INTRODUCTION
Intradialytic hypotension (IDH), the most frequent complication of hemodialysis treatments, is a serious complication associated with ischemic stress to vital organs, poor quality of life, and increased mortality1. Accurate prediction of IDH is a significant challenge for nephrologists because large volumes of data are continuously generated and routinely acquired, including dialysis treatment parameters, hemodynamic factors, and vital signs during the dialysis session. In addition, patient multiple medications, dietary restrictions, lifestyle modifications, and other variables of complex nature contribute to individual variation during the dialysis treatment2. Thus, the effective management of individual patients undergoing dialysis requires novel tools to perform real-time analysis and visualization of large data volumes in a secure, reliable, and efficient way. Artificial intelligence (AI) and, particularly, machine learning (ML) have already been demonstrated to be useful to dialysis patients in the adjustment of the erythropoiesis-stimulating agent dosage for renal anemia3, prediction of the occurrence of IDH4, and evaluation of fluid volume for patients undergoing dialysis5 among other tasks6.
AI technology is advancing rapidly, and new and innovative tools are continuously emerging. In figure 1, we provide a comprehensive overview, illustrating the remarkable contributions of AI to healthcare. As part of these breakthroughs, the advent of generative AI holds the potential to improve patient care with a personalized approach based on real-time monitoring and increase efficiency through automated-guided dialysis sessions to detect eventual anomalies from dialysis-related data. These models can also play a role as assistants in the design of personalized educational materials for dialysis patients.
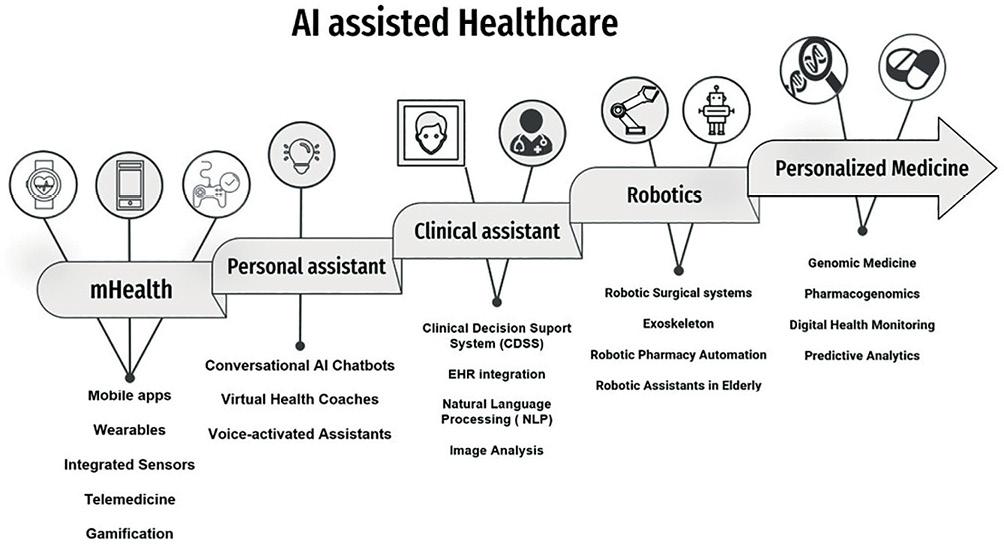
Figure 1. Diagram of artificial intelligence (AI)-driven healthcare design. This image shows the various approaches and levels at which AI can contribute to healthcare. (1) mHealth: health-care services through mobile devices, enabling individuals to access healthcare resources, track their health, and receive personalized recommendations. (2) Personal health Assistants: AI-powered technologies designed to provide personalized support and guidance empowering individuals to manage their health effectively. (3) Clinical assistant: AI systems that assist healthcare professionals in clinical settings, providing real-time decision support, streamlining workflows, and enhancing patient care. (4) Robotics: Utilizing advanced robotic systems and AI algorithms to enhance medical procedures assist health-care providers, patient rehabilitation or medication delivery. (5) Personalized Medicine: by considering factors such as an individual's genetic profile, lifestyle, and environmental influences, personalized medicine aims to optimize treatment selection, dosage, and preventive measures.
Generative AI is instantiated on ML techniques such as generative adversarial networks (GANs) and large language models (LLMs). LLMs have demonstrated a remarkable performance in natural language processing (NLP) tasks since can be adapted to capture the nuances of the complexity of medical language and the diversity of medical contexts. Considering the exponential growth in electronic health records (EHRs) deployment, LLMs are bound to transform medical practice7. The integration of generative AI tools in hemodialysis poses significant challenges, though. This position paper aims to provide a comprehensive overview of the potential benefits and challenges of using generative AI in nephrology and to identify key considerations for their successful implementation.
UNDERSTANDING GENERATIVE ARTIFICIAL INTELLIGENCE
From a general point of view, AI systems can be divided into discriminative AI and generative AI. Discriminative AI focuses on learning the boundaries that separate different classes or categories of data. Discriminative models aim at solving tasks such as classification, regression, and object recognition. On the other hand, generative AI models aim to understand and replicate the underlying distribution of the training data. They focus on learning the joint probability distribution of the input features and the corresponding labels. For this reason, generative models can create new instances that resemble the original data and enable tasks such as data synthesis, text generation, or image generation.
Generative AI must be distinguished from two complementary concepts: (i) Artificial general intelligence or strong AI refers to a type of AI that possess the ability to understand, learn, and apply knowledge across a wide range of tasks, which remains a still theoretical objective. On the contrary, generative AI is a more specific and limited application of AI. (ii) General purpose AI (GPAI) refers to AI systems and algorithms that can be applied to a wide range of tasks and problems without being specifically designed for a particular application. An example of GPAI is transformer-based LLMs like GPT-4. Generative AI can be considered a form of GPAI.
Unlike other AI tools that are primarily focused on classification and prediction tasks, generative AI goes beyond that to generate something new, rather than just making decisions on the basis of existing information. Generative AI systems have more autonomy in making sense of medical data without significant guidance from human operators, allowing it to process vast quantities of relevant data to achieve more versatile machine intelligence expressed mostly so far in linguistic form. These models leverage complex algorithms, often based on deep learning neural networks, to learn patterns and derive meaning out of a vast amount of high-quality structured and unstructured data. However, generative AI models require continuous and accurate data updates, expert supervision, and address potential biases and limitations.
There are different approaches to generative AI8, such as (i) Variational Autoencoders, which are models that generate new samples by learning from a low dimensional representation, known as latent space, of the training data and decoding it back into the original data domain. (ii) GANs consisting of two neural networks, a generator and a discriminator that compete against each other. The generator generates new samples, while the discriminator tries to distinguish between real and generated samples. (iii) Autoregressive models that generate data by modeling the conditional probability of each data point given the previous ones. They generate sequences of data, one element at a time, considering the dependences on previous elements.
METHODS FOR ADAPTING LARGE LANGUAGE MODELS TO DIVERSE MEDICAL DOMAINS: TRANSFER LEARNING AND DOMAIN ADAPTATION
Transfer learning allows LLMs to leverage pre-trained models as a starting point for further training and adaptation to medical domains, such as dialysis. We can ensure that the models are updated and capable of delivering accurate medical knowledge by applying a domain-specific fine-tuning, which involves training over pre-trained LLMs on relevant medicine-specific data such as a dialysis session7. In addition, through domain adaptation developing models trained in one domain can be adapted to other different contexts to work effectively without requiring extensive retraining. One example of a successful domain-specific model is clinical BERT, which has been fine-tuned on the MIMIC-III dataset, which consists of EHRs from intensive care unit patients, demonstrating enhanced performance in clinical NLP tasks, including patient mortality prediction, de-identification, and diagnosis classification8. Another example is BlueBERT, also based on a BERT architecture, which has achieved state-of-the-art performance on various biomedical NLP tasks, including named entity recognition, relation extraction, and biomedical question-answering9.
Alternative methods for scenarios in which domain-specific training data are scarce or unavailable include, for instance, few-shot learning and zero-shot learning, that allow LLMs to adapt to new medical domains more efficiently. Few-shot learning aims to train models to perform well on new tasks with very limited labeled data by leveraging knowledge learned from another tasks10. On the other hand, zero-shot learning focuses on training models to perform tasks without any labeled data for the target task, relying solely on knowledge learned from other tasks11.
CHAT-BASED GENERATIVE PRE-TRAINED TRANSFORMERS (CHATGPT), A USEFUL LARGE LANGUAGE MODEL
The recently developed and deployed LLM named ChatGPT can perform a broad range of natural language tasks12. ChatGPT is powered by GPT3.5, an OpenAI 175 billion parameter model that is trained on a large corpus of text data from the Internet through reinforcement and supervised deep learning algorithms. To the purpose of the scope of this paper, it is important to note that ChatGPT can cover all topics of medical knowledge from basic science, clinical knowledge, and management to bioethics12. However, the performance of ChatGPT in answering specific questions about nephrology is still limited. When assessing the accuracy of ChatGPT on 183 core questions in glomerular diseases, the model only achieved a rate of 45% on the first run and 41% on the second run, that is, far below the passing threshold of 75% and 76% required for nephrologists by nephrology self-assessment program and kidney self-assessment program of the American Society of Nephrology, respectively. However, ChatGPT's performance will continue to improve in real-world clinical situations, as the underlying AI models become more sophisticated and trained with a more specialized data corpus. Consequently, generative AI may improve medical treatment planning offering a new opportunity to personalize hemodialysis treatments.
ChatGPT can serve as a virtual assistant, providing patients with personalized information about their condition, treatment options, and self-care strategies. This AI-driven support can help patients to better understand their disease and improve adherence to their treatment plans, ultimately improving their quality of life. Moreover, from the medical expert standpoint, ChatGPT and alternative LLMs can analyze individual patient's data, identify the risk of complications, or make a checklist of their main problems requiring a solution by nephrologists (a schematic illustration of the process in Fig. 2).
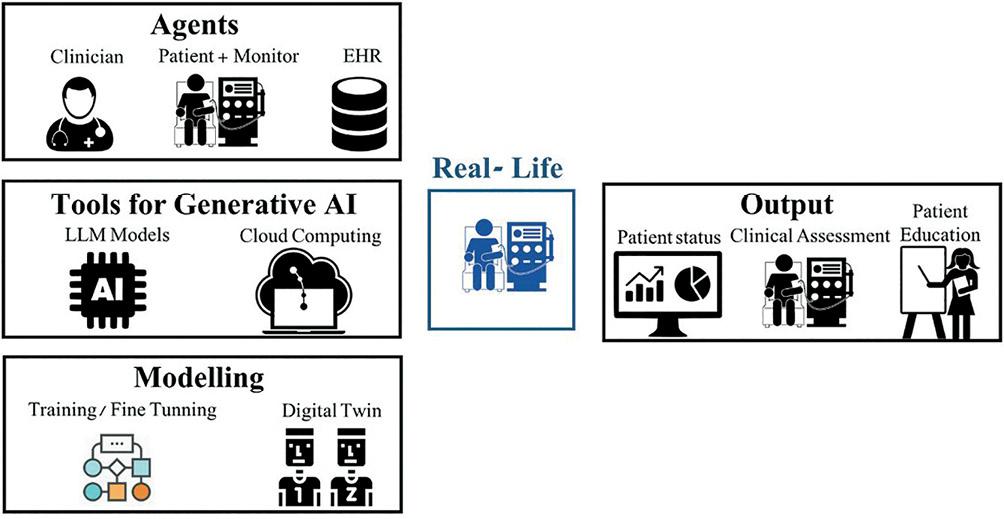
Figure 2. Schematic illustration of large language model use in an artificial intelligence (AI)-driven personalized dialysis model. This image shows the different elements needed for the model, grouped in different steps: (A) Agents: fundamental components collaborating to medical dialysis therapy. (B) Tools: innovative strategy for personalized dialysis approach based on generative AI models powered by cloud computing. (C) Modeling: model training for personalization of dialysis treatment using in silico methods, including the utilization of Digital Twins, to ensure their accuracy and efficacy before their practical implementation in real-life settings. (D) Output: real time patient status data analysis, data visualization to improve data comprehension by clinician and patient. Explicability of the output of the model, enhancing the interpretability of the results.
LARGE LANGUAGE MODELS CAN USE REINFORCEMENT LEARNING FOR FINE-TUNING AND TRANSFER LEARNING
Reinforcement learning (RL) is an ML approach where an agent learns to make optimal decisions through a process of trial and error, based on interactions with the environment, without requiring prior knowledge of optimal performance13. During this interaction, the agent receives feedback in the form of rewards or penalties, based on its actions, to guide, and refine its decision-making process. ChatGPT uses a method known as RL from human feedback as a fine-tuning tool. To apply RL for fine-tuning LLMs, the problem needs to be formulated as an Agent-Environment setting where the agent can interact with the environment to get reward for its action. In the context of chat-based models, the environment represents the dialog with a user. The model takes actions (generating responses) and receives a reward based on the quality or desired outcome of those actions. These rewards are then used as feedback to train the model.
In clinical practice, the treatment of chronic conditions often takes the form of a recurrent trial-and-error process. For example, to find an adequate therapeutic approach, an initial medical intervention is performed first, and the patient is observed for a specific response. Subsequently, the treatment is adjusted to either improve the response or eliminate potential side effect. Thus, there are many potential applications of RL in nephrology just starting to make inroads into real clinical settings. For example, RL can be used to individualize dialysis adequacy14, management of IDH15, and management of therapies for chronic kidney disease (CKD) and its complications such as anemia16, bone mineral disease17, and in the use of medications such as sodium-glucose cotransporter-2 inhibitors, renin-angiotensin system inhibitors, glucagon-like peptide-1 receptor agonists, non-steroidal mineralocorticoid receptor antagonists, and combination therapies to slow the progression of CKD. In addition, RL can be used to individualize the management of fluid balance, electrolytes, and hemodynamic support. RL can be used to learn the optimal dosing of erythropoietin (EPO)18 and fluids based on each patient's individual characteristics and response to treatment19.
We illustrate how RL can be applied in practice in more detail using the case of a computer-based system for decision support in EPO dosing for anemia management20. The authors formulated the model in terms of a Markov Decision Process and simulated the estimation of EPO dosing strategy using the on-policy RL method, SARSA. The fundamental idea behind this model was to minimize a cost, defined as the sum of the square differences between desired responses and the response predicted by the model over a time period after administering a dose. The dose that minimized the cost function was administered to the patient, and this process was repeated at the next dosing interval. This model's most useful feature is its ability to handle non-linear control problems with time delays. The authors demonstrated that the proposed algorithm performed as well as the clinical protocol for anemia management in terms of mean hemoglobin levels. In addition, the algorithm improved the protocol by reducing hemoglobin variability.
A PERSONALIZED PATIENT-COMPUTER INTERFACE TO ENHANCE PATIENT ENGAGEMENT AND SELF-CARE
Since patients and dialysis machines work together, there is an unavoidable connection between them. In addition, data from wearables technology (including variables such as heart rate, sleep, physical activity, electrocardiography, oxygen saturation, and glucose monitoring) and smartphone-enabled self-reported questionnaires, in the context of user-centered design technologies and the development of the internet of things, have provided insights into the usefulness, usability, and fit of technology into daily human life and can also play a significant role in personalized medicine21. Thus, the development of patient-computer interface (PPCI) in dialysis would aim to integrate computer technology and patient-specific data in the management of dialysis treatments to enhance patients' experience by providing them with a personalized and interactive interface. The interface would allow patients to have a more active role in their dialysis treatment since updated information about their health status, symptoms, and preferences can be used to tailor the dialysis treatment to their specific needs. A personalized approach can help optimize treatment outcomes and improve patient satisfaction. Moreover, PPCI can provide educational materials, and interactive tools to monitor their treatment progress and receive real-time feedback on their health to enhance patient engagement and self-care. The most common human-computer interaction technologies currently applied are those under the umbrella concept of mHealth. The potential of data from wearable sensors to predict laboratory results22 and in remote patient monitoring21 has been highlighted. For example, the combination of ambient sensors (such as depth cameras and microphones) with wearables data has the potential to improve the reliability of fall detection systems, while keeping a low false alarm rate23. Consequently, the number of studies using digital products is growing rapidly24.
In this situation, the integration of a conversational AI LLMs such ChatGPT with PPCI should help to further enhance the patient experience by providing a natural language interaction, since patients could ask questions about dialysis, receive personalized recommendations, and get support for any concerns about treatments; all of it without requiring any specific technical skills. However, virtual health assistants (digital AI-enabled coaches that could advise people on their health needs, have not been developed widely to date21. However, considering the rapid advances in conversational AI and the concurrent development of increasingly sophisticated multimodal learning approaches, we anticipate that future digital health applications will fully embrace the potential of AI to deliver precise and personalize medicine.
PERSONALIZED DIALYSIS AND DIGITAL TWINS
Another emerging technology further enabling the concept of personalized medicine is that known as digital twins (DT). A DT is a virtual model of a physical entity, with dynamic, bidirectional links between the physical entity and its corresponding twin in the digital domain25. This virtual model dynamically pairs the physical and digital world and leverages innovative technologies in smart sensors, data analytics, and AI to detect and prevent system failures, improve system performance, and explore innovative opportunities. Conceptual models involve three components, namely, (i) the physical (source) product in the physical space, (ii) the digital representation in the virtual environment, and (iii) connections between the two: data and information flowing between the physical and digital products. In dialysis, the goal is to iteratively model, test, and optimize a dialysis treatment in the virtual space until that model meets the expected performance. In this context, LLMs such as ChatGPT could enhance data analysis and interactive monitoring from DT, providing real-time updates, interpreting complex data, and presenting data in a graphical and understandable format, thus fulfilling the promise of making complex ML models interpretable and, to some extent, self-explaining26. Thus, nephrologists can obtain insights, decision-supported recommendations, and predictions based on the information extracted from the DT and ask questions about the status, performance, or behavior of the simulation. In addition, the nephrologist could explore alternatives through simulation of what-if scenarios and obtain predictions on the future behavior of the dialysis.
GENERATIVE AI AND MHEALTH
The integration of smartphones into the current clinical practice has increased the networking possibilities between patients and clinicians. Over recent years, efforts have been made to develop this technology to improve the effectiveness and efficiency of healthcare through innovative approaches and strengthen the opportunities for self-care, self-management, and patient participation27. Mobile apps are being increasingly used in nephrology, most of those currently available provide information on the complex treatment of kidney diseases28, drug or food interactions, adverse reactions, and patients' education29. To provide a more detailed illustration of mHealth in hemodialysis, we show a case of a customized app named Di Care for improving dietary and fluid adherence in dialysis patients30. This app offers educational material, self-tests to assess levels of adherence to recommendations, and the ability to record medications for reminders. In this study, it was compared to a face-to-face training. The authors observed significant improvements with Di Care app, including a decrease in interdialytic weight gain, and in the levels of potassium, phosphorus, cholesterol, triglycerides, and ferritin. However, the study also identified several technical limitations and barriers. For example, some features of the app did not work properly due to compatibility issues with the Android operating system. In addition, problems with the app's connection were detected, which impacted its overall usability. Furthermore, some participants faced challenges due to the lack of digital abilities and others declined to participate in the study. Nevertheless, recent clinical trials using mobile electronic devices have been proven successful in real-world and real-time monitoring and have been proposed to improve treatment adherence31. This technology has predominately targeted younger patients, but there is also a need to develop mHealth for older dialysis patients and their care partners32. The intersection of generative AI and mHealth has several potential applications that include providing personalized health recommendations, answering patients' queries, assisting in medication management, and offering emotional support. In addition, they can enhance patient engagement, improve access to healthcare information, and provide real-time assistance.
CHALLENGES AND LIMITATIONS
Generative AI models require continuous and accurate updates of data, and expert supervision and must address potential biases and limitations. Recent evidence stresses that the importance of the validation of their results in real clinical contexts suggests that it is paramount to test newly developed algorithms before trying to deploy them33. Despite the potential benefits and promising results, clinical translation is not always guaranteed and presents several issues, namely, fairness, model, and results interpretability34 and the lack of validated models. As an example, when studying the feasibility of providing an automated electronic alarm for AKI in different clinical settings, substantial heterogeneity in the findings among hospitals was described, with the worrying results of a significant increased risk of death for some hospitals35. Thus, there is a concern about result interpretability for models that could have a significant impact on patients' health that reflects the inability to explain which aspects of the dataset used in the training phase led to a predicted result36. It has been suggested that AI models should be reported using best practice reporting guidelines such as the Transparent Reporting of a Multivariate Prediction Model for Individual Prognosis or Diagnosis (TRIPOD)37 or Minimum Information for Medical AI Reporting (MINIMAR)38. Continuously updating LLMs with new data by means of a dynamic model training process is necessary for a clinical decision support system model to remain up-to-date and relevant.
ETHICAL AND LEGAL CONSIDERATIONS
The new wave of generative AI LLMs has generated a perhaps unprecedented wave of attention from mass media and its impact has reverberated throughout society. In doing so, it has become yet another element of contention about the societal impact of AI-based technologies as harbingers of data-centered sciences. Unsurprisingly, health and medicine are domains in which societal impact is undeniable and nephrology is nothing but part of it39. The medical domain has a well-honed systematic management of ethical issues, but data-centric medical science and point-of-care decision-making are still in their infancy in terms of implementation, and Hospital Ethical Committees are not yet prepared to handle issues stemming from the use of AI-assisted, data-based decision support systems. As very expressively stated in40, "in the long term, we will need transdisciplinary training programs that teach computer science alongside health science […] These degrees should also require coursework in medical ethics."
Generative AI and LLMs only exacerbate ethical risks due to their combination of seamless linguistic capabilities and lack of inherent trustworthiness since we do not have a complete understanding of their inner workings and limitations, with concerns to be broadened to include privacy issues and potential and varied biases. Ethics though are only the tip of the iceberg of a less subjective concern, which is the potential collision between LLMs and law and regulations, both in general and indeed in the particular case of clinical medicine and nephrology as part of it. This problem has been discussed in detail in26, at two levels: general laws and domain-specific regulations. A general law such as GDPR, for instance, mandates a "right to explanation" of decisions made on citizens (and in the case of health systems, patients) based on "automated or artificially intelligent algorithmic systems." This has a straightforward impact on any dialysis decision support system based on AI and ML. The forthcoming European AI Act (AIA) makes it even more explicit, as it veers from general algorithmic models to obligations ranked by the potential risks of particular application areas. At the time of writing, on June 14, 2023, the AIA just moved from draft June 14, 2023, to a "parliament negotiating position on the AI Act," which should lead to EU countries talks in the Council with a target of finalizing the text within 2023. Concerning Generative AI, its use would have to comply with transparency requirements: including disclosing that the content was generated by AI; designing the model to prevent it from generating illegal content; and publishing summaries of copyrighted data used for training. The amendments from the original draft include, for instance, that, in recital 27, "In order to ensure alignment with sectoral legislation and avoid duplications, requirements for high-risk AI systems should take into account sectoral legislation laying down requirements for high-risk AI systems included in the scope of this Regulation, such as Regulation (EU) 2017/745 on Medical Devices and Regulation (EU) 2017/746 on in vitro Diagnostic Devices […] AI systems identified as high-risk should be limited to those that have a significant harmful impact on the health […] of persons in the Union." Note also that Amendment 724 of Annex III (referring to high-risk AI applications) now includes amongst them "emergency healthcare patient triage systems."
Overall, these ethical and regulatory considerations aim to raise awareness about the fact that technologies such as the new Generative AI-based methods are less of a technical problem than a societal one, about which medical practitioners should be made aware due to its impact in the foreseeable future.
CONCLUSIONS
Generative AI has the potential to contribute to personalized dialysis treatment and increase the quality of life of patients. The combination of technology, patient-specific data, and AI will contribute to creating a more personalized and interactive dialysis process, improving patients' quality of life. Nephrologists' collaboration with AI academia and companies to develop algorithms and models that are more transparent, understandable, and trustworthy will be crucial for the next generation of dialysis patients.











 text new page (beta)
text new page (beta)


