Introduction
Anthuriums (Anthurium andreanum L.) are plants native to Central and South America that belong to the genus Anthurium; these are in high demand because they produce very showy flowers all year round and have a long postharvest life, characteristics that give them great acceptance in the national and international market (Castillo-Diego, 2012). Anthuriums are the second most important tropical cut flowers, after orchids (Buldewo & Jaufeerally-Fakim, 2002).
The Netherlands is the world's leading producer of anthuriums, growing 25 thousand stems a year, followed by Hawaii (11 thousand) and Mauritius (10.2 thousand) (Guillot-Ortíz, 2008). In Mexico, anthuriums are grown in approximately 20 ha, distributed in the states of Veracruz, Chiapas and Mexico (Gallaga, 2000). Anthurium andreanum and Anthurium scherzerianum are the most cultivated species in Mexico and in the world (Gantait & Mandal, 2010). A. andreanum is the most important species from an economic stamdpoint, since it has the largest number of comercial varieties (López-Puc, Ramírez-Mosqueda, & Lee-Espinosa, 2013).
The main varieties of anthurium used in the world are of Dutch origin and have been formed by hybridization (Hernández, 2004), as well as by genetic transformation methods; however, developing a new cultivar by these methods may require 8-10 years (Azadi, Bagheri, Nalousi, Nazari, & Chandler, 2016; Su et al., 2019). The genetic material of the main species that are commercialized in Mexico is imported from the Netherlands, which creates a complete dependency on germplasm (Ramírez-Zea & Chávez-Servia, 2014). Likewise, the development of new production areas in developing countries requires new varieties with the ability to adapt well to new growing conditions. In the same way, European producers are constantly looking for novel cultivars as a market strategy to be competitive, so the need for new varieties has shown an unprecedented increase at international level (Gupta & Agnihotri, 2017).
Induced mutagenesis is a technique that has been used to improve traits such as resistance to diseases and pests, and the generation of genetic variability (Ahloowalia, Maluszynski, & Nichterlein, 2004). This technique is based on the use of physical stimuli or chemical substances (mutagens) (González-Jiménez, 2004; Messmer et al., 2015). Chemical mutagenic agents such as colchicine generate genetic variations, such as chromosome duplication (polyploidy) (Eng & Ho, 2018). Polyploidy can generate variants with desired characteristics such as increased flower size, flowers with more showy colors and shapes, increased postharvest life, and greater resistance to abiotic or biotic stress (Roughani & Mehdi, 2018). However, the use of colchicine can cause changes in the chromosome number (aneuploidy or euploidy) and genetic damage to germ cells (Firbas & Amon, 2014).
Even though induced mutagenesis has shown to be an efficient method in the generation of ornamental varieties in different countries, in Mexico, the studies that report the generation of mutants in ornaments are scarce and have been directed to species such as tuberose (Polianthes tuberosa), chrysanthemum (Dendranthema grandiflora), wild poinsettia (Euphorbiache pulcherrima), sunflower (Helianthus annuus), Tigridia pavonia and Laelia autumnalis, all of them using physical mutagens (Estrada-Basaldua et al., 2011; Canul-Ku et al., 2012; Castillo-Martínez, de la Cruz-Torres, Carrillo-Castañeda, & Avendaño-Arrazate, 2015; Díaz-López, Morales-Ruíz, Olivar-Hernández, Hernández-Herrera, & Juárez-Cortes, 2017). In the case of anthurium, there are only three reports on induced mutagenesis in the world, of which only one used colchicine (Puchooa, 2005; Chen, Hou, Zhang, Wang, & Tian, 2011; Suraninpong & Wuthisuthimethavee, 2015). The objective of the present work was to induce mutations in A. andreanum by exposing explants obtained from vitroplants to colchicine.
Materials and methods
Regeneration of vitroplants
Anthurium leaves grown in the greenhouse were disinfested with a 1 % fungicide solution (0.2 % Prozycar® and 0.2 % Promyl®) for 30 min; then, they were placed in a solution with 1.2 % sodium hypochlorite (active ingredient) plus 45 µL of silver nanoparticles (Agrovit®) for 30 min. The leaves were rinsed three times with sterile distilled water. From these leaves, segments (1 cm2) were obtained, which were placed in 90 X 15 mm Petri dishes with 30 mL of induction medium containing Murashige and Skoog (1962) salts (MS), 1 mg·L-1 2,4-dichlorophenoxyacetic acid (2,4-D), 1 mg·L-1 benzylaminopurine (BAP), 30 g·L-1 sucrose and 7 g·L-1 agar. The pH of the medium was adjusted to 5.8 and autoclaved for 20 min at 121 °C, before being poured into Petri dishes. The cultures were incubated in a controlled environment chamber at 26 + 2 °C in complete darkness.
After three months, explants with adventitious shoots were transferred to a differentiation medium containing MS salts, 1 mg·L-1 6-furfurylaminopurine (kinetin), 0.3 mg·L-1 naphthaleneacetic acid (NAA), 0.3 mg·L-1 gibberellic acid (GA3), 30 g·L-1 sucrose and 7 g·L-1 agar. Then, the regenerated shoots were cultured for four weeks on a medium containing MS salts, 0.5 mg·L-1 kinetin and 0.3 mg·L-1 silver nitrate (AgNO3) (growth medium). The clumps of shoots were divided and subcultured (every four weeks) on the same medium described above, until they reached 6-8 cm long. The cultures were incubated at 26 + 2 °C and 16 h of cool white fluorescent light (light intensity 60 µmol·m-2·s-1).
Exposure of explants to colchicine
Leaf, node and root (tips) explants (0.5 cm2) obtained from regenerated vitroplants (seven months old) were submerged for 0, 2, 3 or 4 h in a 0.1 % colchicine solution, previously sterilized by filtration (45 µM Millipore® membrane filter), according to the protocols reported by Tian and Ma (2008) and Matos (2014). The explants were removed from the solution, washed with sterile distilled water and then allowed to dry on sterile absorbent paper. These explants were placed in Petri dishes with induction medium, on which they remained for 12 weeks in darkness at 26 + 2 °C.
The explants with adventitious shoots were removed from the induction medium and transferred to the differentiation medium; the regenerated shoots were then cultured on growth medium described above. The cultures were incubated at 26 + 2 °C and 16 h of cool white fluorescent light (60 µmol·m-2·s-1). Twelve weeks after the explants were exposed to colchicine, the following were evaluated: explant survival, the mean lethal dose (LD50), the number of explants that generated callus and the number of explants that formed shoots; at 20 weeks, the number of shoots formed by explant was quantified.
Experimental design
To test the effect of colchicine, a completely random factorial design with two factors (exposure time and type of explant) and 12 treatments was used. Each treatment consisted of ten repetitions; a Petri dish with five explants was a repetition. Means comparison was carried out by Tukey test (P ≤ 0.05) using the statistical package SAS version 9.4; the LD50 was determined by a Logistic Regression Model (LRM).
Karyotype and chromosome count
Chromosomes of root tip meristematic cells (ca. 1 cm long) of the vitroplants regenerated from the nodes and exposed to different treatments with colchicine were counted by squash technique according to García-Velázquez (1990). Briefly, the roots were incubated at room temperature with 0.05 % colchicine solution for 5 h in complete darkness, fixed in a mixture of acetic acid and absolute ethanol (3:1), and kept at 4 °C until counting. At moment of examination, the root tips were treated for 10 min at 60 °C in 1 N HCl, immersed in Schiff´s reagent for 5 min and placed on a slide. Approximately, 1 mm from the root tips was stained with 2 % acetic orcein and after used to apply the squash technique.
The preparations were observed in a optical microscope (model Primo Star, Zeiss) with a 40x and 100x objective, the chromosomes were counted and the images were captured with a digital camera (DCM130E model) adapted to the microscope. In each treatment, the chromosomes of at least ten metaphase cells were counted. For the karyotype, the three photographs that showed the best resolution were selected and processed with the Karyo Type software (Altinordu, Peruzzi, Yu, & He, 2016). Total length = long arm + short arm (TL = LA + SA), centromeric index = short arm/ total length x 100 (CI = (SA/TL x 100), arms ratio = long arm/ short arm (AR = LA/SA), chromosome type (metacentric, submetracentric or subtelocentric) were determined (Levan, Fredga, & Sandberg, 1964); likewise, the length of the haploid genome, the asymmetry degree of Stebbins (1971) and karyotypic formula were obtained.
Results
Vitroplant regeneration
The protocol developed in the present investigation allowed obtaining anthurium vitroplants. Callus formation in the leaf explants was observed after 12 weeks of culture on the induction medium. At 17 weeks of culture, multiple buds began to form, which when were subcultured on growth medium for 12 weeks, reaching 6-8 cm long; these shoots regenerated roots in the same medium.
Effect of exposing leaf explants to colchicine
Leaf explants were the most affected by colchicine, with their damage and mortality directly proportional to the exposure time. These explants showed low survival and null response in terms of callus and adventitious shoot formation. All colchicine-treated leaf explants died after 16 weeks.
Effect of exposure of nodes to colchicine
The survival and percentage of nodes that formed shoots decreased as exposure time increased. Significant differences for these two variables between the nodes exposed for 4 h to colchicine (46 and 46 %, respectively) and the control treatment (86 and 86 %, respectively) (Table 1) were observed. The LD50 for this type of explant was 3.89 h. The explants that survived this time of exposure to the mutagen regenerated fewer shoots (1.1) than the root explants. The basal zone of the nodes exposed for 2 and 3 h thickened considerably and acquired a rosette appearance; after five months of culture, the nodes began to form adventitious shoots. The number of shoots formed by the explants exposed for 2 h to the mutagen was significantly higher (4.4) than that of the control and 4 h (Table 1).
Table 1 Interaction between the type of explant of anthurium (Anthurium andreanum L.) and time of exposure to colchicine on survival, callus formation and regeneration of adventitious shoots.
| Explant | Time | Variables | |||
|---|---|---|---|---|---|
| Survival (%) | Callus formation (%) | Explants with shoots (%) | Number of shoots per explant | ||
| Node | 0 | 86.0 abz | 0.0 b | 86.0 a | 1.0 c |
| Node | 2 | 76.0 abc | 76.0 a | 76.0 ab | 4.47 a |
| Node | 3 | 56.0 bc | 56.0 a | 56.0 ab | 3.25 ab |
| Node | 4 | 46.0 c | 0.0b | 46.0 b | 1.68 bc |
| Root | 0 | 60.0 bc | 40.0 a | ND | ND |
| Root | 2 | 100.0 a | 68.0 a | ND | ND |
| Root | 3 | 100.0 a | 56.0 a | ND | ND |
| Root | 4 | 100.0 a | 44.0 a | ND | ND |
ND = Not determined. zMeans with different letters between rows are significantly different (Tukey, P ≤ 0.05).
Effect of exposure of root explants to colchicine
No significant differences were observed in the survival of the root explants exposed for 2, 3 and 4 h, but some were observed with respect to the control treatment (Table 1). Survival at 12 weeks was higher than 60 %, so it was not possible to determine the LD50 within this period of time. The roots exposed for 2, 3 and 4 h formed callus (44 to 68 %), which orginated in the midrib; this callus was friable and beige in color and as the exposure time to colchicine increased, the size of the cell mass was smaller. When the explants were cultured on differentiation medium and light, the calli began to take on a dark brown color and died. Only 4 % of the root explants exposed for 2 and 3 h to colchicine survived after 16 weeks and formed shoots (120 and 100 shoots, respectively).
Karyotype and chromosome count
Cytological analysis of anthurium plants (wild type) showed that their chromosome number is 2n = 2X = 30 (Figure 1a). Likewise, it was posible to determine that anthurium has 11 submetracentric (sm) and four metacentric (m) chromosomes (Table 2; Figure 2). The individual length of the chromosomes ranged between 2.13 and 5.08 µm, generating a total haploid length of 46.8 ± 0.2 µm obtained with the karyotypic equation 2n = 2x = 6 metacentric (2 satellite) + 24 submetacentric. The mean cetromeric index was 33.86 ± 2.31, which indicates a greater number of submetacentric chromosomes. Based on the inter and intrachromosomal variation, the karyotype is characterized as asymmetric type 3A (Stebbins, 1971), due to the fact that the size of the chromosomes ranges from small to medium (Table 2). A pair of satellites were observed on the second pair of chromosomes, which are very close to these. Likewise, it was possible to observe two B-chromosomes structures-like (Figure 1a).
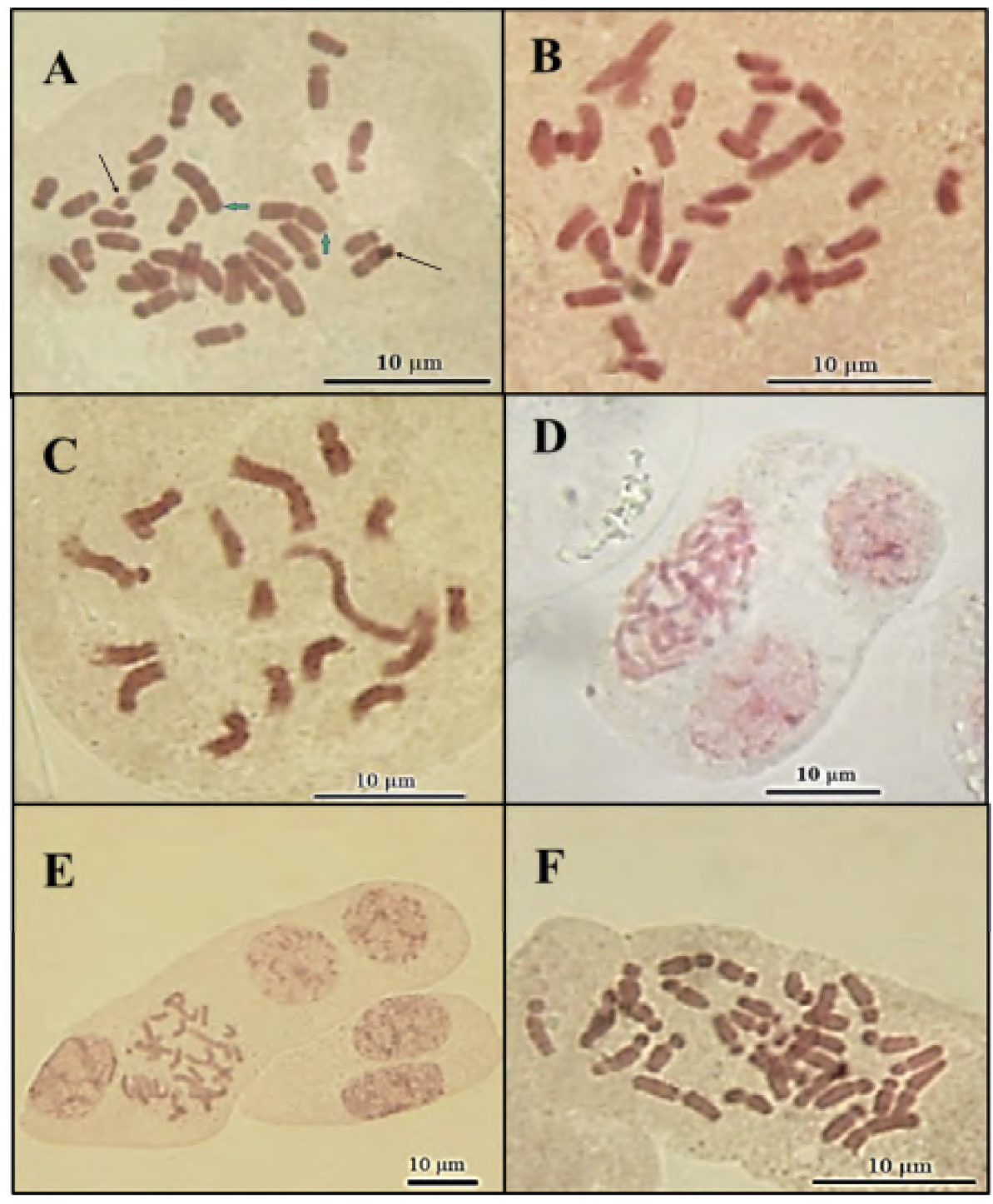
Figure 1 Metaphase chromosomes from anthurium (Anthurium andreanum L.) plants, regenerated from nodes exposed to colchicine: a) Control, b) 2 h, c) 3 h, d) cells with three nuclei, e) cells with four nuclei, and f) 4 h. Green arrows: chromosomes with satellites. Black arrows: B-chromosomes structures-like.
Table 2 Karyotypic parameters of the haploid genome of anthurium (Anthurium andreanum L.).
| HChr | TL (µm) | CI (%) | AR (µm) | Type |
|---|---|---|---|---|
| 1 | 5.08 ± 0.4 | 43.04 ± 5.5 | 1.32 ± 0.3 | m |
| 2 | 3.99 ± 0.4 | 38.82 ± 3.7 | 1.58 ± 0.2 | m |
| 3 | 3.74 ± 0.4 | 29.72 ± 7.7 | 2.36 ± 0.7 | m |
| 4 | 3.48 ± 0.2 | 31.21 ± 3.4 | 2.20 ± 0.3 | m |
| 5 | 3.35 ± 0.1 | 25.95 ± 2.8 | 2.85 ± 0.4 | sm |
| 6 | 3.28 ± 0.1 | 30.76 ± 4.0 | 2.25 ± 0.4 | sm |
| 7 | 3.12 ± 0.3 | 30.41 ± 3.0 | 2.29 ± 0.3 | sm |
| 8 | 3.01 ± 0.2 | 29.38 ± 6.7 | 2.40 ± 0.7 | sm |
| 9 | 2.84 ± 0.2 | 31.48 ± 2.3 | 2.18 ± 0.2 | sm |
| 10 | 2.77 ± 0.1 | 34.36 ± 6.9 | 1.91 ± 0.5 | sm |
| 11 | 2.62 ± 0.1 | 31.19 ± 4.7 | 2.21 ± 0.5 | sm |
| 12 | 2.57 ± 0.1 | 34.81 ± 5.1 | 1.87 ± 0.4 | sm |
| 13 | 2.44 ± 0.1 | 33.63 ± 3.1 | 1.97 ± 0.2 | sm |
| 14 | 2.39 ± 0.2 | 39.73 ± 6.0 | 1.52 ± 0.4 | sm |
| 15 | 2.13 ± 0.1 | 31.56 ± 3.0 | 2.17 ± 0.3 | sm |
HChr = haploide chromosome; TL = total length; CI = centromeric index; AR = arms ratio, sm = submetacentric; m = metacentric.
The results observed in the present work showed that plants regenerated from nodes exposed to colchicine were aneuploid. The plants obtained from explants that remained for 2 and 3 h in presence of colchicine contained 16 to 30 chromosomes, being 29 chromosomes the predominant ones (Figures 1b and 1c), originating a mixoploid population; cells with two or more nuclei sharing the same cytoplasm were also observed (Figures 1d and 1e). On the other hand, the plants regenerated from explants that remained for 4 h in the colchicine solution contained 30 to 32 chromosomes, predominatly those with 31 chromosomes (Figure 1f).
Morphological characteristics of the mutants obtained
In the first months of their in vitro growth, the plants obtained from explants exposed for 2 and 3 h to colchicine were shorter and had fewer leaves and roots than the control plants; also, the roots were observed to be shorter (Figures 3a -c). On the other hand, the plants that regenerated from explants exposed to colchicine for 4 h showed larger leaves with a darker green color and reddisher petioles than the control plants (Figure 3d). When the aneuploid anthurium plants were grown in ex vitro conditions for three months, they showed a slight reduction in the size of the leaves and the length of the petioles with respect to the control plants (Figures 3e-g). After seven months of cultivation in the greenhouse, it was possible to observe the formation of spathe and spadix in the regenerated plants from control explants and those exposed for 4 h to colchicine (Figures 3h-i). The mutant plants were smaller than the control ones.
Discussion
In this work, explants of anthurium vitroplants were exposed to colchicine to induce mutations. The sensitivity to mutagen was different based on the type of explant and the time of exposure. Leaf explants were the most affected. The severe damage to leaf explants could be due to the characteristics and physiological state of the vitroplants and the leaves themselves (Valle-Sandoval, Mascorro-Gallardo, Gil-Vázquez, & Iturriaga-de la Fuente, 2008), unlike the leaves of plants grown in greenhouse or field, the leaves of the vitroplants had a very thin cuticle, which could cause dehydration stress, and severe mechanical damage from cutting, which compromised their survival. Torres and Sanabria (2011) mention that leaves of plants regenerated in vitro can present anatomical and functional abnormalities such as a reduction in the number of cells in the mesophyll, a weak vascular system, and less thickness of its lamina, epidermis and cuticle. Also, it is likely that the exposure of the leaves to colchicine has increased their deterioration; thus, the leaves of young anthurium vitroplants are not considered a good source of explants for future mutagenesis studies.
On the other hand, the better morphogenetic capacity observed in the nodes with respect to the leaf and root explants could be due to the fact that in the nodes the meristem is covered with leaf primordia, making it less susceptible to damage, which allowed determining the mean lethal dose. LD50 indicates the dose to which 50 % of the individuals (explants) die from the effect of the mutagen; its importance lies in the fact that at this dose there is a greater probability of obtaining useful mutations in a genetic breeding program (Álvarez-Holguín et al., 2018). In contrast, the calli generated by root explants changed color and died when they were transferred to the differentiation medium. A similar result was reported by Chen et al. (2011) in root explants (callus masses) of anthurium “Arizona” exposed to colchicine. Root meristematic cells are known to respond rapidly to drugs, suffer DNA damage, and accumulate proteins (ERF115) related to the production of ethylene (stress hormone) (Heyman et al., 2016). However, it should be noted that the surviving calli formed a greater number of shoots than the node explants.
The karyotype and chromosomal number of diploid anthurium plants are similar to those reported by Chen et al. (2011) and Lakshmanan et al. (2015) who found that the number of chromosomes for anthurium is 2n = 2x = 30. Likewise, it was possible to detect the presence of satellites in a pair of anthurium chromosomes comparable to those reported in A. affine, A. bellum and A. pentaphyllum var. pentaphyllum (Pires-Cotias-de Oliveira, Silva-Guedes, & Cerqueira-Barreto, 1999). Satellites are very useful for the description of katyotypes, taxonomic delimitation and comparative cytogenetics (Tapia-Pastrana & Tapia-Aguirre, 2018). B-chromosomes structures-like were also recorded in our examination of metaphase cells. B-chromosomes had been also reported in other anthurium species such as A. affine (Pires-Cotias-de Oliveira et al., 1999). Farhan and Martins (2019) mention that the B chromosomes are karyotypic components that show non-Mendelian characteristics and have a non-standard inheritance behavior; their presence can change or disappear in an aneuploid condition.
In relation to aneuploidy and multinucleate cells, it is known that the feasibility of generating desirable mutants can be affected by the ocurrence of multiple mutational events in individual genomes. The use of chemical mutagens such as colchicine for polyploid induction frequently causes aneuploidy (de Storme & Mason, 2014), which refers to the removal or addition of individual chromosomes from a basic chromosome set (Huettel, Kreil, Matzke, & Matzke, 2008). This variation in the chromosome number could be result of irregularities in the formation of the mitotic spindle and cytokinesis, two mechanisms that depend on the biogenesis of the microtubules (Akhmanova & Steinmetz, 2015). Microtubules are crucial cell structures of cytoskeleton and they are formed by the consecutive assembly of αβ-tubulin dimers.
Because colchicine has high affinity for these dimers in vitro, their joining gives rise to complexes of tubulin-colchicine that can also bind to the growing end of the microtubules (Hardham & Gunning, 1980). When dimers join in vivo, the biogenesis of the microtubules is arrested because of tubulin depolymerization (Leung, Li, Hui, & Kraus, 2015). The speed and outcomes of this disruption depend on colchicine concentration and duration of immersion as it has been shown elsewhere. In this study we tested 0.1 % colchicine during 2, 3 or 4 h in leaf, node and root explants. It was clear that the concentration of colchicine assayed was not enough to duplicate the chromosome set. Instead, the duration of immersion had some intriguing outcomes such as the aneuploidy and the presence of multinucleate cells.
At 2 and 3 h of immersion, multinucleate and aneuploid cells with 16-29 chromosomes were observed. The source of this variation could come from failures in the assembly of the mitotic spindle or from irregularities in the formation of the cortical microtubules. It is likely that microtubules of both structures become unable to reorganize themselves due to depolymerization by colchicine, in such a way that the short-time periods to colchicine exposure compromise the formation of the spindle and the phragmoplast giving place to multinucleate cells. These types of cells along with multipolar anaphases and spindle failures are frequent in cells treated with low concentrations of colchicine (Compton, 2011). The extension of this effect is also tissue-dependent; for instance, Ruíz and Vázquez (1982) observed interphase cells with large nuclei or micronuclei as well as binucleated and polynucleated cells when they exposed barley embryos to 0.3 % colchicine for 24 h. Consequently, it is likely that, during the mitosis of these multinucleated cells, the total set of chromosomes is unevenly distributed among the daughter cells, which could lead to numerical chromosomal abnormalities such as those observed in this study and those by Matos (2014) in Aloe vera plants treated with 0.1 % colchicine for 48 h.
Similarly, observations in the chromosome number in the longest-time exposure (4 h) indicated that the main effect was the aneuploidy, with a chromosomal variation of one or two extra chromosomes. Here, it has been reported that longer exposure periods (or high colchicine concentrations) stimulate new tubulin-containing structures to emerge. These new structures are like a “tubulin cage” wavy network that temporally replaces the true cortical microtubules (Caperta et al., 2006). Possibly, this new structure of polymerized tubulin, the reversibility of the tubulin-colchicine complexes, the low concentration of colchicine and the time of exposure were factors that allowed the elongation of microtubules to resume once the cells were no longer in contact with the mutagen. Therefore, the balance among all these factors is key for ploidy to be able to occur.
During the in vitro growth, the aneuploid plants regenerated from explants exposed to colchicine showed an evident reduction in their size and vigor with respect to the control plants. In this regard, Zhu, Shao, Pan, Ge, and Li (2015) observed that aneuploid (nullisomic) plants of Brassica napus presented phenotypic changes; they were smaller with small light-green leaves and had smaller flowers and non-apical dominance. In contrast, the monosomic plants exhibited similar morphology to normal plants, but they flowered about 10 days earlier. Likewise, Matos (2014) mentions that in some cases positive aneuploidy may occur, which promotes changes at the morphological level such as slight increases in the foliar dimensions of the plants, but without having any visible effect on the increase in biomass accumulation. In some cases the aneuploidy, such as monosomy and nullisomy, can cause mortality due to the destabilizing effect on gene expression caused by duplication or deletion of some chromosomal regions (de Storme & Mason, 2014).
Sun et al. (2013) point out that when the chromosomal segments or chromosomes are reduced from two to one, dose compensation occurs (increased expression of the genes of the remaining chromosome) for most of the affected genes; in trisomic there is also a reduction as a result of inverse dose (the higher the dose, the lower the expression). With both positive and negative dose effects, the reduction in the expression of target genes will occur in both monosomic and trisomic. Birchler (2013) mentions that the variation in chromosomal number could be eliminated by selective advantage of normal diploid cells; if a large number of genes are affected, gene expression normalizes and the effect is silenced.
Information on how aneuploidy affects the characteristics of plants is limited. The regulatory aspects of the genome have a stoichiometric relationship, which is why it is inferred that aneuploidy produces harmful effects; however, in various tetraploid species (corn, barley, datura, lettuce, rye), aneuploid individuals have been detected in percentages ranging between 15-50 %. The above suggests that some species can produce aneuploid gametes and exhibit different levels of tolerance to aneuploidy. It has been determined that variations in gene expression depend on multiple factors such as dose of regulatory molecules, epigenetic factors, sensitivity of repetitive regions and gene silencing mechanisms (Birchler, 2013).
Plant speciation and diversification depend on structural changes in the nuclear genome, both at the whole ploidy and individual chromosome level (de Storme & Mason, 2014). Aneuploidy has not been considered as a factor in speciation and karyotype evolution, due to the destabilizing effect on gene expression caused by duplication or deletion of some chromosomes or chromosomal regions (Guerra, 2008). However, aneuploidy may confer possitive effects on cellular growth and proliferation; it could also be an intermediate stage in the establishment of novel euploid karyotypes and contribute to the establishment of new karyotypes.
In Malus, aneuploid gametes may have an advantage over euploid gametes, contributing to increased heterozygosity and genetic variation (Considine et al., 2012). Likewise, in plants that reproduce asexually, somatic aneuploidy may be tolerated at high levels, and chimeric aneuploid sectors may contribute to formation of new plants through vegetative propagation (de Storme & Mason, 2014). Oleszczuk, Rabiza-Swider, Zimny, and Lukaszewski (2011) highlighted the importance of aneuploid plants in the formation of double haploid lines in xTriticosecale Wittmack; likewise, they affirm that aneuploid individuals can be used in the assignment of markers on chromosomes, which can be an important tool in DNA marker-assisted breeding programs.
As mentioned above, aneuploid plants can show a reduction in size (dwarfism) relative to diploids. Dwarf phenotypes have been reported in aneuploid plants of Musa, Brassica napus and wheat (Roux, Toloza, Radecki, Zapata-Arias, & Dolezel, 2003; Zhu et al., 2015; Jiao et al., 2020). In the present investigation, the anthurium aneuploid plants were smaller than the diploid plants (control), which indicates that the in vitro mutagenesis protocol developed allows regenerating dwarf plants or smaller variants, which retain their ability to form flowers. Currently there are dwarf anthurium phenotypes on the market whose commercial demand is increasing. However, the anthurium germplasm that is marketed in Mexico comes from the Netherlands, since in the national catalog of plant varieties there is no record of any variety that has been generated in our country (Servicio Nacional de Inspección y Certificación de Semillas [SNICS], 2021).
The aneuploid plants generated with the protocol developed in the present work could be incorporated into an anthurium breeding program, identifying those mutants that present outstanding or novel characteristics of commercial interest. Since aneuploid plants can present high rates of mortality and infertility (de Storme & Mason, 2014), tissue culture (micropropagation) is a viable alternative to avoid the loss of plant material and maintain its aneuploid condition, as this technique allows cloning and multiplying a genotype from a small section of the donor plant (mother plant) (Teixeira-da Silva, Dobránszki, Winarto, & Zeng, 2015). From the selected genotypes, it is possible to obtain mutant lines, which could be used in vegetative propagation or as progenitors to generate hybrids. The combination of mutagenesis and tissue culture has been widely used in the improvement of vegetative propagated varieties and offers the possibility of generating a greater number of mutants with outstanding agronomic characters (Hernández-Muñoz, Pedraza-Santos, López, Gómez-Sanabria, & Morales-García, 2019).
Oladosu et al. (2016) highlight the potential of mutation plant breeding as a flexible and practiced approach applicable to any crop as long as the appropriate selection objectives and methods are used. In Mexico, induced mutagenesis has allowed the generation of varieties of different ornamentals crops (Polianthes tuberosa, Dendranthema grandiflora, Euphorbiache pulcherrima, Helianthus annuus, Laelia autumnalis), among which anthurium is not found (Hernández-Muñoz et al., 2019). Induced mutagenesis could become a powerful tool for the genetic breeding of anthurium in Mexico, since it would allow the development of new varieties that adapt to the conditions that prevail in this country, as well as adjust to the needs of the national and international market, thereby reducing the country’s current dependence on germplasm.
Conclusions
The effect of colchicine on anthurium explants was a function of the type of explant and the dose used. The greatest ability for regeneration of anthurium mutants ocurred in node and root explants. Exposure of the explants to colchicine at the doses used gave rise to aneuploid plants (monosomic and trisomic), which showed different morphological characteristics from those of the wild genotype. Colchicine can be used in anthurium breeding programs to generate genetic variability.











 texto en
texto en 





