Introduction
The rapid growth of the global population and advances in civilization have resulted in an exponential increase in demand for clean and sustainable energy, creating a growing concern about an environmental crisis. The search for sustainable energy has been in progress for many decades, but fossil fuels still account for more than 80 % of current energy consumption [1]. Although the usage of fossil fuels is not sustainable, they cause severe environmental contamination, which is a crucial issue for human health and the economy [2,3], and they still remain the main contributors worldwide to the energy sector. Hydrogen has played a key role throughout the industrial life of humankind, and the global demand for H2 has continuously increased. Today’s global H2 production is from methane through the process called Steam Methane Reformation (SMR) which exceeds 70 million metric tons (MMTs)/year and is consumed by the oil and gas industry and by metal refineries or turned into value-added products such as ammonia and chemicals. Reducing reliance on fossil fuel and increasing the use of renewable energy is an urgent priority. The concept of a “Green hydrogen society” is the integration of renewable hydrogen production, storage, and utilization into the global energy system, expected to serve as a greener alternative to fossil fuel utilization and mitigating the climate crisis [4]. However, challenges concerning sustainability in the production of hydrogen need to be resolved to fulfill the development of a hydrogen society. The development and utilization of renewable energy sources, such as solar and wind energy, are regarded as the most plausible alternative to be converted into chemical energy, which is essential to resolve problems of climate change and environmental pollution [5]. Hydrogen is widely considered an attractive environmentally friendly material due to its zero-carbon emission when reacting. The hydrogen economy is a proposed sustainable energy system comprising hydrogen production, distribution, storage, transportation, diverse stationary/mobile applications, and public policy formulation [6]. To attain these goals various classes of materials have been reviewed and developed searching for higher durability, efficiencies, and utility, paying particular attention to catalyst materials that enable the green production of hydrogen from water, chemical, and physical storage and distribution systems [7]. However, difficulties in storage and hydrogen transportation could render a hydrogen society inviable, due to the thigh hydrogen generation and storage cost.
There are three representative water electrolysis technologies: alkaline water electrolyzer (AWE), proton exchange membrane water electrolysis (PEMWE), and solid oxide electrolysis cell (SOEC). AWE from renewable energy plants has been regarded as the promising green hydrogen production at the industrial scale due to its high technology maturity which should be expanded to prepare for the upcoming hydrogen society. [8]. However, there is controversy surrounding this direction. An article published by Griegoriev et al., [9], and by Miller et al., [10] reported that ion-exchange membrane-based water electrolysis has two primary benefits over traditional alkaline liquid electrolysis: a membrane-based cell allows a zero-gap configuration that enables a compact cell arrangement, and a non-porous membrane can be used, allowing differential pressure operations that reduce energy consumption for hydrogen production. Hydrogen storage in liquid or gaseous form presents safety and transportation challenges. Hydrogen storage remain a challenging for its use in various energy devices and applications, and nowadays hydrogen storage materials are under extensive research, with emerging trends, and perspectives [11]. Alanates [12] and nanostructured metal hydrides are also considered an important class of solid-state hydrogen storage materials associated with the improvement of rapid heat transfer characteristics. Safety performance with significant energy storage technology remains priority [13,14]. Important current and future costs, as well as environmental burdens of large-scale hydrogen production system have been reported, considering a daily hydrogen production rate of hydrogen production costs, life cycle greenhouse gas emissions, material utilization, and land transformation [15]. Recently, it has reported by Kevin Li et al, a method of direct hydrogen production from the air, through the capture of freshwater from the atmosphere using hygroscopic electrolyte and electrolysis powered by solar or wind [16]. Using renewable energy, Zetian Mi et al. reported a sun-powered water-splitting hydrogen production with a new type of solar panel that has achieved 9% efficiency in converting water into hydrogen and oxygen, which can drive down the cost of producing sustainable hydrogen [17]. This has been achieved using a semiconductor composed of nanostructured indium gallium nitride, grown onto a surface of silicon that withstands high temperatures and concentrated light equivalent to 160 suns. Solar water splitting, as a typical artificial photosynthesis process, is widely investigated for converting directly solar energy into chemical energy with a focus on the production of hydrogen. Recently, Guant et al., reported the harvesting a two-electron process in solar water splitting with simultaneous production of H2 and H2O2 as a practical route toward water splitting with spontaneous product separation with additional value-added products [18]. This solar process relies on fast kinetics of both water reduction and water oxidation on the surface of the photocatalysts.
Why hydrogen
Hydrogen is a colorless molecular energy vector with the highest specific energy density, making it the best alternative to batteries and fossil fuels used in conventional energy generation. Fossil fuels have 120 kJ/g of energy density, compared to gasoline 45.25 kJ/g and natural gas with 50.19 kJ/g. Hydrogen emits no carbon dioxide after burning an energy density that is around 175 times higher than Li-ion batteries. However, hydrogen does not exist in nature in its elemental form and requires energy to separate it from another atom. There are different sources and processes to produce hydrogen using non-renewable and renewable sources, which are indicated with different colours based on various factors [19]: i) the technology and the conversion process, ii) the amount of carbon emitted during these processes and iii) the source of the electricity that powers the process, especially for electrolysis. Literature reports [20, 21] that grey hydrogen is assigned when obtained from SMR with an approximate carbon footprint of 10 kgCO2 /kgH2; brown/black hydrogen is from gasification of coal; blue hydrogen from fossil fuels; purple/pink when produced by electrolysis using nuclear energy; turquoise when produced by methane pyrolysis; yellow, when hydrogen is produced using grid electricity; white colour when hydrogen is produced from byproduct of industrial processes and green colour is when hydrogen is produced by electrolysis of water and (photo)thermal processes. The transition from non-renewable hydrogen to renewable green hydrogen, which has low carbon intensity is being facilitated by the electrochemical process. Electrolysis has made major advances in recent decades, to the point where it is becoming feasible with novel electrocatalysts and membranes for low temperature applications. The generation of green hydrogen from electrolysis of water splitting is considered an environmentally sustainable clean energy driver, which can be directly used in domestic heating, as a feedstock in industry and in fuel cells for vehicular transport. The hydrogen in the Mexican Industry is reduced, with an annual production of 220,650 Mt/year where 97 % is by SMR (536 NCMH, cubic feed/min) and by electrolysis 50 NCMH. The industrial plant with the major capacity is located in the south of Mexico with a hydrogen production of 3908 NCMH8 [22]. Additionally, a recent study analyzed the importance of the legality and legitimacy of the current conditions of the hydrogen market in Mexico [23].
Electrolysis of water
The electrolysis of water is one of the best-known versatile methods for producing high purity green hydrogen on a small or large scale. It can be generated using photovoltaic, wind, hydropower, or decarbonized grid electricity. Electrolyzers operate with few moving parts including catalysts, membranes, and membrane electrode assemblies and when alkaline media these is known as zero-gap alkaline water electrolyzers [24]. The hydrogen and oxygen produced are physically separated during the gas evolution on each electrode surfaces. There are two types of electrolyzers differentiated by the polarities of the electrodes. Currently, only a small amount, less than 5% of global hydrogen production originates from water electrolysis and results are crucial to develop electrocatalysts for water splitting based on low-cost and land-rich elements [25].
The efficiency of water splitting widely depends on the intrinsic activity, selectivity, and stability of the oxygen evolution electrocatalysts, currently based on noble metals. Water electrolyzers consist of two electrodes, an anode, and a cathode, dipped in water and separated by a semipermeable separator. Water in the electrolyzer is subjected to an electrical current, causing it to split into hydrogen (cathode side) and oxygen (anode side). A summary of reported electrocatalysts and activities for overall water splitting is reported in [5,26], also considering proton exchange membrane combined with anion exchange membrane bipolar interface [27].
Different highly noble-metal free electrocatalysts have been reported as electrodes for the hydrogen evolution reaction, where nonprecious metal borides present a promising alternative based on their rich compositional and structural diversity [28]. Mu et al. reported recent progress of metal-organic frameworks (MOF) electrocatalysts for electrolysis of water and fuel cells based on different advantageous reasons such as porosity, catalysts-electrolyte contact and enhanced electronic conductivity by the construction block subunits of the MOF [29]. The generation of green hydrogen through the photocatalysis and (photo)electrocatalysis process has been explored and their potentiality opens opportunities for a circular economy, even if their potential applications remain unexploited due to hydrogen generation cost [30].
Cathode materials are of paramount important for microbial electrolysis cell (MEC) development and their contribution to achieving a circular hydrogen economy and hybrid materials have become the most popular catalyst candidate while nickel materials also attract increasing interest and exploration [31]. Recently, Chand et al. [32] reviewed in an article the development of membranes for the hydrogen production by water electrolyzers. Bystron et al. reported the state of the art of materials and components used in proton exchange membrane water electrolyzers single cell and cell stack construction [33].
Bessarabov et al. reported the utilization of various support materials for decreasing the noble metal loading, reducing costs of the technology, and enhancing the catalytic activity and durability for the oxygen evolution reaction in a Polymer Electrolyte Membrane Water Electrolysis [34]. Anion exchange membrane electrolysis combines the benefits of alkaline electrolysis, stability of the cheap catalyst and advantages of proton-exchange membrane systems. Faid et al., reported in recent review the development and outlook of cost-efficient transition metal catalysts for hydrogen evolution reaction and oxygen evolution reaction in alkaline electrolyzers, ionomer catalyst-electrolyte interaction, and membrane-electrode assembly performance and stability [35].
In this review authors remarqued that anionic alkaline water electrolysis needs to pursue several new and innovative concepts at the electrode materials, component, stack, and system levels to attain performance and durability compared to state of art platinum catalysts. Current and future trends by using earth-abundant electrocatalysts for water splitting has analyzed by Shamsah et al. [36].
Hydrogen and oxygen in a vehicular transport prototype
With the rapid growth and development of the hydrogen proton-exchange membrane fuel cell technology, there is an increasing demand for clean and sustainable global energy solutions with lower carbon footprints, compared to conventional industrial chemical technology. Identifying the right alternative propulsion technologies to replace fossil-fuel based powertrains is a matter of technological importance. In the green hydrogen economy, fuel cell in electrical vehicular transport is critical for low-carbon emission transport, and the well-to-wheels greenhouse smoke emissions are expected to be reduced to near zero when hydrogen from intermittent renewables solar and wind power and oxygen from air are used [37,38]. Fuel cell vehicles are considered of utmost importance in the development of new green hydrogen electric energy vehicles, regarded as one of the ultimate solutions for actual transportations due to its advantages such as fast filling speed, high efficiency, low noise, and zero-emission collective transport [39]. Polymer electrolyte membrane fuel cells (PEMFCs) have attracted attention owing to their advantages for automotive propulsion in electric novel vehicular transport and their remarkable technical progress in the last decade [40-45].
While some opinions suggest that battery electric vehicles could be a bridging strategy towards the final implementation of automotive hydrogen technology or that of pure battery electric vehicles must be made and cover the automotive market, batteries are energy storage devices that store electricity produced from different sources, whereas fuel cells are energy conversion devices that typically use green hydrogen from renewable sources for energy storage. As mentioned previously, hydrogen has advantages over lithium-ion batteries because hydrogen exhibits higher energy density and reduced refueling time compared to Li-ion arrangement devices [46]. In conventional electric hybrid vehicle prototypes, it is necessary to use Li-ion batteries, accepting their restrictions in terms of weight, costs, energy losses, limited lifetime, and environmental constraints.
As a group of research, we have designed and manufactured a hybrid electric transport prototype, incorporating electric motors to the hydrogen/oxygen PEMFC, following a schematic diagram reported for two wheels drive electric cars. A power interfaces to integrate PEMFC sources to hybrid electric vehicular (HEV) transport well-to-wheel pathway is illustrated in Fig. 1. The PEMFC is an unregulated energy source depending on diverse parameters such as cell temperature, atmosphere humidity, and fuels supply availability. Therefore, it needs a power converter unit (PCU) to connect with loads. A direct-current to direct-current (DC-DC) converter works upon the electric power from the PEMFC source to provide tracking of the PEMFC operating point, to fulfill the nominal voltage for load demand under normal conditions, and during PEMFC transients.
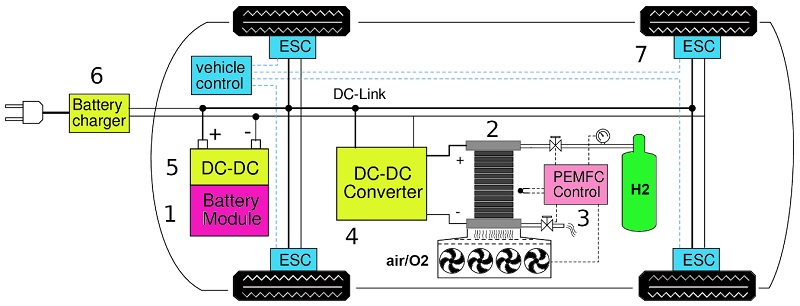
Fig. 1 Schematic diagram of a plug-in hybrid electric vehicular transport (HEVT). 1) Li-ion battery module. 2) PEMFC energy source. 3) PEMFC electronic control for monitoring cells temperature, stack humidity, stack purge, and H2 pressure. 4) Power converter ties the PEMFC to DC link powertrain. 5) Bidirectional power converter for battery charging and battery connection to DC-link. 6) Plugging battery charger. 7) Electronic motor-drive controller on wheels.
The resulting prototype includes a H2/air PEMFC sized of 48 MEAs. The green hydrogen tank with a 136 atm pressure capacity feed hydrogen and oxygen from air to the power generator and, the electricity produced of 0.6 kW (20V, 30A) is converted into DC/DC boost to charge an arrangement of Li-ions batteries. The arrangement includes 11 modules of batteries connected in series with 36 batteries connected in parallel to get 39.6V, 48 A (1.9 kW, 2.6 horsepower). The regulated current and voltage are distributed to the brushless motor controller and to the electric motor wheels. The recent version of our electric vehicular transport was designed using Solid works software, Ansys Engineering Simulation, and 3D design by finite elements. For the design it was considered different aspects were considered during the design process, such as: i) the distance between wheels; ii) the internal capacity; iii) the total weight of the prototype, passengers included; iv) the speed and v) the frontal impact factor. The electric hybrid vehicle weighs 350 kg has a consumption of deliver 2.6 horsepower and can drive at approximately 35 Km h-1 and a half. A solar power of 400W is incorporated at the roof of the prototype. A power system is structured upon the connectivity of power generators with lightweight structural materials in the chassis and transport body. Also, a power interfaces with a DC-DC converter topologies to integrate PEMFC system to the hybrid electric vehicular prototype was developed. At least four national patents have been granted and three in industrial design of the resulted hybrid hydrogen-fuel cell-batteries prototype [47-50]. Based on the experimental performance of the entire electric vehicle powertrain of the PEMFC stack was estimated and the design of the power conditioning unit PCU was adjusted to suit a suitable driving cycle. Recent contributions of the above-mentioned green hydrogen production pathways and application technologies are reported recently [51-54].
Conclusions
In this short communication a recent relevant work publications with orientation in green hydrogen production for vehicular application to reduce the usage of fossil fuels for automotive applications is presented. The objective is to demonstrate that green hydrogen and oxygen from air feeding to a CINVESTAV hybrid electric vehicle transport prototype produce electricity for the transport mobility. Experimental results carried out using Pt-based catalysts in H2/air PEMFC (0.6 kW)) and Li-ion batteries corroborate the performances of an energy management strategies during motor drive cycles of the vehicle prototype. The future investigation of this energy generation system for different applications including the electrification of the powertrain is now in route in the electrochemical group of research.











 nueva página del texto (beta)
nueva página del texto (beta)


