INTRODUCTION
Magnesium is an essential element for metabolic reactions in the human body and is known for its good biocompatibility, good mechanical properties (very close to human bone) and is considered a biodegradable material with potential in biomedical applications in the case of osteosynthesis, which is a process of intervention of fractures or fissures [1][2]. Its high electrochemical activity makes surface modification necessary in order to reduce corrosion rates. Thereby prolong its necessary mechanical properties throughout the recovery time of the bone tissue. Current investigations have focused on addressing this problem by implementing different strategies so that the rate of degradation of magnesium can be controlled within the physiological environment. Some of these are the use of magnesium alloys or protective techniques such as superficial modification treatments and protective recoveries [3]. However, in the surface modification of Mg, the required time for degradation depends on the targeted application, for instance in the design of coatings for tailor degradation is the unknown between the number of degradation test in the lab with the behavior of the coating in test in-vivo [4].
The main reactions reported for the Magnesium corrosion process are[5][6];
Chitosan membranes (CH) have excellent properties, such as antimicrobial activity, biocompatibility, good bio adhesiveness, it is non-toxic, it optimizes wound healing, bone formation, as well as a vehicle and/or drug releaser, and they are very efficient in guided bone regeneration [7][8]. Biodegradability is a property sought in these materials, however, pure chitosan has low mechanical properties due to its brittleness, it is soluble only in acidic media, and it loses its antibacterial activity at pH > 6.5.
To overcome these drawbacks, the most efficient method is to mix it with other polymers to provide it with fluidity such as gelatin which, in addition to fulfilling its function as an additive, enhances the antimicrobial property of chitosan in the form of a flexible film [9][10][11][12][13].
There are many ways to produce these membranes; one example is to produce them using the electrospinning technique as electrospray, which generates micro-thin films with properties such as microporosity, large surface area, and biodegradable. Also, can be obtained by this technique as nanoparticles from a polymer in solution with a conductive solvent [14][15] [16].
The first appearance of this technique was reported by Nollet from the 17th century, when he observed that “a person, electrified by connection to a high-voltage generator, would not bleed normally if cut; the blood would come out of the wound in the form of drops”. After that, Rayleigh defined a limit on the charge carried by a drop in 1882, Zeleny reported various modes of operation of electrospray during 1914, and Taylor determined the shape of the cone formed by the fluid at the capillary tip in an electric field in 1964. Widespread use of electrospray began after Dole introduced it as a method of generating gas-phase ions for mass spectrometry analysis in 1968 [17].
The present work focuses on evaluating the corrosion rate of structural Mg with a coating made of an electrosprayed chitosan membrane (CH) modified with gelatin (GE) and/or glutaraldehyde (GL). The electrochemical evaluation was carried out using the techniques of electrochemical impedance spectroscopy (EIS) and linear polarization resistance (LPR), applied by means of a potentiostat to the Mg samples bare and coated with the membranes, to evaluate the process of degradation of the membrane and the anticorrosive protection to the Mg. Also, by means of surface modification treatment and evaluating in a Kokubo solution (BFK) at 37 °C body temperature. In addition to the challenges in this job, still hard research is being carried out for the control of biodegradation of Mg alloys, and for developing new type of surface modification approaches [4].
MATERIALS AND METHODS
Mg discs of 1 cm in diameter and 3 mm thick were used. They were roughened up to 600 grit sandpaper and ultrasonic washed together with chemical roughening with ethanol and acetic acid, as shown in Figure 1.
Kokubo Saline Solution (BFK)
All the used reactives were grade ACS and prepared for one L of solution following the methodology of Tadashi Kokubo [7] [18] [19] as shown in Table 1.
Table 1 Chemical composition of the Kokubo solution (BFK) and the sequence order for the preparation.
| Order | Reactive | Quantity, g | Conc., mM |
|---|---|---|---|
| #1 | NaCl | 7.996 | 136.7 |
| #2 | NaHCO3 | 0.350 | 4.16 |
| #3 | KCl | 0.224 | 3 |
| #4 | K2HPO4·3H2O | 0.1764 | 0.7737 |
| #5 | MgCl2·6H2O | 0.305 | 1.5 |
| #6 | 1 kmol/m3 HCl | 40 cm3 | 40 |
| #7 | CaCl2 | 0.278 | 2.5 |
| #8 | Na2SO4 | 0.071 | 0.5 |
| #9 | (CH2OH)3CNH2 | 6.057 | 48.46 |
| #10 | 1 kmol/m3 HCl | pH Adjusting |
For the formation of the membrane, chitosan solutions were prepared at 1.5 % diluted concentration of a mixture of acetic acid and distilled water in a ratio of 9:1, mixing everything at a temperature of 60 ± 5 °C until dissolved. To compare with chitosan modified with gelatin and/or glutaraldehyde, it was carried out by adding 2 % gelatin in the solution together with chitosan, the modification with glutaraldehyde is done after the formation of the membrane, placing a drop of it on the coating, letting it absorb and evaporate.
The membranes were made with the Fluidnatek LE-100 used as electro spraying equipment, placing 3 ml of the chitosan solution in a syringe, as shown in Figure 2. The images show the membranes a) Chitosan, b) Chitosan modified with gelatin, c) Chitosan modified with glutaraldehyde and d) Chitosan modified with gelatin + glutaraldehyde. The Figure 3 show an image SEM with the dispersion of the microparticles contained in drops of chitosan at the contact with the Mg substrate. The processing parameters were as follows: applied voltage, −30 kV; feed solution flow rate, 0.3 ml/hr; distance between the nozzle and the substrate, 10 cm; deposit time, 30 min.
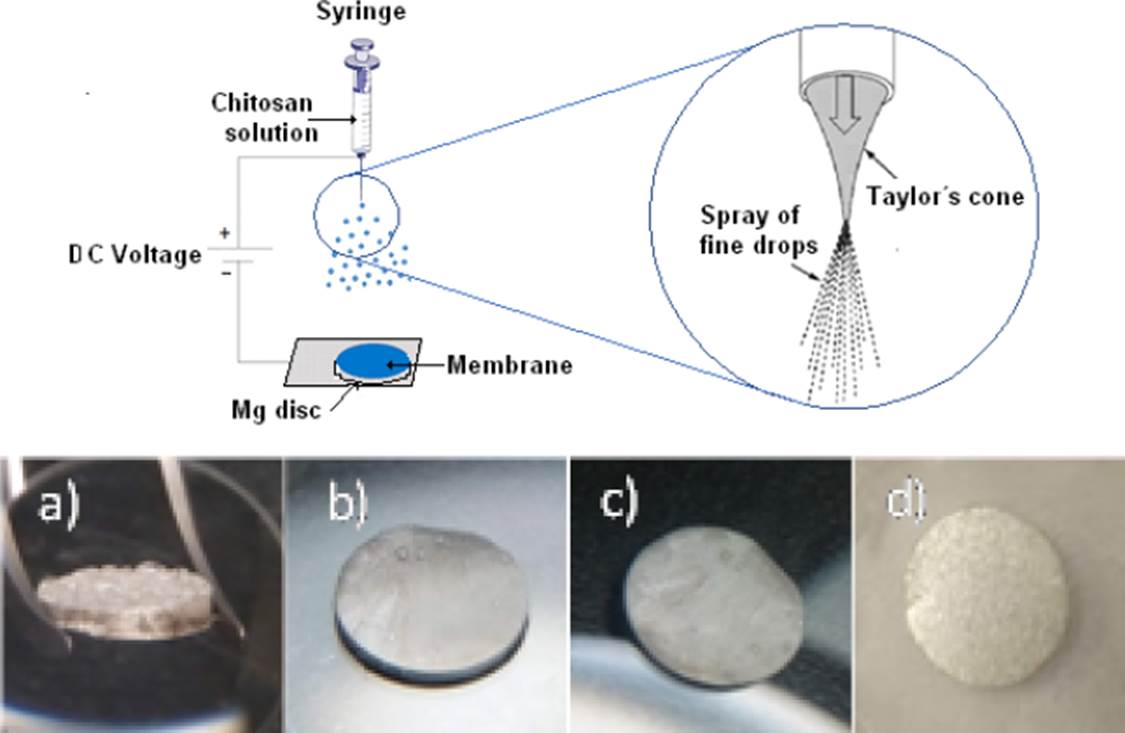
Figure 2 Scheme of the spray process and samples with the sprayed membranes a) MgCH, b) MgCH+GE, c) MgCH+GL and d) MgCH+GE+GL over discs of Mg.
Once the samples with the membranes were ready, an electrochemical cell was prepared in a simulated Kokubo physiological body solution (BFK) and using a temperature of 37 °C as shown in the cell arrangement in Figure 4. The impedance spectroscopy technique (EIS) was carried out, using a frequency sweep from 10kHz to 0.01 Hz, with an amplitude of ± 10 mV peak-to-peak and 7 points per decade of frequency and the linear polarization resistance (LPR) where a bias of ± 20 mV/Ecorr was used. The corrosion potential (Ecorr), was monitored with respect to time. The electrochemical tests were carried out with a BioLogic brand potentiostat model SP-150, where the electrochemical monitoring was carried out from the first hour of immersion and subsequently measured every 24 hours for a period of 30 days.
Some of the main chloride reactions expected in the Mg -BFK solution are described below;
For the analysis of the surface morphology of the samples before and after exposure, a high-low vacuum scanning electron microscope, model SEM 6000 NEOSCOPE with a voltage of 150 keV, was used to perform the elemental chemical analysis of the membranes, the X-ray scattering technique known as EDS was used.
RESULTS AND DISCUSSION
The corrosion potential (Ecorr) and corrosion rates (CR) results are shown in Figure 5 and Figure 6 respectively, where we can see the same trend for bare Mg, Mg with chitosan membrane (Mg CH) and Mg with gelatin-modified chitosan (Mg CH+GE) and glutaraldehyde (Mg CH+GL), however, for Mg with the membrane modified with gelatin and glutaraldehyde (Mg CH+GE+GL) was observed that the Ecorr tends to rise more positive values, which implies an energy gain. This translates into thermodynamic stability for the reactivity of the species involved in the interface of the evaluated systems and the tendency of CR to decrease, implying that the metal-coating systems tend to improve the resistance against the corrosiveness of the environment.
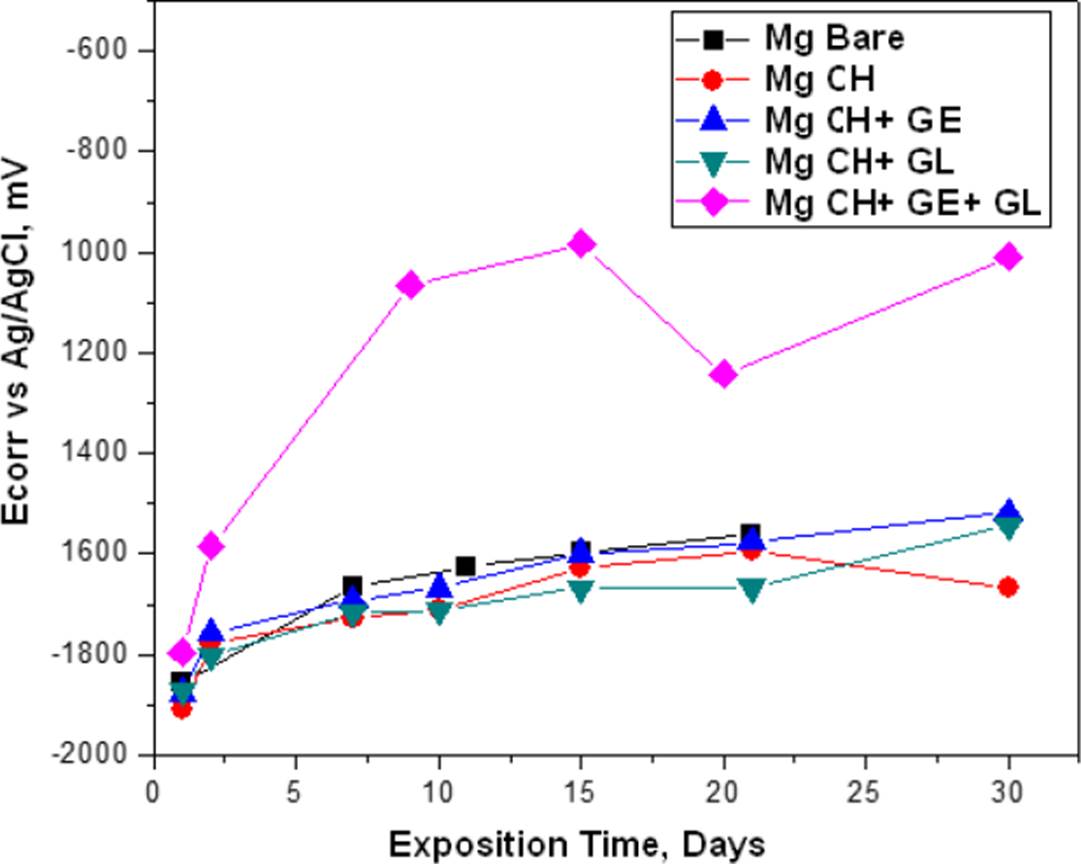
Figure 5 Ecorr of bare and coated Mg at the immersion time measurements in the BFK solution at 37 ºC.
Corrosion mechanism of Mg alloys in simulated body fluid always has been complicated because of many factors like the complex chemical composition influence. However, the obtained CR values between 0.1 to 2 mm/y are in coincidence for in vitro conditions reported values [4].
The Figure 7 is the Nyquist diagram of bare Mg and clearly shows the corrosion process suffered by magnesium with resistance up to 1.4 kΩ-cm2 before the presence of the coating. In the case of Mg coated with chitosan, we can see an increase in the corrosion resistance except for day 30, where it decreased due to the total degradation of the membrane and that exposed to the metal. For the chitosan membrane shown in the Nyquist of Figure 8, the resistance was increased by twice and no degradation is observed in the membrane.
For the gelatin-modified chitosan membrane shown in the Nyquist of Figure 9, the corrosion resistance was 3 times higher, and less degradation after 30 days as observed in the membrane. The Mg with chitosan modified with glutaraldehyde which is shown in the Nyquist of Figure 10 shows that the corrosion resistance is similar to the bare Mg and if a degradation of the membrane is observed on day 30.

Figure 9 Nyquist diagrams of the Mg coated with chitosan and modified with gelatin and immersed in BFK solution.
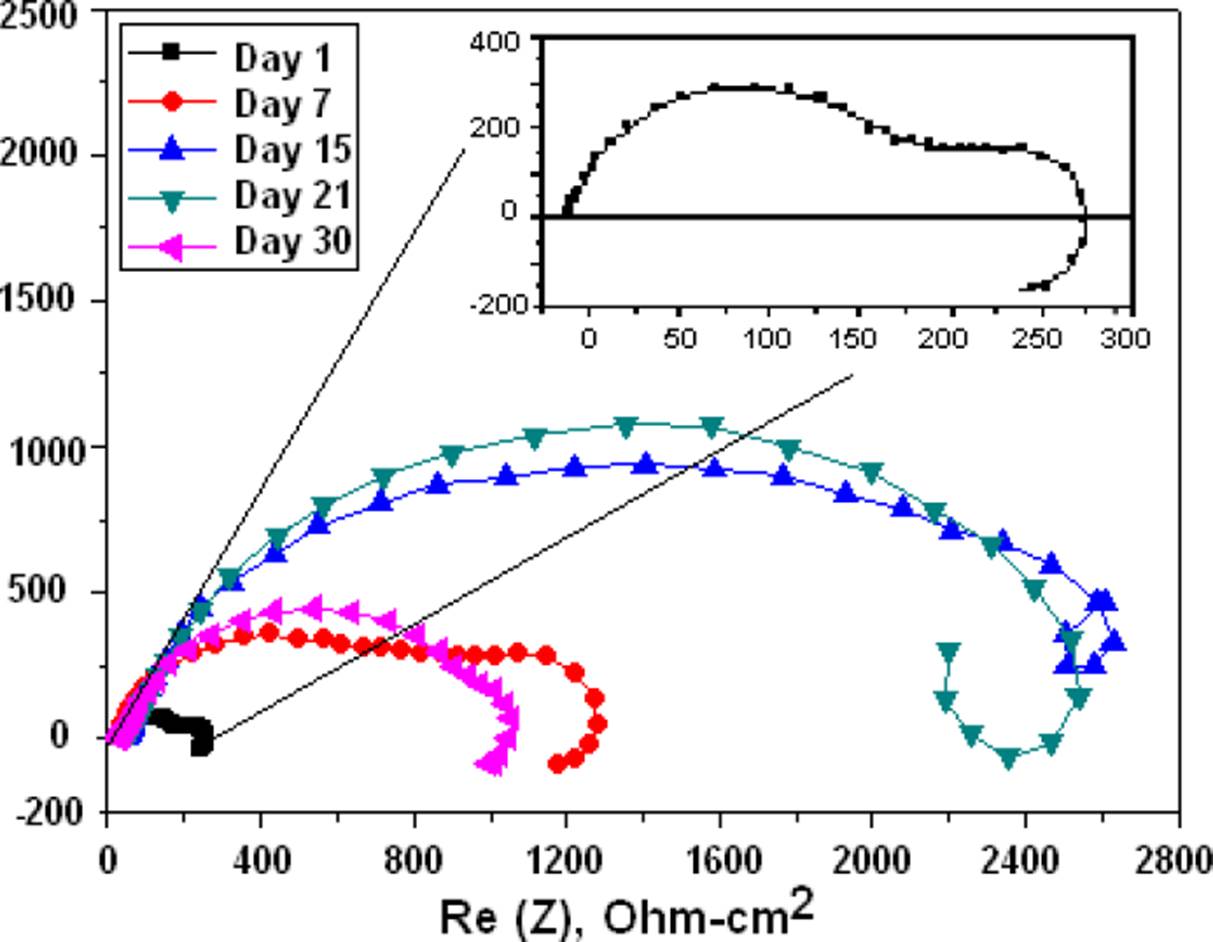
Figure 10 Nyquist diagrams of the Mg coated with chitosan and modified with glutaraldehyde and immersed in BFK solution.
The Nyquist diagram for the gelatin and glutaraldehyde modified chitosan coated Mg is shown in the Nyquist of Figure 11, it can be clearly seen that the corrosion resistance is extremely large compared to the other membranes, the corrosion process becomes slower and the membrane makes it difficult for chloride ions to reach Mg. In the Figure 12 the spectra obtained from Raman Spectroscopy of bare Mg, chitosan, and the different Mg+ membrane systems are shown, and observing the covering capacity of the additives CH, GE and GL over the Mg substrate. The diagram shows the peaks of 1050 cm-1 for Mg and 2885 cm-1 reported in the literature as characteristic of chitosan [20].
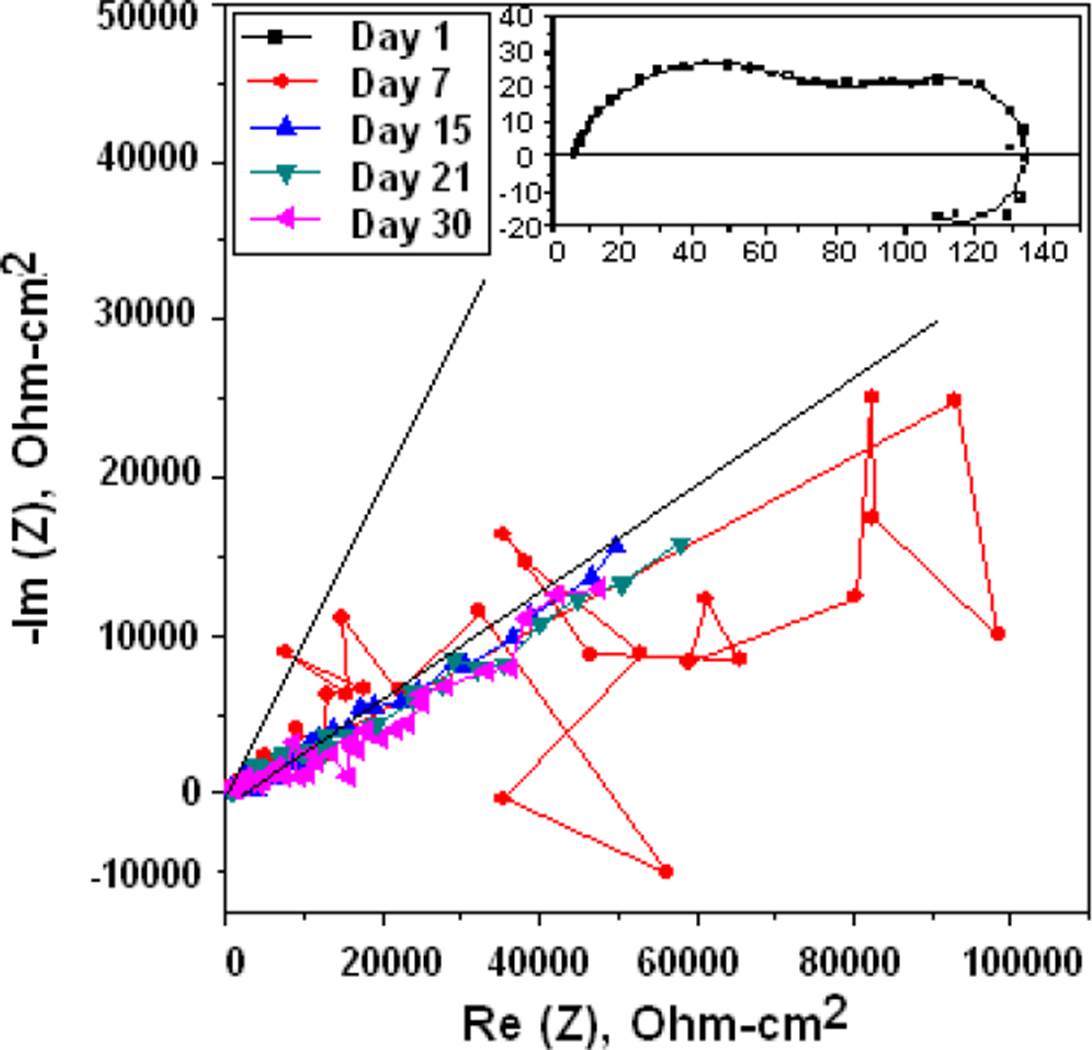
Figure 11 Nyquist diagrams of the Mg coated with chitosan modified with gelatin plus glutaraldehyde and immersed in BFK.
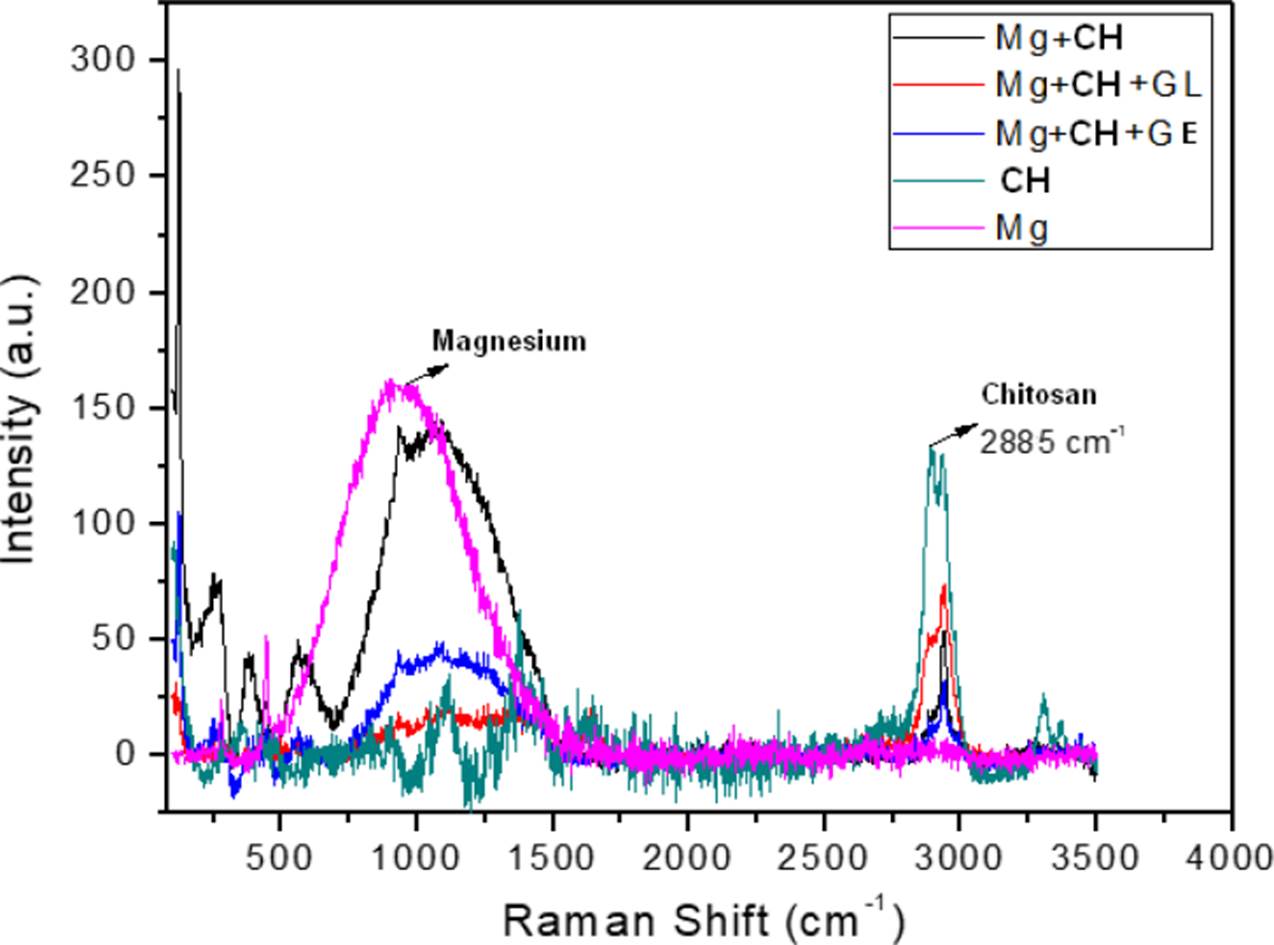
Figure 12 Raman Spectroscopy response obtained from the bare and coated Mg with membranes of chitosan modified with gelatin and glutaraldehyde.
The Figure 13 show the SEM images of (a) corrosion products on the bare Mg, (b) coated Mg with chitosan, showing morphology with less corrosion than the bare Mg, (c) Mg coated with chitosan plus the gelatin, showing the dispersion of microspheres of the membrane, controlling the embrittlement of the coating and reducing the corrosion rate on the substrate and (d), the formed membrane practically intact, well formed and smooth, over the entire surface due to the cross-linking reaction produced by glutaraldehyde with chitosan, in addition to increasing mechanical resistance and flexibility given by the gelatin, showing the best anticorrosive effect over the Mg substrate.

Figure 13 SEM microscopy of samples after exposition to the BFK solution a) Bare Mg, b) Mg with chitosan, c) Mg with chitosan modified with gelatin and d) Mg with chitosan modified with grenetine plus glutaraldehyde.
Table 2 shows the results of the elemental chemical composition for the Mg samples and chitosan membranes, where it is observed that in the exposed Mg samples it registers the main elements (O, P and Ca) of the BFK solution, while the chitosan membranes raise the C, O, P and Ca content, lowering the detection of Mg due to the coating and the gelatin involves the presence of elements such as C, O, Cl, and Ca mainly, and when the coating system includes the glutaraldehyde the Si, Al and Ca increases such a bit, maybe due the trapping effect of such elements in the formed lattice. Then, as was observed in the EIS response, the best results were observed with the membrane made of CH+ GE+ GL.
Table 2 Elemental chemical composition detected in % mass by EDS on the surface of samples of coated Mg before and after the exposition to the BFK solution.
| Ele men t | Mg witho ut attack | Mg attac ked | MgCH | MgCH+ GE | Mg- CH+ GE+ GL |
|---|---|---|---|---|---|
| C | 25.17 | 27.05 | 31.03 | 44.52 | 43.8 |
| O | 1.6 | 36.08 | 42.2 | 30.55 | 32.2 |
| Mg | 73.23 | 32.2 | 12.1 | 4.9 | 5.3 |
| Si | 0.05 | 0.03 | 0.08 | 0.21 | |
| P | 1.08 | 6.2 | 4.15 | 4.5 | |
| S | 0.28 | 0.08 | 0.2 | 0.07 | |
| Cl | 0.06 | 0.05 | 6.85 | 0.03 | |
| K | 0.13 | 0.17 | 0.69 | ||
| Na | 0.28 | 0.87 | 0.26 | 0.97 | |
| Ca | 1.98 | 6.1 | 5.7 | 11.8 | |
| Al | 0.10 | 0.04 | 0.05 | 0.19 | |
| Total | 100% | 100% | 100% | 100% | 100% |
In the literature there are many in-vitro studies that deal with a variety of topics, some evaluate and compare electrolytes, others evaluate the surface morphology of coatings such as chitosan[21]. others report the behavior of Mg alloys with other metals such as Zn, Ca, and others [4]. There are even several review-type papers on the applications of Mg and its alloys as a biomaterial [22], but in no work has the application of chitosan membranes modified with gelatin and glutaraldehyde been studied, and due to the results obtained, it is considered a contribution that deserves further studies with a view to its application at invivo conditions, even the membranes can also be used as a vehicle for the transport of drugs in post-operative treatments [23].
CONCLUSIONS
The membrane that degraded the least and had the best results with the lowest corrosion rates is that of the Mg-CH+GE+GL system and it is observed almost intact in the SEM results. The Raman information of the membranes, helped in the interpreting of the covering properties of the additives.
The corrosion protection also can be attributed to the synergistic effect of such additives; the gelatin serves as a means for the chitosan to have greater resistance since it provides viscoelasticity, flexibility and mobility properties. Glutaraldehyde serves as a cross-linking agent between the chitosan polymer chains, creating greater cohesion in the membrane, improving its mechanical properties and its resistance to protect the Mg substrate against the corrosiveness of the medium.
With the results of the electrochemical evaluation, it was verified that the chitosan with the gelatin and glutaraldehyde acting independently, do not reach the anticorrosive protection properties and only by acting the whole system together is a synergistic effect achieved. The challenge in both strategies is to combine control of the corrosion behavior with the membrane biodegradation and desired biological performance for the in-vivo applications.
The electrospray process of the polymeric substances is another important factor due to the distribution and better coverage area provided by the microparticles that form the membrane. Also is recommended the chemical characterization of the membranes in the near future.











 text new page (beta)
text new page (beta)









