Introduction
The nopal is a xerophytic plant belonging to the genus Opuntia that has physiological and morphological adaptations that allow it to live and grow under water-deficit conditions (Marín-Bustamante et al., 2018). Its flattened stems perform the photosynthetic process and are technically known as cladodes. The plant has mechanisms that allow water retention and low loss through evaporation and transpiration (Guevara-Figueroa et al., 2010).
Cladodes are vegetables rich in dietary fiber, carbohydrates, proteins, amino acids, vitamins, and minerals such as potassium, magnesium and calcium (Contreras-Padilla et al., 2016; Shedbalkar, Adki, Jadhav, & Bapat, 2010). Numerous medicinal properties have been documented in the literature, including as a treatment for diabetes, gastritis and hypocholesterolemia (Avila-Nava et al., 2014). Additionally, they have been reported to be useful in the treatment of burns, wounds, edema, and indigestion (Park & Chun, 2001). Other authors report properties such as antioxidant, anti-ulcerogenic (Galati et al., 2003), anti-viral, anti-inflammatory and healing activity (Aruwa, Amoo, & Kudanga, 2018).
The drying of nopal cladodes offers an alternative for their preservation, storage, handling and consumption, as well as an ingredient in a wide range of food products (López, de Ita, & Vaca, 2009). However, this operation is impeded by the cuticle (García-Saucedo, Valdez-Morales, Valverde, Cruz-Hernández, & Paredes-López, 2005). Microwave drying has been shown to provide uniformity in energy application, high thermal conductivity, reduced drying time, energy savings and advantages such as space utilization, easy cleaning, precise process control and quick start-up (Chahbani et al., 2018; Chandrasekaran, Ramanathan, & Basak, 2013; Chizoba-Ekezie, Sun, Han, & Cheng, 2017; Dadali, Demirhan, & Özbek, 2008; Guo, Sun, Cheng, & Han, 2017; Pereira, Marsaioli, & Ahrné, 2007; Pu, Li, Hui, & Raghavan, 2016; Therdthai & Zhou, 2009; Vongpradubchai & Rattanadecho, 2009; Wang et al., 2019). However, microwave drying can cause excessive heating, which can result in physical damage to the product such as carbonization, color loss and uneven temperature distribution (Vadivambal & Jayas, 2007), causing negative effects on the structure, color, porosity and elasticity of the product (Fernandes, Rodrigues, Law, & Mujumdar, 2011), and a possible change in chemical composition.
Adsorption isotherms allow optimizing drying processes, dehydrator design, microbiological control, evaluation of storage stability, prediction of the useful life of the product by determining the change in moisture during its shelf life and selection of packaging material (Amiri-Chayjan & Esna-Ashari, 2010; Ciro, Osorio, & Cortés, 2008; Vega-Gálvez, Lara-Aravena, & Lemus-Mondaca, 2006). Net isosteric heat (qst) is a measure of the attraction forces between the water molecules and the surface of the food substrate (Al-Muhtaseb, Al-Harahsheh, Hararah, & Magee, 2010). The qst provides an idea of the microstructure associated with the food and the physical interpretation of the food-water interface (McMinn & Magee, 2003; Moraes, Rosa, & Pinto, 2008; Toğrul & Arslan, 2006). In the literature, qst has been determined for a great diversity of foods, finding values between 10-75 kJ·mol-1, varying according to the drying method, the contact area of the materials, and the resulting mass and heat transfer phenomena (Chenlo, Moreira, Prieto, & Torres, 2011; Miranda et al., 2012; Rohitha-Prasantha & Amunogoda, 2013; Singh, Mishra, & Saha, 2011; Yogendrarajah, Samapundo, Devlieghere, de Saeger, & de Meulenaer, 2015).
Although nopal is a widely accepted food in Mexico, it is mainly consumed fresh, so there is very little scientific information on processes to prolong its shelf life. Medina-Torres, Gallegos-Infante, González-Laredo, and Rocha-Guzman (2008) evaluated the effect of convective and osmotic drying on mechanical properties of nopal, and reported that nopal samples dried by the former method preserved their mechanical properties in a better way. However, some other reports point out that use of convective drying may cause quality alteration of dried foods (Chahbani et al., 2018; Giri & Prasad, 2007). In view of the above, the aim of this research was to elucidate the effect of microwave drying, at two specific powers (75 and 158 kW·kg-1), on the microstructural characteristics of dehydrated nopal. In addition, isotherms at different temperatures (10, 20, 30 and 40 °C) and net isosteric heat (qst) were used as tools to explain the interaction of water with the matrix of the nopal cladodes.
Materials and methods
Raw material
The nopal cladodes used in the experimentation were from the Atlixco variety, which were acquired in Otumba, State of Mexico. Homogeneous pieces weighing between 120-130 g, with thickness of 5-7 mm, intermediate maturity and no mechanical damages, were selected. The cladodes that comprised the sample were washed and peeled (the spines were removed, and the tips were trimmed), in order to finally obtain pieces of 12.5 cm in length and 64.2 ± 0.38 g in weight.
Microwave drying
Samples with 92.91 ± 0.19 % moisture were intermittently dehydrated in a microwave oven (Ms 1446SQP, LG brand, with internal dimensions of 0.37 x 0.36 x 0.23 m, a voltage of 120 V, and frequency of 2 450 MHz) by applying powers of 75 and 158 kW·kg-1, until reducing the moisture to 7.72 ± 0.515 %. Once dry, the samples were stored in plastic bags at room temperature until the analyses were performed. The surface temperature of cladodes was measured using an infrared thermometer (Fluke 561, Fluke®, USA). For each drying condition three replicates were used.
Microstructure analysis and microanalysis
The study of the microstructure and elemental analysis were performed using a scanning electron microscope (JSM-6390 LV, JEOL, Japan), equipped with an energy-dispersive X-ray (EDS) detector and a Peltier cooling stage. Dried nopal samples were mounted on brass sample holders using double-sided carbon tape and coated with gold under vacuum sputtering (Desk IV, Denton Vacuum). Elemental analysis of the dried samples were obtained by EDS. In the case of fresh samples, these were placed on a cooled specimen holder and their temperature was gradually decreased until reaching -25 °C. The equipment was operated using an accelerating voltage of 20 kV and the images were obtained at different magnifications.
Drying kinetics
Drying kinetics were obtained at the two specific powers (75 and 158 kW·kg-1). Weight loss in the cladodes was monitored when they were being dehydrated; to do this, the sample was pulled out every 10 s and the weight and temperature were determined to later obtain the moisture content by weight difference.
Adsorption isotherms
The materials dehydrated by applying a power of 75 kW·kg-1 were used to plot the adsorption isotherms, since it was the best drying condition to preserve the structures constituting the cladodes. The adsorption isotherms were obtained at four temperatures (10, 20, 30 and 40 °C) in a water activity (a w ) range from 0.461 to 0.988, using a gravimetric method based on the a w generated by saturated salt solutions (potassium carbonate, magnesium nitrate, sodium nitrite, sodium chloride, ammonium sulfate, potassium chromate, potassium nitrate and potassium sulfate) (Lee & Lee, 2008). A known amount of dry sample (2 g) was placed in the different solutions provided in hermetically sealed containers for 14 days. They were then removed and the a w and equilibrium moisture (x e ) content were determined. The a w was measured using an Aqualab CX-2 water activity meter (Decagon Devices, USA), calibrated prior to use with deionized water and adjusted according to the working temperature. The x e was obtained based on weight differences in accordance with the official method of the Association of Official Analytical Chemists 934.06 (AOAC, 1990), following the method reported by McMinn and Magee (2003). The Equations used to model the isotherms are presented in Table 1.
Table 1 Used equations for isothermal modeling.
| Model | Mathematical equation1 | Reference |
|---|---|---|
| Peleg |
|
(Sopade, Xun, Halley, & Hardin, 2007) |
| GAB |
|
(Singh et al., 2011) |
| Halsey |
|
(Lopes-Filho, Romanelli, Barboza, Gabas, & Telis-Romero, 2002) |
| Oswin |
|
(Shivhare, Arora, Ahmed, & Raghavan, 2004) |
| BET |
|
(Chenlo et al., 2011) |
| Iglesias and Chirife |
|
(Lee & Lee, 2008) |
| Kuhn |
|
(Ayranci & Duman, 2005) |
| Smith |
|
(Miranda et al., 2012) |
1A, B, C, CB, c, d, k, and no are constants of the models.
Isosteric heat of adsorption
The sorption isotherms obtained at different temperatures allow the qst of sorption to be calculated (Simal, Femenia, Castell-Palou, & Rosselló, 2007). This parameter can be determined by the Clausius-Clayperon equation (Chenlo et al., 2011; Moreira, Chenlo, Prieto, & Torres, 2012; Singh et al., 2011):
Statistical analysis
The nonlinear regression of the models applied to the experimental values was carried out with Sigma Plot 10 software that uses the Marquardt-Levenberg algorithm to find the parameters that minimize the difference of the sum of squares between the observed and predicted values of the dependent variable. The quality of fit of the proposed models was evaluated using the coefficient of determination (R 2 ) and standard error (SE) using the nonlinear regression model, with an experimental probability of 95 % (P ≤ 0.05).
Results and discussion
Microstructural changes due to microwave application
Cuticule
Figure 1 shows the cuticle of fresh and dehydrated nopal (at powers of 75 and 158 kW·kg-1). The fresh nopal (Figure 1a) has a waxy layer that protects the epidermis, with slightly sunken stomata formed by two occlusive cells and the ostium where the gas exchange of the photosynthetic process is carried out. This morphology is similar to that reported in the analysis of 10 materials of the genus Opuntia (Silva, Acevedo, & Silva, 2001). When applying low power during drying (158 kW·kg-1), the cuticular surface showed wrinkling and the contour of the stomata emerged from the surface forming eruptions, which allowed seeing the opening of the ostium (Figure 1b). Figure 1c shows that when applying a greater amount of energy (158 kW·kg-1), the cuticular surface exhibited less pronounced eruptions, so it tended to be a more flattened surface. In the dehydrated nopal cladodes, white granular formations were observed, which may have been formed by the transfer of vapor and the entrainment of calcium oxalate to the surface.
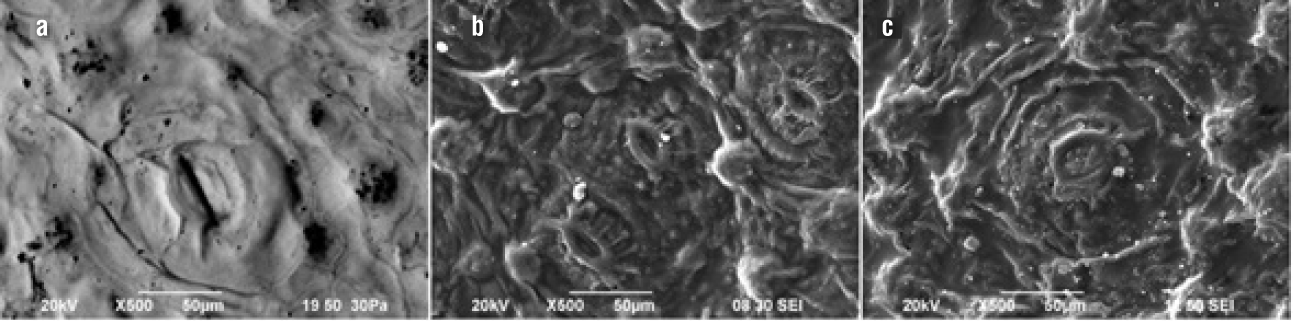
Figure 1 Cuticle microstructure: a) fresh nopal, b) nopal dried at 75 kW·kg-1 and c) nopal dried at 158 kW·kg-1.
Elemental microanalysis to determine the composition of the white granules formed in the cuticle showed high concentrations of calcium and potassium (Table 2). The presence of these elements may be due to the migration to the cuticular surface of calcium oxalates, mucilage polysaccharides and organic acids present in the vacuoles of the parenchyma and chlorenchyma. At a higher power, there was a lower surface concentration of salt-forming minerals such as calcium, potassium, chlorine, magnesium and phosphorus. This phenomenon may be due to their concentration in the parenchyma and not in the cuticle.
Internal tissues
Figure 2 shows the inner layers of the fresh and dehydrated cladodes. In fresh nopal, four layers were distinguished: cuticle, epidermis, chlorenchyma and parenchyma (Figure 2a). The chlorenchyma is composed of cylindrical, elongated cells in multilayers, with small intercellular spaces in agreement with what was observed by Silva et al. (2001). Andersen, Lucchini, Moriconi, and Fernández (2006) studied the anatomy of Lippia turbinata “poleo” leaves, considering the multilayers of the palisade cells as a xeromorphic feature. These cells are responsible for the photosynthetic process, so they are rich in chlorophyll, which gives them a green color.
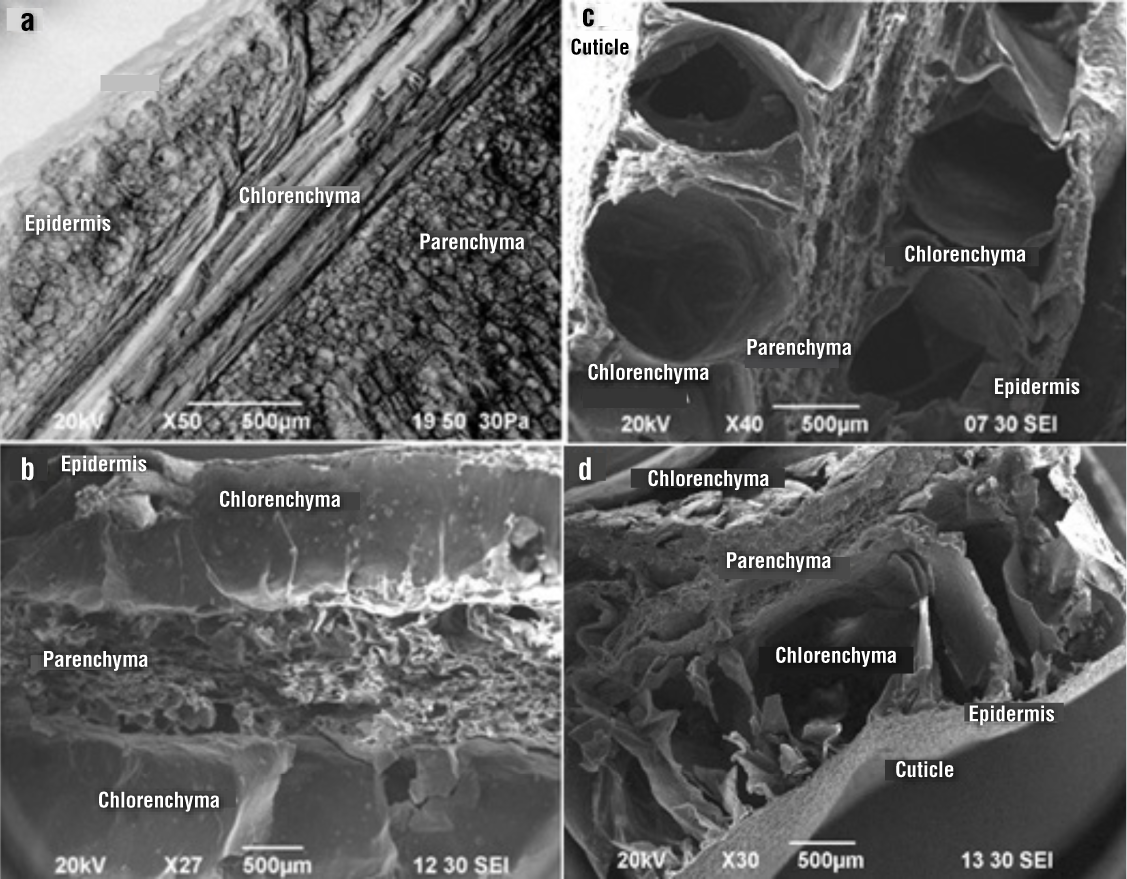
Figure 2 Inner microstructure: a) longitudinal cut of fresh nopal, b) longitudinal cut of nopal dried at 75 kW·kg-1, c) cross section of nopal dried at 75 kW·kg-1 and d) cross section of nopal dried at 158 kW·kg-1.
In the drying of the nopal cladodes by applying a power of 75 kW·kg-1, water-permeable areas were formed by opening the cell membranes of the chlorenchyma and the parenchyma (Figures 2b and 2c). In the chlorenchyma, circular orifices were observed in the xylem ducts and in the parenchyma holes were observed at the sites where the vacuoles were found. By increasing the drying power to 158 kW·kg-1, water evaporation occurred more quickly (according to the drying kinetics), causing the rupture of cell membranes, indicating that by applying a greater amount of energy the tissues suffer greater damage (Figure 2d). Therdthai and Zhou (2009) determined that by increasing the working power, more and larger pores are generated, due to the rapid and massive vaporization of the water.
Parenchyma
The parenchyma of fresh and dehydrated nopal (75 and 158 kW·kg-1) is shown in Figure 3. The parenchyma is the tissue of the inner or central portion of the fresh cladodes. Microstructural analysis verified that the cells comprising it have a spongy isodiametric geometry (Figure 3a), whose function is the accumulation of mucilage and water. Silva et al. (2001) indicate that the closer cells are to the center of the parenchyma, the more they lose their structure and the thinner their walls become. The cells are white due to the reduced number of chloroplasts and the presence of large vacuoles occupying 95 % of cell volume. Cell vacuoles have circular drusen formed by calcium oxalate crystals in accordance with what was observed by Soares-da Silva et al. (2010) and Silva et al. (2001).
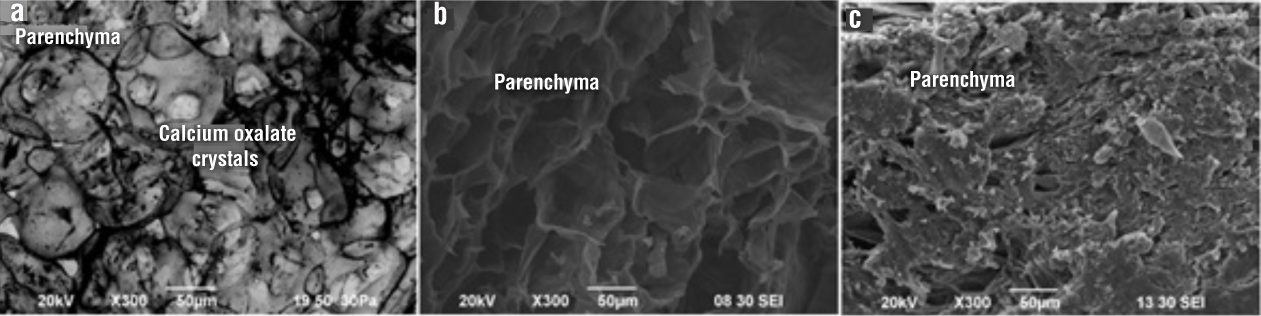
Figure 3 Parenchyma microstructure: a) fresh nopal, b) nopal dried at 75 kW·kg-1 and c) nopal dried at 158 kW·kg-1.
The parenchyma of the material dehydrated at low power showed holes, open and porous cell membranes (generated by removing the water stored in the vacuoles), and the conservation of the cellular geometry (Figure 3b). Figure 3c shows the parenchyma of the nopal dehydrated at a higher power in which it can be seen that the cells were compressed and impermeable. When rapid vaporization took place, compounds such as oxalates were not mobilized causing their concentration in situ, and causing the cell membranes to compress, without showing cavities or holes.
The power applied in the microwave drying of nopal had a direct impact on the microstructure of the samples. Using the lower power (75 kW·kg-1), there was greater preservation and differentiation of the structures constituting the cladodes (epidermis, chlorenchyma and parenchyma), while increasing the power to 158 kW·kg-1, resulted in greater structural damages due to the rupture of the membranes. This can be attributed to the rapid and violent vaporization of water during the transport phenomena of the fluid during drying. In the drying kinetics at the two specific powers it was observed that the temperature increased with the power and that the drying time was reduced, indicating a greater speed in the moisture loss. The dehydrated nopal cladodes at lower specific power had water-permeable areas and a wide contact surface, providing good interaction at the food-water interface.
Drying kinetics
The drying kinetics of the nopal varied according to the power applied (Figure 4a). At 75 kW·kg-1, the material reached a constant moisture content at 310 s and maximum temperatures of 98 °C; thus, the heat treatment was 23 250 kJ·kg-1. By applying 158 kW·kg-1, the temperature increased rapidly, reaching maximum temperatures of 107 °C, and the material reached a constant moisture content at 260 s, with which 41 080 kJ·kg-1 were applied, indicating a greater speed in the loss of moisture when increasing the power.
Adsorption isotherms at low specific power
Adsorption isotherms were obtained in an a w range from 0.461 to 0.988, since the a w of the dehydrated product was 0.478 ± 0.003 (Figure 4b). Isotherms were obtained with the typical sigmoidal shape of Brunauer type III curves. These types of curves indicate weak interactions between the adsorbate and the adsorbent (Brunauer, Deming, Deming, & Teller, 1940), maintaining low amounts of water at low a w and large amounts at high relative humidity levels (Samaniego-Esguerra, Boag, & Robertson, 1991).
The type III sigmoid curve has been obtained in various foods such as chocolate powder (Medeiros, Bartolomeu-Ayrosa, de Moraes-Pitombo, & da Silva-Lannes, 2006), dehydrated soursop powder (Ceballos, Giraldo, & Orrego, 2009), naranjilla pulp powder (Gabas, Telis-Romero, Giraldo-Gómez, & Telis, 2009), mango pulp (Rangel-Marrón et al., 2011) and yam (Montes et al., 2009). Characteristics shared by these products are their broad exposure surface when in powder form, their porous or membranous materials, and in some cases their high sugar content.
In the adsorption isotherms at different temperatures, it was observed that the moisture content decreases when the temperature increases at the different a w levels. Shivhare et al. (2004) explained that the kinetic energy associated with the water molecules present in food increases with increasing temperature; this, in turn, results in the decrease in attractive forces and therefore the escape of the water molecules, which translates into a decrease in the degree of adsorption with the increase in temperature at an a w specific.
The model with the best fit, based on the coefficient of determination, was the Peleg one; the Equations obtained were:
The values of the correlation coefficient (R 2 ) and the standard error (SE) of the nonlinear regressions applied to the experimental values of the adsorption isotherms are shown in Table 3.
Table 3 Goodness of fit of the different adsorption isotherm models.
| Model | R 2 | Standard error | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 °C | 20 °C | 30 °C | 40 °C | 10 °C | 20 °C | 30 °C | 40 °C | ||
| Peleg | 0.9917 | 0.9962 | 0.9965 | 0.9955 | 0.0905 | 0.0417 | 0.0328 | 0.0358 | |
| GAB | 0.9898 | 0.9984 | 0.9980 | 0.9950 | 0.0984 | 0.0262 | 0.0245 | 0.0369 | |
| Halsey | 0.9905 | 0.9978 | 0.9950 | 0.9945 | 0.0927 | 0.0307 | 0.0376 | 0.0380 | |
| Oswin | 0.9899 | 0.9970 | 0.9931 | 0.9941 | 0.0959 | 0.0358 | 0.0440 | 0.0394 | |
| BET | 0.9882 | 0.9954 | 0.9936 | 0.9948 | 0.1036 | 0.0439 | 0.0423 | 0.0368 | |
| Iglesias and Chirife | 0.9868 | 0.9945 | 0.9940 | 0.9944 | 0.1097 | 0.0481 | 0.0411 | 0.0384 | |
| Kuhn | 0.9865 | 0.9943 | 0.9939 | 0.9944 | 0.1076 | 0.0490 | 0.0415 | 0.0382 | |
| Smith | 0.8693 | 0.8769 | 0.8987 | 0.9220 | 0.3445 | 0.2277 | 0.1689 | 0.1428 | |
Net isosteric heat of nopal dehydrated at low specific power
The qst curve was calculated using the equations provided by the Peleg model in a moisture content range from 0.05 to 1.00 kgH2O·kg-1. This curve allows the estimation of the energy additional to the enthalpy of vaporization at a specific temperature to achieve the separation or union of the water at the food interface.
In Figure 4c, it can be seen that at high moisture contents the qst is low, so the energy demand at this stage is mainly for water vaporization (enthalpy of vaporization). In the monolayer where the water is adsorbed by chemisorption and has a strong bond with the surface of the food, the qst rises and is precisely the additional energy to break the union.
The qst for the dry material was 7.51 kJ·mol-1 at a moisture content of 0.05 kgH2O·kg-1. This value is relatively low compared to that obtained in other materials; this may be due to the drying method as observed by Lee and Lee (2008). These authors compared the qst obtained by freeze, vacuum and hot-air drying of a mushroom (Innotus obliquus). Particle size may also be the cause of a low qst value. Moreira et al. (2012) and Singh et al. (2011) observed that qst decreases as the contact surface increases since lower values are obtained in ground grains than in whole ones. The dehydrated nopal has a large exposure area which can generate a similar behavior.
The qst values obtained for different moisture contents were modeled using decreasing exponential equations with different parameters. Equations 14-16 allow calculating the qst value as a function of moisture content. The four-parameter exponential model was the one that had a better fit (R 2 of 99.9). The equations obtained were as follows:
Conclusions
The power applied in the microwave drying of nopal had a direct impact on the microstructure of the samples. When using a power of 75 kW·kg-1 there was greater preservation and differentiation of the structures constituting the cladodes (epidermis, chlorenchyma and parenchyma), while increasing the power (158 kW·kg-1) resulted in greater structural damages due to the rupture of the membranes. The net isosteric heat value was low, probably due to the material’s wide contact area and the high conductivity of the heat generated by the microwaves from inside the sample. It can be concluded that microwave drying of nopal is an alternative method that reduces drying time and allows the preservation of the material’s structural properties when a power of 75 kW·kg-1 is used for a time of 310 s.











 texto en
texto en 



