Introduction
The content of antioxidant phytochemicals in many fruits has been the subject of recent research (Jagadeeshwar 2005, Salem et al. 2018, Taher et al.2018), such as capulin (Prunus serotina subsp. capuli), a species that grows wild or under cultivated conditions in several regions of the Americas, and which has attracted considerable attention as a potential source of phenolic compounds that are plentiful in the fruit skin (Ordaz-Galindo et al. 1999, Rivero-Cruz 2014). The plant and fruit of the Prunus serotina species have been used since pre-Hispanic times as food and for medicinal purposes due to the antioxidant, antihypertensive and antimicrobial properties that have been attributed to them (Jiménez et al.2011, Luna-Vázquez et al.2013).
Excessive production of reactive oxygen and nitrogen in the human body are responsible for the pathogenesis of some chronic diseases. It has been reported that the ingestion of antioxidant phytochemicals, such as the phenolic compounds present in many fruits, is inversely associated with many chronic diseases caused by oxidative stress (Zhang et al. 2015). Natural antioxidants are prized because they can be used to design functional or nutraceutical foods that provide health benefits (Villanueva-Tiburcio et al. 2010, Gürbüz et al. 2018). The changes in chemical composition and content of phytochemical compounds with the antioxidant power in fruits mainly depends on the development stage of the plant. In non-climacteric fruits, such as capulin, it is an important condition because the optimal harvest time depends on it (Kader 2002). Most of the antioxidant capacity in Prunus serotina species belongs to the group of phenolic compounds and vitamins. Some authors report that the content of these compounds in this fruit can be affected quantitatively by several factors, such as genetic differences, environmental production conditions, harvest maturity and postharvest handling conditions (Lekala et al.2019, Zadernowski et al.2005). To better exploit the antioxidant potential of capulin fruit, it is necessary to determine the stage of development when the highest antioxidant activity occurs, and that allows establishing a harvest index for the fruit. Therefore, the aim of this research is to evaluate the concentration changes in the compounds that contribute to the antioxidant capacity in capulin (Prunus serotina) fruit during the ripening process, in order to determine when the maximum value is reached.
Materials and methods
Identification of herbarium specimen and fruit development evaluation
A wild phenotype of capulin (Prunus serotine subsp. capuli (Cav). McVaugh) from Zacatlán (19°55’ 47.1” N, 98° 00’ 00.4” W; 2 531 m), Puebla State, Mexico was studied. This species was sampled in a pine-oak forest area. A voucher specimen (No.25499) was identified, following the Calderón and Rzedowski (2005) protocol, and kept in the Herbarium of the Universidad Autónoma Chapingo. After the trees in the study area bloomed, five branches were selected for measuring fruit growth at 0, 30, 45, 60,75, 90, and 97 days after anthesis (DAA). During sampling days, 50 fruit were taken for weight and size evaluation.
Analysis of fruit growth
The results for fresh weight, polar diameter and equatorial diameter of the fruit were subjected to polynomial regression analysis, from which models that best explain the growth of the fruit based on these variables were generated. The estimation of the models was performed using the Sigma Plot program (11.0) and selected based on the value of R2.
Chemical characterization of fruit during ripening
For chemical fruit characterization during ripening, five harvests were taken and identified as stages (S1-S5). The fruit were collected in five stages of maturity, based on the color of the external surface: green (S1), yellow-red (S2), reddish-brown (S3),100% reddish (S4) and reddish-purple (S5) (Figure 1). Around 200 g of fruit, representative of the entire population, were collected in each harvest. Skin and pulp were separated from seed, freeze-dried and ground (NutriBullet 600w 8) for storing at -5 °C. Total soluble solids were measured by refractometry on a portable refractometer with a temperature correction. A drop of pure juice from each of the three replicates containing 15 fruit was measured. The results were expressed in °Brix. Titratable acidity was measured by diluting 1mL of pure juice in 10 mL of distilled water and titrating this solution with 0.01 M NaOH at pH 8.1
Extract preparation for phenolic and flavonoid contents and antioxidant capacity
Extraction was performed based on the method reported by Hernández-Rodríguez et al. (2016), using ethanol/water (1:1, v/v) as solvent. Each sample was adjusted at pH 3.3 with 5% HCl (Jiménez et al. 2011) and stirred by vortex for 3 min at 252 g (Vortex Synergy, WVR International). Then, the sample was treated under sonication (to exhaustively extract the phenolic compounds) for 15 min at 20 °C, using ice to maintain the temperature (Ultrasonic Cleaner 8890, Cole-Palmer). Then, the sample was shaken in an incubator (Orbital incubators Prendo INO-650 M) for 30 min. Finally, the sample was sonicated again for 15 min and then centrifuged for 15 min at 700 g. Each sample was analyzed in triplicate. Phenolic compounds are polar substances and therefore polar organic solvents are used for their extraction and the extraction medium is acidified to favor their protonated form.
Total phenolic content
Total phenolic compounds content was determined using the Folin-Ciocalteu reagent (Singleton and Rossi 1965) adapted to microplates. The absorbance was measured at 760 nm in a microplate reader (Synergy 2 Microplate reader, Biotek International, software Gen5). A gallic acid standard curve with a linear range (2.5-29.0 µg GA mL−1) was prepared from a freshly made 0.5 mg mL−1 gallic acid stock solution. Results were expressed as mg gallic acid equivalents (GAE) per gram on dried basis (mg-GAE gdb −1).
Tota flavonoids content
The total flavonoids content was determined according to Kubola and Siriamornpun (2011) adapted to microplates. Extract (0.5 mL), distilled water (2.5 mL) and 5% NaNO2 solution (0.15 mL) were mixed. After 6 min, 10% AlCl3 6H2O solution (0.3 mL) was added; after five min, 5% NaOH solution (1 mL) was added and stirred by vortex (3 min,252 g). Absorbance of an aliquot (200 µL) was measured at 510 nm in the microplate reader (Synergy 2 microplate reader, Biotek International, software Gen 5). A catechin standard curve within the linear range (0.7 to 34.5 µg CE mL−1) was prepared from a freshly made 300 µg mL−1 catechin stock solution. Results were expressed as mg of catechin equivalents (CE) per gram on dried basis mgCE gdb−1
Ferric-reducing antioxidant power (FRAP)
The FRAP assay was carried out according to the method of Benzie and Strain (1996) adapted to microplates. The FRAP reagent was prepared fresh each time it was needed, from sodium acetate buffer (300 mM, pH 3.6), 10 mM TPTZ solution (40 mM HCl as solvent) and 20 mM iron (III) chloride solution at a ratio of 10:1:1, respectively. An aliquot (20 µL) of a suitably diluted extract was mixed with 180 µL of FRAP solution and 60 µL of distilled water. The absorbance was measured at 595 nm in a microplate reader (Synergy 2 microplate reader, Biotek International, software Gen 5). A Trolox standard curve with a linear range (3.8 to 46 µM) was prepared from a freshly made 1 mM Trolox stock solution. The results were expressed as micromole Trolox equivalents pergram on dried basis (µmol TE gdb −1).
Total anthocyanin content
Monomeric anthocyanin content of the capulin fruit was measured using a spectrophotometric pH differential protocol (Giusti and Wrolstad 2001). One portion of 20 mg of the sample was mixed thoroughly with 5 mL of a pH 1 buffer (0.025 M KCl). The absorbance of the mixture was measured at 510 and 700 nm using a microplate reader (Synergy 2 microplate reader, Biotek International, software Gen5). Another 25 mg portion of the sample was combined similarly with 5 mL of a pH 4.5 buffer (4.5 M CH3COONa); the absorbance of this solution was measured at the same wavelengths. The anthocyanin content was calculated based on Cyanidin-3-O-glucoside, molar extinction coefficient of 26,900 and a molecular weight of 449.2 gmol−1. Results were expressed in terms of mg Cyanidin-3-O-glucoside equivalent per gram on dried basis (mg Cyanidin-3-glucoside gdb−1)
Statistical analysis
All assays were carried out in five replicates. The experimental unit was a capulin tree. A completely randomized simple factor design was applied to data; when suitable, means were compared by Tukey’s test at p ≤ 0.05. All statistical analyses were made using the program SAS version 9.1. The data were presented as the mean ± standard error of the mean.
Results
Fruit development
Fruit growth with respect to polar and equatorial diameter was best described with the use of third-degree polynomial models (Figure 2). Fruit weight was best described by a second-degree polynomial model (Figure 2). In general, the growth of the fruit for evaluated variables presented three stages: the first of maximum or exponential growth (0-45 DAA); the second of less growth (45-75 DAA) and the third, again, of fast growth (75-97 DAA). This last stage of growth belongs to the change of color in the fruit. The growth patterns of the two diameters were similar; however, during fruit development and maturation, the growth of the polar diameter exceeded the equatorial diameter (1.1 and 1.2 cm, respectively).
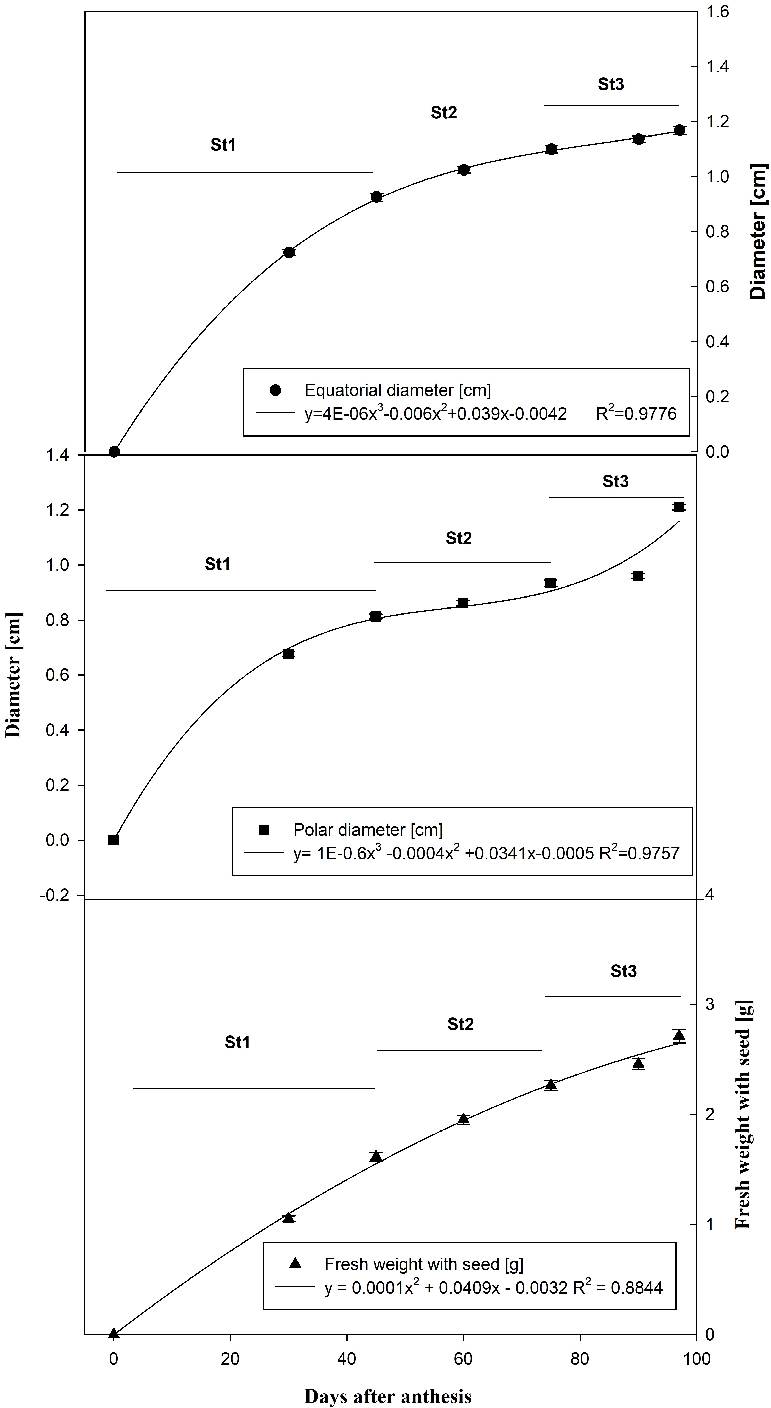
Figure 2: Fresh weight and equatorial and polar diameters of capulin (Prunus serafina subsp. capuli (Cav). McVaugh) fruit during development. St1: exponential growth, St2: less growth and St3: fast growth.
The titratable acidity of capulin fruit changed significantly from 1.2 to 0.5 mg of citric acid equivalents per gram of fresh fruit (Table 1). The total soluble solids (TSS) content increased significantly from 6.3 to 10.1 °Brix from S1 to S5 (89 to 97 DAA, Table 1). The TSS/TA ratio ranged significantly from 5.52 to 19.63, and an increase was observed in fruit ripening.
Table 1: Content of total phenols, total flavonoids, antioxidant capacity, anthocyanins, total soluble solids, titratable acidity and TSS/TA in fruit of Capulin (Prunus serotina subsp. capuli (Cav). McVaugh) at different stages of ripening.
| Stage of ripening (coloration) | Total phenols (mg GAEdb -1) | Total flavonoids(mgCEgdb -1) | Antioxidant capacity(µmol TE gdb -1) | Anthocyanins (mg Cyn-3-) glucoside gdb -1 | Total soluble solids (TSS) (°Brix) | Titratable acidity (TA) (mg CA gfw -1) | TSS/TA |
|---|---|---|---|---|---|---|---|
| S1 | 26.96a | 14.79a | 42.58a | 0.04a | 6.30a | 1.24a | 5.52a |
| S2 | 23.47a | 15.93a | 54.88ab | 0.20b | 7.82a | 0.96b | 8.54b |
| S3 | 23.60a | 16.56a | 63.56bc | 0.34c | 8.52b | 0.78bc | 11.26c |
| S4 | 14.40b | 9.98b | 50.04cd | 0.66cd | 9.55b | 0.64cd | 15.22d |
| S5 | 14.77b | 9.23b | 32.80d | 1.41d | 10.13c | 0.51d | 19.69e |
| HMSD | 4.68 | 2.79 | 11.83 | 0.18 | 1.02 | 0.24 | 2.56 |
S: stage of ripening. Means followed by the same letters in the column do not differ significantly according to Tukey’s test (p ≤ 0.05).
Changes in the concentration of antioxidants during maturation
The total phenolic content reached a maximum of 26.96 mg GAE gdb−1 at S1 and thereafter remained without significant changes until S3; however, from this time it began to decrease until reaching an average concentration of 14.40 mg GAE gdb −1 at S4 (Table 1). The total flavonoid content had no significant variation (p > 0.05) up to S3; the maximum value was 16.56 mgCE gdb −1. Then, the content decreased significantly to 9.23 mgCE gdb −1. Total anthocyanins increased significantly (p < 0.05), from 0.04 (S1) to 1.4 mg Cyanidin-3-glucoside gdb −1 (S5), during the ripening period of the berries in the tree. The highest increase (approximately 1 mg Cyanidin-3-glucoside gdb −1) was observed in the last stage of ripening (S4-S5), which corresponds to a change in color, from reddish to purple on the fruit skin. The antioxidant capacity of the fruit had a maximum value of 63.7 µol TE gdb −1 at S3. From that date it fell significantly to 32.7 µmol TE gdb −1 at the S5 ripening stage (day 97).
Discussion
Fruit development
The behavior observed is typical of stone fruits, where the first stage has greater growth because there is an overlap in the growth of the stone and pulp (Díaz et al. 2017). According to Hernández et al. (2002), fruit growth begins with the cell division process and results in a weight increase. In peach fruit three growth stages were also detected: stage I lasted from flowering to the beginning of stone hardening. This phase was characterized by a period of intense cell division, starting from fruit set. Stage II was characterized by a slow increase in the mesocarp, since the most relevant process is lignification (stone hardening). Stage III started once the stone hardened completely and was characterized by a marked elongation of the cells. Therefore, there is an accelerated growth in size and fruit weight (Ojer 2011).
The observed titratable acidity behavior can be explained according to Neves et al. (2015) who reported that the decrease in titratable acidity during fruit ripening can be attributed to both the use of organic acids in the respiration process and the result of the dilution effect that occurs during fruit growth. The TSS content had the opposite behavior to titratable acidity. According to Eskin and Hoehn (2013), as the maturation process occurs in fruits, the TSS content increases due to the action of the polygalacturonases and cellulases enzymes involved in the degradation of the pectin and cellulose in the cell wall. The TSS/TA ratio had an increase throughout fruit ripening; this coincides with Vursavuş et al. (2006) who reported a TSS/TA ratio ranging from 20.09 to 29.49 for three varieties of sweet cherry (Prunnus avium L.) harvested at commercial maturity. According to Siegmund (2015), the development of the flavor in fruits is due to an increase in sweetness and a decrease in acidity resulting in a suitable sugar/acid ratio.
Changes in the concentration of antioxidants during maturation
The behavior observed in total phenolic content is similar to the ones reported for fruits of raspberries (Rubus idaeus L. cv. Polka) (Ali et al. 2011), Vaccinium corymbosum L. (Castrejón et al. 2008) and Eriobotrya japonica Lindl. (Ding et al. 2001). It is common, during fruit development and maturation, to find strong variations in the phenolic compounds. Those variations depend on: the availability of precursors of phenolic molecules, especially phenylalanine; the activity of enzymes involved in the biosynthesis, interconversion and degradation of phenolic molecules; the changes that occur in the cell structure during fruit maturation and the effect of external factors (Macheix and Fleuriet 1990).
Regarding flavonoid content, Lou et al. (2012) reported a similar trend during fruit development in the species Morus alba L., where the most abundant flavonoid was proanthocyanidin, which decreased during fruit development but increased during the maturation stage. Similarly Kennedy et al. (2002) reported that there is an increase in the amount of anthocyanidin incorporation into proanthocyanidins in the skin of Vitis vinifera L. berries during the ripening stage, which results in an increase in the amount of total flavonoids at this stage. On the other hand, the increase in anthocyanin content is strongly related to reddish coloration. Research on strawberries found an increase in anthocyanin content when the fruit change to a reddish or purple color in the final stage of ripening (Castrejón et al. 2008, Villanueva-Tiburcio et al. 2010, Alí et al. 2011).
The antioxidant capacity trend observed during ripening was similar to other studies on fruits of Vaccinium corymbosum L., Morus alba L. (Castrejón et al. 2008) and Myrciaria dubia (H.B.K) McVaugh (Villanueva-Tiburcio et al. 2010); in all cases it was found that antioxidant activity decreased in the final stage of fruit maturation. When comparing the results found in this work, during the fruit development stage, with those reported for the different varieties of Rubus sp., Rubus idaeus L., Rubus occidentalis L. and Fragaria × ananassa D, it was observed that in both studies, in the final maturation stage, there was a downward trend in total phenolic content, flavonoids and antioxidant capacity, while total anthocyanins increased significantly (Table 1). The appearance of color in the fruit is explained by the final stage of the anthocyanin biosynthetic pathway, where the enzyme flavonoid-4-reductase reduces dihydroflavonols to leucoanthocyanidins, which undergo oxidation, dehydration and glycosylation with sugars such as D-glucose, lactose, L-rhamnose and D-xylose to form anthocyanins (Eskin and Hoehn 2013).
Thus, the incorporation of flavonoids and sugars to biosynthesis anthocyanins may explain the decrease in total phenolics and flavonoids, as well as the increase in anthocyanins. Kähkönen et al. (2001) and Kader (2002) indicate that the different stages of fruit development and maturation affect the phenolic profile, as these phenolic compounds have a high content in young fruits, but decrease during maturation, with the exception of anthocyanins that generally accumulate in this last stage.
The contribution of phenolic compounds to antioxidant activity is variable; in fruit of the species Vaccinium L. sp. (Connor et al. 2002), it is reported that the antioxidant capacity is strongly related to the content of phenolic compounds and anthocyanins, while in the species Morus alba L. (Lou et al. 2012) it is reported that there are other components, different from anthocyanins and flavonols, that contribute to this activity in the last stages of fruit ripening. In Vaccinium corymbosum L., it is reported that phenolic compounds other than anthocyanins can contribute positively to total antioxidant activity (Castrejón et al. 2008).
The antioxidant capacity showed a higher correlation with the total flavonoid content (0.72, Pearson’s correlation). Flavonoids are positively related to total phenolic content and negatively related to anthocyanin content (0.92, -0.84, Pearson’s correlation). Although the behavior of the anthocyanins is ascending, the proportion in which they are found with respect to the flavonoid content is lower. In a capulin fruit study, a total of 2433 phenolic compounds were identified and quantified, with only 89 corresponding to cyanidin glycosides, whereas most correspond to catechin, dimeric epicatechin, trimeric proanthocyanidins, and quercetin glycosides (Vasco et al 2009). Considering the above, it is probable that in this study the presence of phenolic compounds is responsible for the antioxidant capacity in the fruit. The results in this research showed the importance of establishing a harvest time, especially to take advantage of the antioxidant power of the fruit. In this sense, it is important to determine a suitable harvest index for the fruit, because as already shown, over-ripening contributes to the loss of its functional properties and antioxidant capacity (Gruz et al. 2011). In this case the fruit reached maximum antioxidant capacity when it was completely reddish (S3).
Conclusions
The experimental evidence showed that total phenolic and flavonoid contents, as well as antioxidant capacity decrease in capulin fruit in the last stages of ripening. Therefore, it is suggested that the ideal stage at which the fruit should be harvested is the S3 ripening stage (93 DAA), because in this stage the fruit contained the maximum concentration of phenolics and flavonoids, which means the highest antioxidant capacity.











 nueva página del texto (beta)
nueva página del texto (beta)




