW·m-2) compared with that during the 1999-2000 normal conditions (40.0 ± 1.6 g DW·m-2) (Cabello- Pasini et al. 2003) and the 1992-1993 ENSO event (57.02 g DW·m-2) (Echavarria-Heras et al. 2006). Most of these differences could be attributed to ENSO effects, as shown by the low biomass values during the 1986-1987 ENSO (Ibarra-Obando et al. 1997). The low biomass reported by Ibarra-Obando et al. (1997) during the 1986-1987 ENSO is underestimated because they only analyzed leaf biomass at the intertidal level. Intertidal shoot density in our study was 889.6 ± 152.7 shoots·m-2, which is similar to the 400-1,390 shoots·m-2 found during the 1991-1992 ENSO event by Echavarria-Heras et al. (2006) but slightly different from the 695 ± 54 shoots·m-2 found during 1999-2000 (non-ENSO years) by Cabello-Pasini et al. (2003). These studies show that shoot density was not significantly affected by ENSO events.
Reproductive strategies can result from optimal resource allocation between vegetative growth and sexual reproduction (Freitas-Coelho et al. 2005), with a balance being established between vegetative and reproductive biomass to fit environmental conditions and increase survival or reproduction. In plants RE accounts for most of the energy a population invests in sexual reproduction (Klinkhamer et al. 1990). Zostera marina showed lower RE values in the subtidal environment. At subtidal depths, available energy goes mainly to vegetative shoot reiteration, while in the intertidal environment, a larger amount of energy is channeled to sexual reproduction, as evidenced by the differences in the number of reproductive shoots in each zone. Zostera marina has been shown to increase its RE over a stress gradient or in a disturbed area (Cabaço and Santos 2012, Qin et al. 2014, Blok et al. 2018).
The perennial populations of Z. marina in subtidal and intertidal environments at SQB seem to be well adapted to the local conditions. Their best reproductive strategy is to produce vegetative lateral shoots year-round. Nonetheless, sexual reproduction is important for maintaining the species’ genetic variability, representing a potential tool to insure persistence through a seed bank during catastrophic environmental events: ENSO, extraordinary storms, or climate change.
Seagrass habitats are declining (Cabello-Pasini et al. 2003). Thus, it is important to keep seagrass habitats in good health by maintaining or improving their conservation status. We believe there is a stress gradient that increases from the subtidal to the intertidal environment, with reproductive structures being longer in the subtidal environment as a response to the less stressful environmental conditions. Stress is mainly caused by desiccation due to air exposure and high surface water temperatures. The second most important stress factor would be light intensity at the intertidal level.
Resources are allocated to attain more or faster reproductive growth under harsher conditions (Cabaço and Santos 2012). We recorded lower RE rates in the subtidal environment than in the intertidal environment as a result of less energy being invested in sexual reproduction (Figs. 4, 5). Energy in the subtidal environment is used to increase vegetative shoot density. The sexual life cycle of Z. marina in SQB was shorter in the subtidal environment.
Eelgrass ecosystem structure and function are shifting because of drivers that range from local to global scales. Zostera marina meadows are declining in distribution and total area (Cabello-Pasini et al. 2003), and these changes are attributed to coastal degradation and climate change. These changes are not completely understood, nor do we know what coastal areas will be affected in the short and long terms. In SQB water turbidity has increased by 2-fold in the last 2 decades (Cabello-Pasini et al. 2003). As a consequence, the number of subtidal Z. marina meadows has decreased, and shallower intertidal mudflats have been colonized (Cabello-Pasini et al. 2003). Echavarria-Heras et al. (2006) concluded that the 1986-1987 ENSO event had a negative impact on the SQB eelgrass meadows. Solana-Arellano et al. (2008) mentioned the negative effects of the 1982 ENSO event in Punta Banda Estuary, 160 km north of SQB, and Johnson et al. (2003) reported that off San Diego, California (USA), the 1997-1998 ENSO event had a negative impact on Z. marina but a positive one on Ruppia maritima. The extreme 1997 ENSO event probably caused the 64.2% decrease in Z. marina biomass as water temperature increased by 1.5 ºC, compared with the 0.25 ºC increase associated with other ENSO events (Trenberth et al. 2002). The warmer water conditions increased stress in the environment and forced a 53.8% increase in RE. Blok et al. (2018) suggested that warmer waters could increase the capacity for sexual reproduction at northern latitudes where the waters are colder. This condition reduced vegetative biomass and allowed R. maritima to invade new spaces and displace or stop the expansion of Z. marina (Johnson et al. 2003). The increase in sexual reproduction in Z. marina could be associated with its ability to adapt to new environments and it could enable ecological specialization (Hadany and Otto 2009). It has been suggested that sexual reproduction is an important adaptation mechanism in stressful environments that forces plants to produce seeds in large quantities (Cadenasso et al. 2003), increasing the seed bank and allowing the invasion of new spaces. The evolution of sexual reproduction could be an adaptive strategy to cope with environmental stress and it could be associated with specializations for living under stress. Specialization in a specific stressful environment does not imply that a population will perform well when confronted with other environmental stressors (Hadany and Otto 2009).
We don’t know for how long or which consequences the Z. marina ecosystem will face when affected by the seemingly unpredictable trends in climate change. There are other stressors not considered here: water turbidity, scarce nutrient flux, light attenuation, and human impacts. These stressors can act alone or in conjointly (Cabello-Pasini et al. 2003, Echavarria-Heras et al. 2006). Also, the result of competition with invaders like R. maritima is uncertain. In Chesapeake Bay, Shields et al. (2018) showed that the density of Z. marina exhibited periods of decline followed by recovery, increased reproductive output, and regrowth after water temperature and other water parameters improved but that the invading R. maritima dominated many areas of the bay previously dominated by Z. marina, posing a threat to the survival of Z. marina.
Our data suggests that eelgrass living at -1.90 m produce more vegetative biomass but are less sexually active, that shoots between -0.05 and +0.01 m produce vegetative biomass and are sexually active, and that shoots at +0.10 and +0.20 m show high sexual reproduction. Intertidal Z. marina showed higher vegetative shoot density and RE, while subtidal Z. marina showed high vegetative biomass and long shoots. Shoots on boundary edges are continuously under stress and will probably be the most drastically affected by climate change and catastrophic events.











 texto en
texto en 


