Introduction
One of the most diverse and ecologically important tree genera in the tropics is Ficus L. (Moraceae). Within Ficus, there are ca. 876 species (POWO, 2021), with different growth forms and breeding systems, as well as a wide variety of shapes, sizes, anatomical wall structures, and colors of syconia (Berg, 1989; Kravtsova and Carvajal, 1995; Berg and Corner, 2005; Lomáscolo et al., 2008). Species of this genus are considered a keystone resource in many tropical ecosystems (Janzen, 1979; Herre et al., 2008), since they provide food for a diverse assemblage of mammals, birds, and other fig-eating animals, which also disperse their seeds (Shanahan et al., 2001).
This genus is characterized by having an urn-shaped and nearly completely closed syconium, with an apical entrance called the ostiole (Berg, 1989; Verkerke, 1989). The syconium initially functions as an inflorescence, which contains pistillate and/or staminate flowers; with the development of the fruits, the syconium then becomes an infructescence (Galil and Eisikowitch, 1968; Berg, 1989; Verkerke, 1989). Ficus species are closely dependent on wasps of the family Agaonidae because they are the figs’ sole pollinators (Ramírez, 1974; Janzen, 1979). In turn, the wasps depend on the fig for reproduction because their larvae feed only on gall flowers (Ramírez, 1970a; Jousselin and Kjellberg, 2001). This interaction is considered a classic example of coevolution (Ramírez, 1974; Hembry and Althoff, 2016).
The complex development of syconia reflects adaptation to pollination by agaonid wasps and has been divided into different developmental phases (Galil and Eisikowitch, 1968; Smith and Bronstein, 1996; Piedra-Malagón et al., 2019). However, there is considerable diversity in this obligate mutualism, and fig-pollinator wasp interactions vary. For instance, half of all Ficus species are monoecious, with both wasps and seeds produced in the same syconium (Verkerke, 1989), while the remaining Ficus are gynodioecious, where the syconia of female trees give rise only to seeds, and the functionally male trees solely to wasps (Berg, 1989). Recently, gynomonoecy was discovered in F. umbrae Ezedin & Weiblen; seed syconia have pistillate flowers and non-functional male flowers, while gall syconia have fully functional male and female flowers (Ezedin and Weiblen, 2019).
Five to eight phases of syconium development have been described in monoecious species; phases B (female phase) and D (male phases) are the phases during which the wasps interact most closely with the different structures inside of the syconium (Galil and Eisikowitch, 1968; Verkerke, 1989; Smith and Bronstein, 1996; Piedra-Malagón et al., 2019; Delgado-Pérez et al., 2020). During Phase B, a limited number of female wasps enter the syconium through the ostiole, depending on the size of the syconium. Small syconia have few flowers which are often small (Murray, 1985; Liu et al., 2011), which are probably visited by fewer foundress wasps, and have smaller pollen-transfer genetic neighborhoods than large syconia visited by larger wasps (Borges, 2021).
In addition, there are different pollination behaviors among agaonids; some pollinate actively and others passively (Jousselin and Kjellberg, 2001). In active pollination, female wasps collect pollen from the anthers during the male phase (Phase D) before leaving their natal figs and place it in special thoracic structures called corbiculae and coxal combs to transport them to another syconium with receptive pistillate flowers (Phase B) (Ramírez, 1969). Once inside a receptive fig, the wasp puts a bit of the pollen from its corbiculae on the stigmatic surface each time it lays an egg (Ramírez, 1970a). In the case of passive pollination, the pollinator does not show any pollen collection and deposition behavior and lacks corbiculae (Galil and Neeman, 1977; Kjellberg et al., 2001). Active pollination has probably evolved to improve progeny nourishment and larval survivorship, as fertilized ovules may provide a better feeding substrate for the developing larvae (Jousselin et al., 2003). Also, this type of pollination behavior is likely to be more efficient at transferring pollen than passive pollination (Kjellberg et al., 2001).
Distinctive morphological adaptations in both wasps and figs have been associated with pollination behaviors. In most species with active pollination, the stigmas can be cohesive, forming a common surface for pollen tube germination (the synstigma), which may help ensure seed production in flowers when pollinating wasps do not deposit pollen directly in the pistillate flower (Jousselin and Kjellberg, 2001; Teixeira et al., 2018, 2021). Furthermore, the pollen of actively pollinated fig species is ellipsoid, and the anther-to-ovule ratio is low (A/O<0.16), since they produce less pollen, because wasps can only carry a limited amount of pollen in their corbiculae (Jousselin and Kjellberg, 2001; Wang et al., 2014). Active pollinator wasps present sternal corbiculae, coxal corbiculae, and coxal combs (Kjellberg et al., 2001). However, there can be passive pollinator species within genera that are mostly active pollinators; these species may have reduced sternal corbiculae and lack coxal corbiculae and combs. Examples include Pegoscapus carlosiRamírez, 1970, P. mariae Ramírez, 1970, and Ceratosolen galili Wiebes, 1964. In the case of passively pollinated Ficus species, the synstigma is absent, the pollen is spherical or cylindrical, and the anther-to-ovule ratio varies from 0.29 to 0.92; their pollinator wasps lack corbiculae and coxal combs (Kjellberg et al., 2001; Teixeira et al., 2018).
Phase E of syconium development is particularly relevant in the interaction of Ficus species with their seed dispersers, since the size and coloration of ripe syconia are directly related to the type of frugivore that consumes them, and the distance seeds are dispersed (Shanahan et al., 2001). For example, small syconia, which are often red, are probably consumed by small birds with short dispersion distances, contributing to more local gene movement (Borges, 2021). Meanwhile, larger birds and bats and other mammals prefer large, dull-colored fruits and disperse seeds at longer distances (Shanahan et al., 2001). Nevertheless, the dispersal distance depends on foraging behavior, since bats of the family Phyllostomidae fly to temporary feeding roosts near the fruiting tree, which limits their effective dispersal distance (Heer et al., 2015).
In several monoecious Ficus species, the development of syconia in an individual tree is usually highly synchronized (Janzen, 1979; Cook and Power, 1996). However, in highly seasonal environments where tree reproduction and wasp dispersal are likely to be reduced during cold and/or dry periods, Ficus populations can break within-tree synchrony in order to allow the persistence of small pollinator populations year-round (Ramírez, 1970a; Janzen, 1979; Smith and Bronstein, 1996). In small populations, rather than promoting selfing, it appears that within-tree flowering asynchrony enhances reproductive assurance by increasing opportunities to contribute pollen to and receive pollen from other trees, thus enhancing both the male and female components of fitness (Gates and Nason, 2012). Also, the presence of syconia throughout the year may be an important resource in periods of low fruit availability in seasonal tropical and subtropical forest ecosystems (Shanahan et al., 2001; Bianchini et al., 2015; but see Compton and Greeff, 2020).
The developmental phases and morphological changes of the syconium have been characterized in only a few fig species in the Americas (e.g., Hernández Sosa and Saralegui Boza, 2001; Piedra-Malagón et al., 2019; Delgado-Pérez et al., 2020; Cervantes-Pasqualli and Laborde, 2021). This characterization is essential to identify the critical phases involved in the pollination and dispersion of Ficus species. There are three endemic species of Ficus in Mexico, one of which is F. pringlei S. Watson (Quintana and Carvajal, 2001; Ibarra-Manríquez et al., 2012). Unlike most species of the subgenus Spherosuke Raf., which have a hemiepiphytic or strangler habit (Berg and Corner, 2005), F. pringlei is a rupicolous tree up to 12 m high that grows on rocky outcrops or cliffs in the Mexican states of Colima, Guerrero, Jalisco, Michoacán, Nayarit, and Zacatecas (Serrato et al., 2004; Durán-Ramírez et al., 2010; Carvajal, 2012; Ibarra-Manríquez et al., 2012). The syconia of F. pringlei have a diverse associated community of wasps, but all are undescribed, including its pollinator (Pegoscapus sp.) and several non-pollinating genera of Chalcidoidea. Furthermore, detailed ecological information about the potential distribution and reproductive aspects of F. pringlei (e.g., description of developmental phases of syconia and morphological adaptations related to wasp pollination behavior) is unavailable.
This study therefore aimed to i) describe and illustrate the main changes over the course of the developmental phases of F. pringlei syconia, ii) determine the type of pollination of this Ficus species, and iii) detect areas of suitable environments to locate F. pringlei using ecological niche modeling (ENM). Achieving these objectives is relevant to understanding the reproductive biology of these rare species, particularly their relationships with their pollinators and potential frugivores in the tropical dry forest (TDF) and discovering new locations where F. pringlei has not yet been recorded.
Material and Methods
Sampling and preparation of material
Branches with syconia in the different development phases were collected from five individuals of F. pringlei in August 2021 from three locations: Colima (19.3466°N, 103.8412°W, one individual), Jalisco (21.0308°N, 103.4607°W, two individuals), and Zacatecas, Mexico (21.1783°N, 103.5332°W, two individuals).
The syconia were preserved in 70% ethanol. The characterization of Smith and Bronstein (1996) and Piedra-Malagón et al. (2019) were used to establish typical phases of development. Five figs of each phase of development were cut in half and fixed in 70% ethanol, dehydrated in an ethanol series, and processed in the laboratory of Escuela Nacional de Estudios Superiores (ENES), Universidad Nacional Autónoma de México (UNAM) in Morelia, Mexico.
More than ten mature stamens from two figs were collected to measure pollen size (20 grains), pollen shape based on Reitsma (1970), and detailed exine ornamentation according to Wang et al. (2014). The wasps were collected from closed syconia in Phase D and stored in 70% ethanol. The fore coxae and the ventral and lateral parts of the female thorax were examined to establish whether the fore coxa bore a comb (a line of setae) and corbiculae were absent or present (either fully developed or reduced).
The external morphology of the stigma, pollen, and wasps was assessed using a Jeol JSM-IT300LV scanning electron microscope (SEM; JEOL, Peabody, USA). Previously fixed samples were dehydrated in an ethanol series, critical point dried in an Autosamdri-815 Series A critical point dryer (Tousimis, Rockville, USA), adhered to metal sample holders, and sputter-coated with gold in a Denton Desk V instrument (Denton Vacuum, Moorestown, USA). The A/O ratio was measured by counting male and female flowers in five Phase C figs from five trees.
Ecological niche modeling
Ecological niche models were used to examine the potential distribution of F. pringlei. All analyses were performed with the software R v. 4.0.3. (R Core Team, 2020). Data on the presence of this species were obtained from a critical review of the material deposited in the National Herbarium of Mexico (MEXU, 2019), Global Biodiversity Information Facility (GBIF, 2020), and new records in the field (Appendix). The initial data set was reduced by removing duplicates and records that were not georeferenced or whose coordinates were inconsistent with the locality or municipality. The spThin package (Aiello-Lammens et al., 2015) was used to minimize the spatial autocorrelation of records, leaving only one record within a 10 km range in the final data set.
Nineteen bioclimatic variables available from WorldClim v. 1.4 (Hijmans et al., 2005; WorldClim, 2020) were initially included as predictor variables for the niche model, with a spatial resolution of 2.5 arc-min. Highly correlated variables (r>0.85) were subsequently detected with the function correlation_finder of the package nichetoolbox (Osorio-Olvera et al., 2020), which resulted in the selection of the following nine bioclimatic variables: annual mean temperature, annual mean diurnal range, isothermality, temperature seasonality, annual precipitation, precipitation of driest month, precipitation seasonality, precipitation of warmest quarter, and precipitation of the coldest quarter. The union between 12 physiographic subprovinces (INEGI, 2001) and a buffer area of 200 km around the presence records were used for the calibration area.
Model calibration, evaluation, and ensemble were performed with the package biomod2 (Truiller et al., 2020), which combines multiple algorithms to reduce uncertainty (Araújo and Guisan, 2006; Thuiller et al., 2009). Five algorithms were used: artificial neural network (ANN), generalized additive model (GAM), generalized boosted model (GBM), generalized linear model (GLM), and maximum entropy model (MaxEnt). For each algorithm, except for MaxEnt, default configurations were used (obtained with the kuenm package; Cobos et al., 2019). The combinations of four parameters (FC: linear, quadratic, product, and hinge) were used to determine MaxEnt configurations, which adjust the flexibility in the response of the model and the regularization multipliers (RM: 0.5-2.5 (intervals of 0.5) and 3-6 (intervals of 1, 8, and 10), which penalize the complexity of the model.
The best model was selected based on three criteria: 1) statistical significance (based on partial ROC analysis), 2) predictive capacity (5% omission rate, OR), and 3) complexity of the model (evaluated with AIC). Since the occurrence data set consisted only of presence data, a set of 2000 pseudo-absence data were generated. To calibrate the model developed in biomod2, ten repetitions of each algorithm were used with different calibration and evaluation data; 80% of all data was for calibration. The models with the ROC metric were tested, assembling only those with a ROC value ≥0.8.
The ensemble model was used to project the potential distribution under current climatic conditions. The importance of the variables in the model assembled with three permutations was also determined. This model was validated with statistical significance of the partial ROC (≤0.05) and the dataset of new records that were not used in the calibration process in nichetoolbox. The continuous maps of climatic suitability were transformed into a binary map of presence and absence using the threshold of the probability of occurrence that maximizes the ROC metric (Thuiller et al., 2009). The predicted area was compared with the vector data set of land use and vegetation at a scale of 1:250000, Series VII (INEGI, 2018). Finally, the percentage of the predicted and available area to locate F. pringlei within Natural Protected Areas was evaluated using vector data (CONANP, 2022).
Results
Development of syconia and pollination mode traits
Phase A1. The syconia begin their development as two buds, in pairs, in the axil of a leaf, each protected by a pubescent basal bract (Figs. 1A, 2A, 3A). The first structure to differentiate at the apex of each syconium is the ostiole, which is composed of a series of overlapping bracts. The bracts are divided in three categories: i) external (or superficial), ii) transitional (located in the middle part of the ostiole and horizontally intertwined), and iii) internal or wall (with an arrangement inclined towards the interior of the syconium cavity) (Fig. 2A). In this phase, the internal bracts occupy the interior cavity of the syconium (Fig. 2A).

Figure 1: External characteristics of syconia development phases in Ficus pringlei S. Watson. A. top view syconium in Phase A1 (arrow, basal bract); B. Phase B; C. foundress female wasp in the syconium interior in Phase B; D. non-pollinating female wasps (arrows) on syconium in Phase B; E. Phase C1; F. Phase C2; G. syconium interior in Phase C2; H. Phase C3; I. syconia in Phase D with two exit tunnels (arrows) excavated by males; J. syconium in Phase E with two exit tunnels (arrows); K. syconium probably bitten by birds. L, M. syconia in different phases of development. bb=basal bracts.
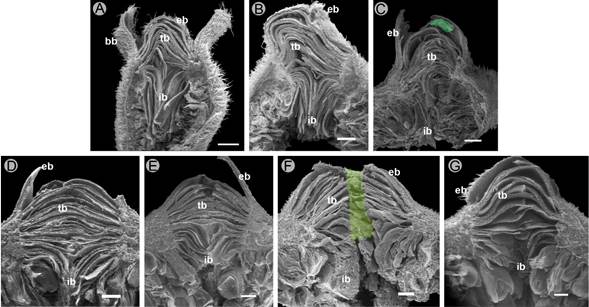
Figure 2: Longitudinal section of the syconium of Ficus pringlei S. Watson, in different stages of development. A. Phase A1; B. Phase B; C. Phase C1 with a pollinating wasp (green); D. Phase C2; E. Phase C3; F. Phase D with one exit tunnel excavated by males; G. Phase E. bb=basal bracts, eb=external bracts, tb=transitional bracts, ib=internal bracts. Scale bars: 500 µm.
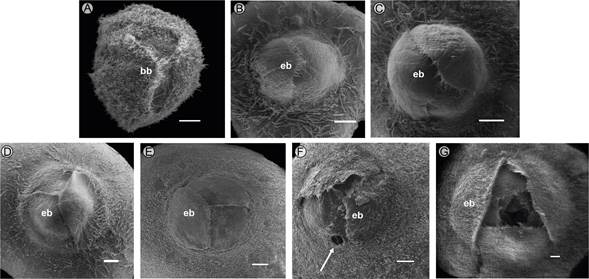
Figure 3: Superior view of the ostiole of Ficus pringlei S. Watson, in different stages of development. A. Phase A1; B. Phase B; C. Phase C1; D. Phase C2; E. Phase C3; F. Phase D with one exit tunnel excavated by males; G. Phase E. bb=basal bracts, eb=external bracts. Scale bars: 500 µm.
Phase A2. The morphological characterization is as described in the first phase, with an increase in the size of the syconium. Inside the syconium, the internal bracts are slightly lax, separated from each other, and their free ends extend towards the interior of the syconium. The stigmas of the pistillate flowers have a uniform distribution in the cavity of the receptacle (Fig. 4A), without developing papillae (Fig. 4B).
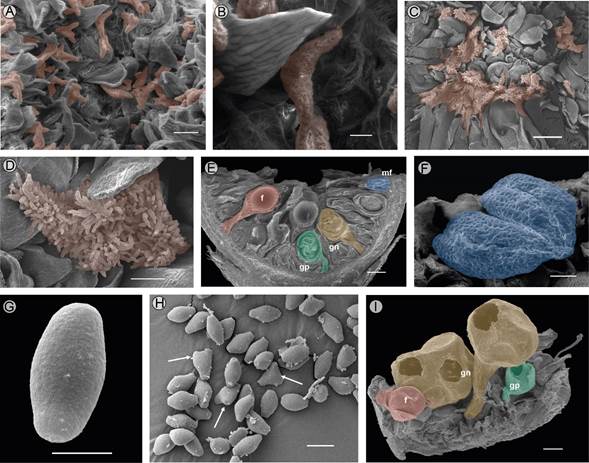
Figure 4: Internal morphology of different stages of development and pollen of Ficus pringlei S. Watson. A. stigmata in Phase A2; B. detail of stigmata in Phase A2; C. synstigma formed by the entanglement of styles and stigmatic papillae in Phase B; D. detail of stigmatic papillae; E. longitudinal section of the syconium in Phase C3; F. stamen with mature anther in Phase D; G. truncate obtuse rhombic pollen with psilate ornamentation; H. biporate and triporate pollen grains (arrows); I. section of syconium in Phase E. f=fruit, gn=galls of non-pollinating wasps, gp=galls of pollination wasp, mf=male flower. Scale bars: A=200 µm, B=50 µm, C=500 µm, D=100 µm, E=500 µm, F=100 µm, G=5 µm, H=10 µm, I=500 µm.
Phase B. The growth of the syconium continues, acquiring pale green macules; the basal bracts partially cover it (Fig. 1B). Inside the syconium, the pistillate flowers mature. The stigmas are receptive, forming a cohesive synstigma, with tight contact between the stigmatic branches through the entanglement of long papillae that compose the stigmatic surface (Fig. 4C-D). During this phase, the pollinator wasp enters the syconium (Fig. 1C) through the bracts of the ostiole, since the transition and internal bracts are less compact (Fig. 2B). It is common to find only one wasp, but up to ten were recorded. In some syconia, it can be inferred that a wasp entered because wings were found on the external bracts. Also, non-pollinating wasps were found ovipositing through the wall of the syconia (Fig. 1D).
Phase C1. The syconium continues to increase in size (Fig. 1E) while the fruits and wasps begin to develop. Dead wasps can be found among the bracts (Fig. 2C), and the ostiole is convex (Fig. 3C). Inside the syconium, the cavity is still well-defined, and no difference is detectable between the ovaries containing immature seeds and those containing wasps. The mean total A/O was 0.08±s.d. 0.03 (range 0.03-0.19). In the middle of the syconium near the ostiole, the mean A/O ratio was higher (0.06±s.d. 0.03) than at the base of the syconium (0.1±s.d. 0.04). The mean total number of pistillate flowers was 561.5±s.d. 138.8 and staminate flowers 43.7±s.d. 17.3.
Phase C2. The syconium continues to grow, and sometimes the external bracts turn reddish (Fig. 1F). The wasp larvae and fruits continue to develop, such that the syconium cavity nearly disappears (Fig. 1G). The transitional bracts are more compact (Fig. 2D), and the ostiole remains convex (Fig. 3D). The exoskeleton of the pollinator female wasp is compressed, dehydrated, and can be found in different degrees of decomposition. Frequently, it is also possible to observe nematodes or fungal hyphae feeding on the wasp.
Phase C3. The ostiole maintains the reddish color on the external bracts (Fig. 1H) and is nearly flattened (Fig. 3E). In the interior of the syconium, the development of the male flowers, fruits, and wasps is nearly complete. The bodies of the new wasps become visible through the ovary walls (Fig. 4E), which acquire a dark coloration, while those containing the fruits are yellowish.
Phase D. The syconium turns yellowish and is slightly soft, and the external bracts turn brown (Fig. 1I). The male wasps of the genus Pegoscapus sp. are the first to emerge; they break the walls of the ovary with their mandibles and copulate with the females, facilitating their exit from the ovary wall. The male wasps excavate tunnels through the walls of the syconium (Fig. 1I-J) or the ostiole (Figs. 2F, 3F), which allows the fertilized and pollen-bearing female wasps to exit the syconium. Internally, the stamens are mature (Fig. 4F). The pollen size is minute: mean polar axis 6.46 (range: 5.59-6.98 µm), mean equatorial axis 12.2 (11.23-13.52 µm), and the mean ratio of both axes 0.53 (0.42-0.61 µm). Based on the shape in equatorial view, pollen was mostly rhombic, with psilate ornamentation (Fig. 4G). Pollen grains are biporate or, more rarely, triporate (Fig. 4H).
Phase E. The syconia are reddish (Fig. 1J), glabrescent, with a slightly sweet taste, and the external and internal walls are very soft to the touch (Fig. 1K). The external and transitional bracts are lax (Figs. 2G, 3G). Internally, the structures within the syconium cavity acquire an overall dark brown color. The staminate and pistillate flowers are senescent; hollow galls are observed without wasps inside them, and the seeds are ripe inside the fruits. The most prominent galls (>1 mm) correspond to non-pollinating wasps (Fig. 4I). The development of the syconia was asynchronous (i.e., we found different development phases of the syconium among different branches of the same tree; Fig. 1L, M).
Pollinating wasps of both sexes of Pegoscapus sp. were collected. The females are winged (Fig. 5A), with lamellae on the mandibles (Fig. 5B). They have pollen pockets, coxal combs on their fore coxae, and an elongated depression that was bordered by the comb (Fig. 5C, D). Males, in contrast, are wingless, blind, and do not have structures to transport pollen (Fig. 5E). In phase D, different non-pollinating female wasps were also observed. These non-pollinating insects are larger than pollinating wasps and have red eyes (Fig. 5F).
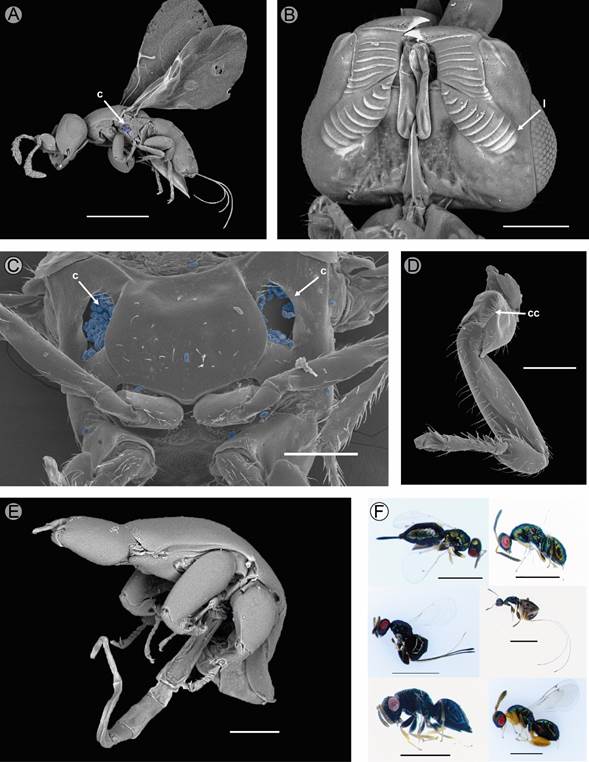
Figure 5: Pollinating (Pegoscapus sp.) and non-pollinating wasps of Ficus pringlei S. Watson. A. female wasp of Pegoscapus sp. (corbiculae, arrow); B. detail of the mandibles (arrow) of the female Pegoscapus sp.; C. corbiculae of Pegoscapus sp., pollen in blue; D. coxal combs on fore coxae and elongated depression bordered by the comb in Pegoscapus sp.; E. male wasp of Pegoscapus sp.; F. female non-pollinating wasps. c=corbiculae, cc=coxal combs, l=lamellas. Scale bars: A=500 µm, B=100 µm, C=100 µm, D=100 µm, E=200 µm, F=1 mm.
Ecological niche model
A total of 169 records were obtained with an elevation between 160 and 1806 m (Fig. 6, Appendix). The mean annual precipitation ranged from 661-1644 mm for these records, and the mean annual temperature was between 17.5-28.7 °C. In kuenm, 341 models were generated, and the best model selected was RM 5 and feature class quadratic and product. However, in biomod with 50 models, GBM and GLM performed best, followed by GAM, ANN, and MaxEnt. Four models generated with GBM (three models, ROC=0.814, 0.828, 0.803) and GLM (one model, ROC=0.805) were selected to build the ensemble model. The partial ROC value for the ensemble model was 0.921 (p<0.05).
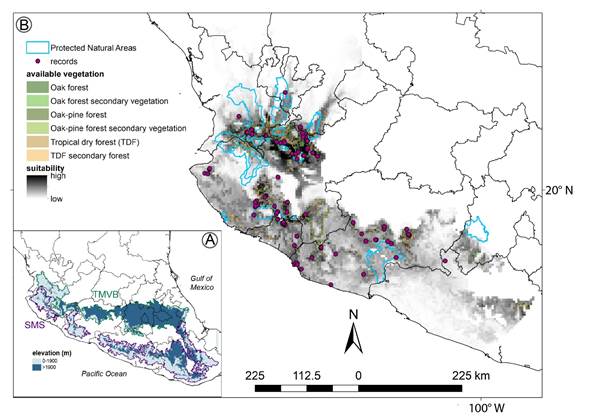
Figure 6: Potential geographical distribution of Ficus pringlei S. Watson, under current climate conditions. A. Trans-Mexican Volcanic Belt (TMVB) and Sierra Madre del Sur (SMS) biogeographical provinces; B. occurrence records, current potential geographical distribution, available vegetation, and Protected Natural Areas for F. pringlei.
Nearly three quarters (74%) of the predicted suitable area to found F. pringlei was in the states of Jalisco and Michoacán. There was a gap in the center of the distribution, corresponding to the highlands west of the Trans-Mexican Volcanic Belt (TMVB) and Sierra Madre del Sur (SMS) (Fig. 6). The most important variable in the ENM was temperature seasonality (0.42), followed by annual mean temperature, annual precipitation, precipitation of the coldest quarter, precipitation of warmest quarter, annual mean diurnal range, precipitation seasonality, isothermality, and precipitation of driest month (Fig. 7). However, only with temperature seasonality, a measure of temperature change over the year, and annual mean diurnal range, is possible to identify the climatic differences between the north and the south of the TMVB; both variables are higher in the former region (Fig. 7).
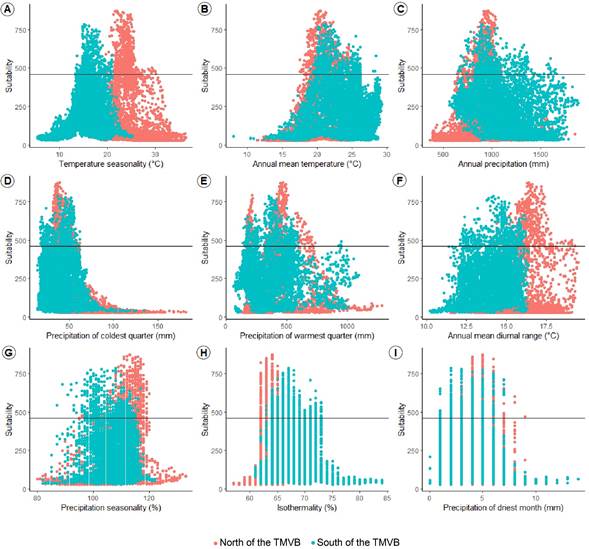
Figure 7: Habitat suitability and environmental variables in the ensemble model of Ficus pringlei S. Watson. The horizontal line indicates the value of the threshold to transform the model on presence and absence data. TMVB=Trans-Mexican Volcanic Belt.
The total predicted area to locate F. pringlei corresponds to 21968 km2; however, this is drastically reduced when considering the land cover type. Agriculture and other types of vegetation cover that are not suitable to find this species cover 57% of the total predicted area. Of the remaining area (9651 km2; 43.9% of the total predicted area), primary (1594 km2) and secondary (3832 km2) TDF vegetation were the vegetation types with the largest area, followed by forests of oak (2426 km2) and oak-pine (1799 km2) (Fig. 6). When considering the suitable area accounting for vegetation types, only 1926 km2 is contained within Natural Protected Areas. Most of this was north of the TMVB, with 74% recorded in the C.A.D.N.R. 043 Natural Resources Protection Area Forest Protection Zone in Nayarit and 7.8% in the La Primavera Forest Protection Zone and Wildlife Refuge. On the contrary, south of the TMVB, despite the existence of more Protected Natural Areas, only 18.2% (350.5 km2) of the total predicted suitable area was within Natural Protected Areas (Fig. 6).
Discussion
Development of syconia and pollination type
The most evident external morphological differences throughout the development phases of the syconium of F. pringlei are the gradual increase in size, changes in color, consistency, and shape of the ostiole (Figs. 1, 3). Transitional bracts are intertwined to form a helicoidal path towards the interior of the syconium cavity, like other representatives of the section Americanae (Miq.) Corner (Ramírez, 1974; Verkerke, 1989). Phase B, which is critical for pollination and lasts a few days, is mainly identified by the internal characteristics of the syconium and by the entry of the wasps. One of the most notorious internal characters was the presence of the receptive synstigma which, as in some species of the section Americanae (Teixeira et al., 2018, 2021), does not form a continuous surface. Rather, the cohesion of the synstigma is due to the intertwining of the stigmatic branches and papillae of a few flowers. The synstigma may act to distribute pollen tubes to the flowers located near those that have been pollinated, thus modifying the fertilization pattern, and maximizing the male reproductive success of the plant when pollen deposition is limiting (Teixeira et al., 2018, 2021).
Although the pollinating wasps of F. pringlei have not been described, it belongs to the genus Pegoscapus Cameron, 1906, since unlike the other genus of wasps Agaonidae found in the Americas (Tetrapus Mayr, 1885), the mandibular appendices are lamellas and have external and coxal corbiculae (Fig. 5B-C) (Ramírez, 1970b). A single foundress wasp was found within the syconia, but sometimes as many as ten were recorded. An extraordinarily high number of female foundresses per syconium could be a consequence of the asynchrony in the reproductive events among the population of Ficus trees, resulting in a very high temporal variability in syconium availability. This variation often has consequences for the reproductive success of both the wasps and the plants. When more than one foundress wasp is found, females may have fewer offspring and females (Herre, 1989), which may imply an increase in interbreeding of the new generation of wasps, but a decrease in the potential for pollen dispersal per syconium. Therefore, pollinators may use the density of broken wings in the ostiole to decide which syconium to enter, preferring an empty one (Ramya et al., 2011).
Conversely, Phase D can be inferred by external characteristics of the syconium, such as slightly soft consistency to the touch, yellow coloration, and the presence of holes in the syconium wall or in the ostiole, through which the female wasps leave the syconium. Male tunneling activity near the ostiole may be driven by a higher proportion of staminate flowers near the ostiole so that pollen can be loaded on females prior to their departure (Galil and Eisikowitch, 1968). From direct observations in fresh syconia, active pollination behavior was recorded in F. pringlei. These observations are supported by a low A/O ratio, the presence of synstigma and psilate pollen, as well as morphological traits to collect and store pollen efficiently in female wasps such as big sternal corbiculae, coxal corbiculae, and coxal combs. Pollen has been studied for few American species (e.g., Ibarra-Manríquez and Martínez-Hernández, 1997), but F. pringlei has pollen shape and ornamentation that is similar to other species that have active pollination (Wang et al., 2014).
A great diversity of non-pollinating wasps was found, some of which colonized the syconia during Phase B. Large galls generated by these wasps were also found. Gall size is usually characteristic of each wasp species and may structure the entire community of wasps occupying the syconium (Cardona et al., 2013; Compton et al., 2018) and even affect the fitness of the host Ficus tree (Zhang and Li, 2020) or the asynchrony of the development of the syconia (Krishnan and Borges, 2014). Therefore, it is essential to delve into the diversity, development, colonization, and temporal variation of these non-pollinating wasps.
Phase E was the most contrasting since the syconium turned reddish and was slightly sweet. The contrast between the syconium color and the foliage suggests that it may be dispersed by birds (Lomáscolo et al., 2008) over long distances (Shanahan et al., 2001). In some New World species, in which the seeds are primarily bird-dispersed, the production and maturation of syconia are less synchronous than in bat-dispersed syconia (Herre, 1996). Indeed, although determining the phenological strategy was not the objective of this work, the field observations throughout the study show that asynchrony in the crown of an individual is possible, since syconia in different phases of development can be recorded on branches (Fig. 1A, L-M). This strategy may allow the persistence of pollinator populations and increase opportunities to provide and receive pollen from other trees in small populations and highly seasonal environments (Ramírez, 1970a; Janzen, 1979; Gates and Nason, 2012; Smith and Bronstein, 1996).
Ecological niche model
In the search for presence data, F. pringlei is frequently sympatric with F. cotinifolia Kunth and they are morphologically similar in the absence of figs. This could lead to erroneous determinations of both species in the herbarium specimens and in the field. However, as Ibarra-Manríquez et al. (2012) point out, F. pringlei can be distinguished because the leaves are always pubescent on the abaxial side, the tertiary venation is very conspicuous, and the syconium has a convex ostiole in the early stages of development.
Ficus pringlei is found mainly in the TDF, a vegetation type that has suffered extensive damage due to its widespread conversion to farmland, accompanied by uncontrolled cattle grazing and fires (Miles et al., 2006). The situation is critical in Mexico, where 73% of the original TDF has been lost and protected areas within this forest type are scarce (Trejo and Dirzo, 2000). In addition to land-use changes, climate change can reduce areas of this vegetation type, as is mentioned by Prieto-Torres et al. (2016) for the Balsas River Basin. Specifically, in the potential distribution area of F. pringlei, 145 km2 of primary and secondary TDF vegetation was transformed into unsuitable habitats for this species, such as agricultural land or grassland, between 2016-2018 (INEGI, 2016, 2018). Therefore, it is important to establish strategies for conserving this endemic species, which is not currently recognized in any risk category of The International Union for Conservation for Nature of Threatened Species Red List (IUCN, 2021) or NOM-059-SEMARNAT-2010 (SEMARNAT, 2010), and only 20% of its potential suitable areas under current conditions is contained within Natural Protected Areas (Fig. 6).
Temperature seasonality was the main environmental factor that explained the distribution of F. pringlei. This climatic variable and others that were significant (e.g., annual precipitation) have been typically associated with Ficus species since they are mostly distributed in tropical forests, and they rarely exceed 2500 m elevation due to their low tolerance of low temperatures (Ramírez, 1969; Janzen, 1979; Ibarra-Manríquez et al., 2012). There was more significant variation in annual mean temperature seasonality north of the TMVB (Fig. 7), which could reflect a greater seasonal variation and affect plant traits, pollinator and parasitic fig wasp reproduction, as well as seed production (Peng et al., 2010; Krishnan et al., 2014; Zhang et al., 2019). For example, in the monoecious species F. racemosa L., although levels of within-tree reproductive asynchrony were similar across seasons, asynchrony had variable effects on pollinator and parasite reproduction, which could be linked to seasonal variation in syconium size (Krishnan et al., 2014). However, future studies are required to corroborate the validity of these associations with seasonality in F. pringlei.
Finally, as has been observed in other groups of plants (e.g., Anguiano-Constante et al., 2021; López-Barrera et al., 2021), it is possible that the TMVB, in addition to promoting a disjunct distribution in the species, acts as a geographical barrier to gene flow. In that case, it is essential to evaluate this possible isolate for F. pringlei, since it could help guide conservation efforts (Coates et al., 2018).
Conclusions
This study showed that the area suitable to locate F. pringlei is only distributed in western Mexico, mainly in TDF and, to a lesser extent, oak and oak-pine forest. Their syconium developmental phases were consistent with those described previously for other monoecious species in the genus. Likewise, through direct observations and different characters (e.g., synstigma, type of pollen, anther to ovule ratio, and the existence of coxal combs and corbiculae in the wasps), it was determined that the pollination behavior performed by wasps is active. Furthermore, it leaves open a series of questions that will be interesting to answer in the future. These include quantifying the degree of asynchrony of the syconia at the individual or population level in localities with different seasonal variations and its effect on plant traits (e.g., seed production and gene flow), describing pollinator and non-pollinating fig wasps and their reproduction, and identifying frugivorous fauna that depends on syconia availability in Phase E and its impact on seed dispersal. Additionally, it will be important to evaluate the TMVB’s role as a possible barrier shaping the genetic structure of F. pringlei and therefore, guide the conservation units to the species.











 nueva página del texto (beta)
nueva página del texto (beta)



