Introduction
The concentration of nitrate (NO3) has increased tremendously in groundwater, causing incessant water pollution in China (Bi et al. 2010). In some regions, nitrate concentrations have soared to several hundred milligrams per liter (Zhang et al. 2012) in groundwater; such precarious concentrations are ten times greater than the Class III standards of Groundwater Quality Standards (GB/T 14848-93). Groundwater is one of the most important sources of drinking water in China; therefore, healthy humans would be adversely affected when they consume groundwater containing high levels of NO3, which is a major contaminant. In a chemically reductive environment, nitrate (NO3) compounds get easily converted into nitrite (NO2) compounds. Furthermore, NO3 and NO2 are important functional groups in various nitrogenous organic compounds. In particular, NO3 and NO2 participate in various chemical reactions and form the following categories of toxic compounds: carcinogenic, and/or mutagenic N-nitroso compounds (Zhang et al. 2008; Li C et al. 2013). According to statistical data, it is dangerous to consume NO3 contaminated drinking water because it is closely linked to cancers of the stomach, intestine, skin, bone, and nervous system (Nathan S. Bryan et al. 2013; Yan 2013). To ensure the safety standards of drinking water, we need to carry out nitrate (NO3) remediation in groundwater.
For NO3 remediation in groundwater, biological denitrification is one of the most suitable methods. Anaerobic denitrification is the most widely used process in the biological environment. In this process, heterotrophic denitrifying bacteria metabolize organic compounds, which are primarily carbon compounds. In such metabolic reactions, the NO3 radical is reduced and converted into free nitrogen under anaerobic conditions (Miso et al. 2014). Since groundwater lacks organic carbon, heterotrophic denitrification is not really effective in removing NO3 contaminants from groundwater. Several previous studies have reported that acetic acid, ethanol, wood chips, compost, cotton, wheat straw, and paper scraps are not adequate carbon sources for heterotrophic denitrification (S. Israel et al. 2009; Jin and Liu 2011; Jian et al. 2013; Li et al. 2014). In 2010, some scholars found that Canadian lignite can be a suitable source for solid-phase organic carbon, which would apparently achieve NO3 removal (Li 2010) more effectively. In another study, scholars reported that luminescent bacteria acute toxicity of Canadian lignite was low (Jiang et al. 2014). Lignite resources are abundant in China (Fu 2012). However, because of low grade coalification, the industrial application value of such lignite is poor (Shen et al. 2012). Therefore, scientific studies must be carried out to determine whether Chinese lignite can be used as an organic carbon source for denitrification of groundwater. Since very few studies have investigated the suitability of Chinese lignite for denitrification, we are not really sure whether Chinese lignite can be considered as a solid-phase organic carbon source. Moreover, very few studies have investigated the impact of Chinese lignite on human health; therefore, we cannot guarantee the safety standards of denitrification achieved by Chinese lignite. In this study, denitrification was performed on groundwater in simulated conditions using Chinese lignite as the organic carbon source. Lignite was obtained from the following places in China: Sanjitun, Lingshi, Wangniutan, and Zhaotong. The lignite samples of Wangniutan showed the highest efficacy (34%) in NO3 removal process. By performing an acute toxicity test, we found that there was no toxicity in the lignite samples of Wangniutan. Furthermore, we investigated the preliminary mechanism through which lignite (the carbon source) initiated the denitrification process. For effective NO3 removal, the organic compounds must have the ability to release static carbon easily; moreover, small molecule organic compounds were used as the main carbon sources for denitrification.
Materials and Methods
Test System
Polyvinyl chloride (PVC) columns (6.0 cm in internal diameter and 60 cm length) were set up with the following components: a water distribution zone, a reaction zone, and catchment areas of 5, 50, and 5 cm thickness, respectively. While designing the columns, we evenly spaced out four sampling ports along each column (Figure 1). The ports were installed at the following points: 15 (P1), 25 (P2), 35 (P3), and 45 cm (P4) from the base of the column. They were fitted with a rubber septum, which enabled sampling through a syringe.
Artificial wastewater was pumped into the column by a peristaltic pump (Figure 1), and samples were obtained from the sampling ports and catchment areas. These samples were subsequently analyzed with standardized techniques.
Fillers
Fillers were prepared from various lignite types, which were obtained from the following places in China: Sanjitun (Heilongjiang Province), Lingshi (Shanxi Province), Wangniutan (Hebei Province), and Zhaotong (Yunnan Province). Typically, lignite material was dark brown in color. After grinding the lignite, we obtained rough, porous particles, with a particle size of 1-2 mm.
Simulated Groundwater
To prepare simulated groundwater containing NO3, we mixed 216 mg of KNO3 (AR, Xilong chemical plant, Guangdong, China) with 1 L of deionized water. The influent NO3-N concentration was 30 mg·L−1. By adding Na2CO3 into the simulated groundwater, we adjusted the pH to 6.5-7.0. A UV/visible spectrophotometer (UV-1200, Mapada, Shanghai, China) was used to analyze the NO3-N and NO2-N concentrations in groundwater.
In the reaction chamber, we controlled the water temperature with a cooling liquid circulating system. As shown in Figure 2, the system included a plastic cooling hose and DLSB-10 (Low temperature cooling liquid circulating pump). During the experiment, the water temperature was in the range 12.0-16.0 °C in the column.
The following parameters were measured primarily: NO3-N, permanganate index (CODMn), pH, water temperature, and microbial toxicity; the measurements were carried out using the methods described in the following reference books: Water and Wastewater Monitoring Analysis Method (fourth edition, 2002) and Water quality - Determination of acute toxicity - Luminescent bacteria test (GB/T 15441-1995).
Test Method
Start and Operation of the Reactor. The lignite was washed with distilled water and dried naturally. The column was filled with a suspension, which was obtained from the denitrification segment of a sewage treatment plant in Beijing (Gaobeidian plant). Denitrifying bacteria were then introduced into the column. In the beginning, the column reactor was operated for initial hydraulic retention time (HRT) of 24 hours, with a flow rate of 0.5 m·d−1. Table 1 presents the operational parameters of the column reactor. The NO3 removal rate and other related indicators were detected periodically. The porosity of the columns was detected by mercury intrusion porosimetry.
Table 1 Operation parameters of the column reactor.
| Number | Filler | Filling volume (cm3) | Porosity (%) | Initial influent NO3 concentration (mg L−1) | Initial hydraulic retention time (h) |
|---|---|---|---|---|---|
| R1 | Sanjitun lignite | 1.41 × 103 | 64.5 | 30 | 24 |
| R2 | Lingshi lignite | 1.41 × 103 | 64.9 | 30 | 24 |
| R3 | Wangniutan lignite | 1.41 × 103 | 63.8 | 30 | 24 |
| R4 | Zhaotong lignite | 1.41 × 103 | 64.6 | 30 | 24 |
Static Carbon Release Test. After washing and drying all the lignite samples, we added 3.0, 7.0, and 12.0 g of lignite to three different conical flasks of 500 ml capacity. Then, 300 ml of deionized water was added into each conical flask, resulting in lignite concentrations of 10.0, 23.3, and 40.0 g L−1. The lignite samples were soaked in the deionized water for 24 hours. For the analysis of CODMn, water samples from the conical flasks were taken after 0, 2, 6, 10, 16, and 24 hours. For CODMn analysis, water sampling was done every 3 hours. Finally, CODMn analysis was stopped when a stable value of COD Mn was observed in water samples. To perform this test, the column water temperature was maintained in the range 13.0-15.0°C.
To describe the release of static carbon, Higuchi model (Brazel and Peppas 2000) was used. In the test solution, the carbon emission was described by CODMn.
where M (mg O2 L−1) is the total carbon released theoretically; M t (mg O2 L−1) is the carbon released at time t; k (mg mg−1 h−n) is the coefficient of carbon release rate; t is the carbon release time, and n is the characteristic parameter for carbon release.
After taking the logarithm of the equation, we found a linear relationship between ln(M t) and ln(t). The parameter n represented the slope of the line, and ln(k) was the linear intercept. For n < 0.45, the carbon release mechanism was Fick diffusion. For 0.45 < n < 0.89, it was a combination of diffusion and structure dissolution, and for n > 0.89, it was structure dissolution (Zhang et al. 2009).
Analysis of the Lignite Molecular Structure. Lignite samples (2.0 g) were taken, washed, and radiosterilized by an ultraviolet radiator (TUV 16W FA, Philips, Shanghai, China). After the treatment, lignite samples were air-dried. Then, the samples were ground to micron-sized particles with an agate mortar; these particles were tested by infrared microspectrometry (NICOLET iN10 MX, ThermoFisher, New York, USA). Table 2 presents the parameters of infrared microspectrometry.
Results and Discussion
Nitrate Removal
At the beginning of the operation, the removal rate of NO3 compounds was unstable because of the following factors: microorganism growth and adsorption of the filler. The NO3-N removal rate became stable only after 15 days. Figure 3 illustrates the NO3-N removal rate in the column reactor average (R1-R4) during the period of stable operation. Figure 4 illustrates the average concentration of NO2 effluents under similar conditions.
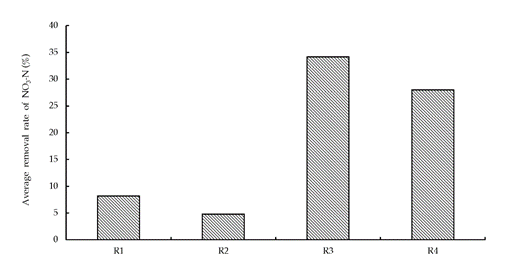
Figure 3 Column reactor average (R1-R4) of the NO3-N removal rate during stable operation conditions.
As shown in Figure 3 and Figure 4, R3 had the highest NO3 removal efficacy (34%), with the effluent NO3 concentration being below 20 mg·L−1. The NO3 removal rate of R4 was 28%. The NO3 removal rate was below 10% in other reactors: R1 and R2. Therefore, NO3 removal efficacies were significant in the reactors R3 and R4, which contained lignite from Wangniutan and Zhaotong, respectively.
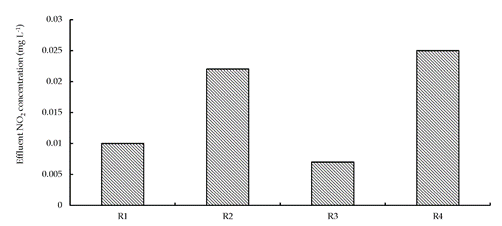
Figure 4 Column reactor average (R1-R4) of the effluent NO2 concentration during stable operation conditions.
In R1 and R3, the effluent NO2 concentrations were below 0.01 mg· L−1. In R2 and R4, the effluent NO2 concentrations were above 0.02 mg· L−1. This indicates that denitrification reaction was almost complete when we used Chinese lignite obtained from Sanjitun and Wangniutan.
The water samples of R1 and R3 showed no-toxicity when we performed an acute toxicity test; however, the water samples of R2 and R4 showed low-toxicity. This implies that lignite obtained from Sanjitun and Wangniutan is far more superior. They can be effectively used as organic carbon sources for reducing NO3 levels in groundwater (Tables 3 and 4).
Table 3 Acute toxicity evaluation standards of the luminescent bacteria test.
| Cytotoxical grade | Relative luminance L (%) | Results |
|---|---|---|
| 0 | L > 90 | No-toxicity |
| I | 70 < L ≤ 90 | Low-toxicity |
| II | 50 < L ≤ 70 | Middle-toxicity |
| III | 30 < L ≤ 50 | Low-high toxicity |
| IV | 0 < L ≤ 30 | Mid-high toxicity |
| V | L = 0 | High-toxicity |
Static Carbon Release
Figure 5 shows that the net carbon release was highest for the lignite obtained from Wangniutan; the CODMn values represented the net carbon release of various lignite types. When the lignite concentration was increased steadily, the net carbon release also increased proportionately. For lignite concentrations of 40.0 g·L−1, the net carbon release was 22.71 and 18.41 mg O2 L−1 for lignite obtained from Wandniutan and Zhaotong, respectively.
The carbon release mechanism of all lignites was structure dissolution (Table 5). For lignite concentrations of 10.0 g L−1, the coefficient of carbon release rate (k) was the highest for the lignite obtained from Sanjitun, followed by lignite obtained from Zhaotong, Lingshi, and Wangniutan. For lignite concentrations of 23.3 and 40.0 g L−1, the sequence was as follows: Sanjitun > Lingshi > Zhaotong > Wangniutan.
Table 5 Results of the dynamic equation fitting for the carbon release.
| Type | Concentration (g L−1) | Linear fitting parameters | Mechanism | |||
|---|---|---|---|---|---|---|
| R2 | n | ln(k) | k | |||
| Sanjitun | 10.0 | 0.9963 | 1.0220 | −3.4429 | 0.0320 | structure dissolution |
| 23.3 | 0.9936 | 0.9725 | −3.4121 | 0.0330 | ||
| 40.0 | 0.9971 | 0.9214 | −3.0814 | 0.0459 | ||
| Lingshi | 10.0 | 0.9983 | 1.0808 | −3.7804 | 0.0228 | structure dissolution |
| 23.3 | 0.9977 | 1.0952 | −3.6801 | 0.0252 | ||
| 40.0 | 0.9965 | 1.0231 | −3.3479 | 0.0352 | ||
| Wangniutan | 10.0 | 0.9972 | 1.6764 | −5.5486 | 0.0039 | structure dissolution |
| 23.3 | 0.9931 | 1.5468 | −5.0687 | 0.0063 | ||
| 40.0 | 0.9948 | 1.4954 | −4.8584 | 0.0078 | ||
| Zhaotong | 10.0 | 0.9956 | 1.1332 | −3.7684 | 0.0231 | structure dissolution |
| 23.3 | 0.9934 | 1.2125 | −3.9289 | 0.0197 | ||
| 40.0 | 0.9941 | 1.1324 | −3.7349 | 0.0239 | ||
Lignite Molecular Structure
For the lignite obtained from Sanjitun, Lingshi, Wangniutan, and Zhaotong, the infrared spectrum showed absorption peaks at 3600-3200, 3000-2800, 1600 and 1440, and 1320-1210 cm−1, respectively (Figure 6). The absorption peaks at 3600-3200 cm−1 were produced by alcohol or phenolic hydroxyl groups, while those observed at 3000-2800 cm−1 were attributed to alkane-CH groups. The absorption peaks at 1600 and 1440 cm−1 resulted from the vibration of the aromatic ring and carboxylic acid-CO group, respectively, while the peaks that appeared between 1320-1210 cm−1 were attributed to the carboxylic acid-OH group. For the lignite obtained from Lingshi, the infrared spectrum showed some characteristic absorption peaks at 3000-2800 and 1600 cm−1; however, absorption peaks were not observed at 3600-3200 and 1320-1210 cm−1. For the lignite obtained from Lingshi, Wangniutan, and Zhaotong, an absorption peak appeared at 1038 cm−1. This peak was probably generated from the vibration of the following functional groups: vibration of alkane-CH and anhydride-COC, the stretching vibration of sulfoxide-S=O, or a combination of the three.
Denitrification Effect
Greatest amounts of NO3 were removed successfully when denitrification reaction was carried out using Chinese lignite obtained from Wangniutan and Zhaotong. For the lignite obtained from Wangniutan, the NO3 concentration was below 20 mg·L−1 in the column effluent; the corresponding NO2 concentration was below 0.01 mg·L−1. Thus, the NO3 and NO2 concentrations completely complied with the Class III water standards of the Groundwater Quality Standards (GB/T 14848-93). For the lignite obtained from Wangniutan and Zhaotong, the net carbon release was high but the rate of static carbon release was low. This indicates that compared with the lignite obtained from Sanjitun and Lingshi, a slower rate of carbon release led to high NO3 removal efficacy. By performing acute toxicity analysis, we found that there was low-toxicity in the effluent of the lignite obtained from Zhaotong. Moreover, there was no-toxicity in the effluent of the lignite obtained from Wangniutan. Therefore, the lignite obtained from Wangniutan was the best solid-phase organic carbon source for denitrification in this study.
Denitrification Mechanism
While using lignite as a solid-phase organic carbon source, we found that NO3 removal was related to the carbon emission and the rate of static carbon release (Zhang et al. 2009). When the net carbon release was high and the rate of static carbon release was low, the denitrification process was more effective (Shao et al. 2011; Wang 2013; Xie et al. 2013). For the lignite obtained from Sanjitun and Lingshi, the net carbon release was low and the rate of static carbon release was high. Therefore, there was a lack of total carbon release and excessive energy was released in a short time (Wang 2013). For the lignite obtained from Wangniutan and Zhaotong, the trend was completely opposite: the net carbon release was high but the rate of static carbon release was low. Thus, these lignite types showed a high NO3 removal rate.
For the lignite obtained from Wangniutan and Zhaotong, we determined the molecular structure. Numerous small organic compounds, such as alcohols, phenols, and carboxylic acid, were detected in the molecular structure of these lignite types. These small organic compounds were the main carbon sources for denitrification, significantly improving NO3 removal. Although numerous small organic compounds were detected in the molecular structure of lignite obtained from Sanjitun, the efficacy of NO3 removal was poor with this lignite type. This must have happened due to the existing form of small organic compounds. In the main molecular structure of the lignite obtained from Sanjitun, there were small molecule organic compounds, such as hydrocarbons and oxygen-containing compounds; these compounds existed in a microporous embedded state or network embedded states. Free organic compounds are released easily by lignite. However, it is difficult to release embedded organic compounds from the molecular structure of lignite because of various reasons, such as physical adsorption, van der Waals forces, hydrogen bonding, and weak complexing (Chen et al. 2011; Han et al. 2014; Zhou et al. 2012). The small molecule organic compounds were mainly embedded in the molecular structure of the lignite obtained from Sanjitun. On the other hand, they were mainly in a free state in the lignite obtained from Wangniutan. As shown in Figure 7, denitrifying bacteria are more likely to use small molecule organic compounds in free states; these bacteria are present on the lignite surface or in an aqueous solution.
Conclusion
We obtained four different lignite types from Sanjitun, Lingshi, Wangniutan, and Zhaotong in China. These four lignite types were used as the solid-phase organic carbon sources for denitrification. Compared to the three lignite types, the lignite obtained from Wangniutan was superior in terms of its efficacy in NO3 removal; it showed the highest efficacy (34%) in NO3 removal, with the following characteristic accomplishments: the effluent NO3 concentration was below 20 mg·L−1 and the effluent NO2 concentration was below 0.01 mg·L−1; moreover, no-toxicity was observed in this lignite type when it was subjected to an acute toxicity test. Thus, the lignite obtained from Wangniutan is a safe organic carbon source for denitrification.
While using lignite as a solid-phase organic carbon source, we observed that NO3 removal was related to the static carbon release from lignite. If the net carbon release was high and the rate of static carbon release was low, the denitrification process was better. From the lignite obtained from Wangniutan and Zhaotong, organic carbon was probably released to the groundwater at a slower rate; therefore, the NO3 removal rates were much higher with these lignite types.
Small molecule organic compounds are the main carbon sources for denitrification. By determining the molecular structure of the lignite obtained from Wangniutan and Zhaotong, we observed numerous small molecule organic compounds, such as alcohols, phenols, and carboxylic acid, in the molecular structure. These small molecule organic compounds were more likely to be used by bacteria when they were in a free state.











 nueva página del texto (beta)
nueva página del texto (beta)








