Introduction
Community structure and species distribution are influenced by intrinsic factors including ontogenetic development of individuals, and extrinsic factors such as environmental traits and resource availability (Bell & Galzin, 1984; Wiens, 1989). These factors are highly variable and may work independently or synergistically to determine habitat quality and establish unique arrangements in biological communities (Ferrari et al., 2018; Smallhorn-West et al., 2017). In intertidal habitats, abrupt changes in environmental features strongly influence the dynamics of the sessile organisms that remain exposed at low tide (Huggett & Griffiths, 1986). Intertidal rock pools do not experience the extreme change of intermittent exposure that occurs for adjacent substrata. Nonetheless, the fluctuations in environmental conditions are more drastic than the ones experienced in the subtidal zone (Metaxas & Scheibling, 1993). The extent of physicochemical oscillation in rock pools is mainly associated with volume and intertidal height (Daniel & Boyden, 1975; Griffiths et al., 2003; Huggett & Griffiths, 1986; Legrand et al., 2018; Metaxas & Scheibling, 1993; Morris & Taylor, 1983). Rock pools with larger volumes in the lower intertidal are more resilient to perturbations and tend to maintain a more stable physico-chemical environment than small rock pools in the high intertidal zone (Cunha et al., 2007; Metaxas & Scheibling, 1993). These differences generate strong zonation patterns for benthic rock pool dwellers that display scattered distributions, rather than a random assortment, and contribute to the complexity of the substrata in the intertidal zone (Huggett & Griffiths, 1986).
Within rock pools, the complexity of the substrata changes largely due to irregularities, crevices, interstices, and fissures that can be used as a refuge and shelter for mobile and sessile organisms (Metaxas & Scheibling, 1993). Furthermore, abiotic and biotic elements such as sand, large rocks, pebbles, shells, algae, corals, and encrusting molluscs or crustaceans create a complex mosaic in the benthos of the rock pools that influence not only the topography, but also the attraction and deterrence of other intertidal organisms (Martins et al., 2007). Consequently, rock pools are a conglomerate of habitats with traits that differ along a spatial gradient. During high tide, fish swim across rock pools (Malard et al., 2016; Marsh et al., 1978) exploiting resources of the intertidal area and eventually selecting a particular rock pool when the tide recedes (Castellanos-Galindo et al., 2010; Faria & Almada, 2006; Griffiths, 2003; White & Brown, 2013). Many fish species are opportunists in juvenile stages (González-Murcia et al., 2012, 2016) spending short periods of time in intertidal rock pools and returning to subtidal areas when they reach a particular size Gibson & Yoshiyama, 1999; Zander et al., 1999). Resident species, however, inhabit intertidal rock pools their entire life and are well adapted to cope with the environmental variability of the intertidal zone. Opportunist and resident species both present high rock pool fidelity with home ranges usually limited to a few pools (Griffiths et al., 2003; White & Brown, 2013). Therefore, the distribution of species and individuals within rock pools is consistent in space and time (Faria & Almada, 1999).
Changes in rock pool intertidal fish community structure have been associated with rock pool environmental and structural features (Andrades et al., 2018; Castellanos-Galindo et al., 2005; Cunha et al., 2007, 2008; González-Murcia et al., 2016; Mahon & Mahon, 1994; Yoshiyama, 1981). Vertical zonation patterns in intertidal rock pool fish assemblages are typically marked by a decrease in abundance and species richness from low to high tide zones (Gibson, 1972; González-Murcia et al., 2016; Griffiths et al., 2003; Zander et al., 1999). White et al. (2015) considered that fish abundance and species richness increased with rock pool depth, but Mahon and Mahon (1994) suggested that those changes are more related to rock pool volume. Additionally, environmental and biological factors can regulate size-length patterns within species. Hernández et al. (2002), experimentally tested water temperature fluctuations and territoriality in intertidal rock pools to explain height distribution and differences in body size in Graus nigra. Their results indicated that smaller fish are well adapted to cope with wide temperature ranges but aggregate in optimal environments until large territorial conspecifics display agonistic behaviour towards them, displacing them to suboptimal environments. Davis (2000) observed that rock pool roughness positively affects abundance of piscivorous fish, providing shelters to hide and ambush prey, but reduces the abundance of herbivorous fishes that potentially avoid these areas because of higher predation risk. High algal cover in intertidal rock pools has also been associated with high abundance of fish, potentially because it provides food and shelter from predators (Galindo & Giraldo, 2008; Coull & Wells, 1983; Griffiths et al., 2006). Thus, features of rock pool habitat can be suitable for some intertidal fishes in particular ontogenetic stages, while having detrimental impacts on others or determining the occurrence of fish guilds.
Whether environmental or biological factors drive the structure of intertidal fish assemblages is still debated. Based on a deterministic approach, community structure can either be influenced by limiting similarity or environmental filters (Götzenberger et al., 2012; HilleRisLambers et al., 2012). Environmental filters exert strong temporal or spatial effects on ecosystems with severe environmental conditions like estuaries (Miyazono et al., 2010), river floodplains (Ortega et al., 2015), or tropical wetlands (Córdova-Tapia et al., 2018) and could be relevant in rock pool intertidal systems. Intertidal fishes actively reside in a rock pool or a set of rock pools that represent habitat traits that can optimise their development and fitness (Faria, 2001; Hernández et al., 2002). Nonetheless, few rock pool intertidal fish studies have extended to rock pools uninhabited by fishes or reported the number of rock pools unoccupied by them (Malard et al., 2016; White & Brown, 2013). Even fewer have recorded habitat complexity variables and structural traits of those rock pools (Malard et al., 2016; White & Brown, 2013). Characterizing and comparing these rock pools is similar to a comparison of extreme treatments, which is the easiest way to enhance the detection of an effect in an experiment. Thus, exploring the traits of rock pools uninhabited by fishes can provide insights about major drivers in fish intertidal dynamics.
Tropical intertidal rock pools harbour a species-rich ichthyofauna with high levels of endemism (Andrades et al., 2018). However, coastal ecosystems are highly susceptible to anthropogenic activities, including sediment and sewage deposition, nutrient runoff, and destruction or alteration by coastal development. Therefore, intertidal habitats are at risk of rapid and abrupt biodiversity loss. Describing the ecology of intertidal fish and characterizing traits that influence their diversity will improve our understanding of current intertidal fish dynamics and set a baseline for comparison with future research. Herein, we studied habitat complexity and physical attributes of intertidal rock pools in El Zonte to describe qualitatively and quantitatively the assemblage of fishes in the intertidal rock pools and to assess the relationship between intertidal rock pool traits (rock pool volume, height, perimeter, surface area, habitat structure, and complexity) and occurrence, abundance, species richness, and size of intertidal fishes.
Materials and methods
We assessed 45 rock pools in the intertidal fish community at El Zonte, Chiltiupán; La Libertad, El Salvador in March, 2018. El Zonte is located on the coast of rocky cliffs on the western coast of El Salvador. There are many small rocky shores in this area; 3 of them were accessible and separated by sandy beaches and the river mouth of the Río El Zonte, at distances of 0.5 m to 2 km (Fig. 1). Tides ranged from 0 to 170 cm and rock pools were counted during low tide. The vertical distribution of rock pools along the shore, hereafter referred to as rock pool height, was recorded during low tide using a tide calendar to verify the lowest level at the interface between outgoing and incoming tides. Average rock pool height in the intertidal zone was 122.3 cm (± 22.11 SD) ranging from 10-130 cm and average rock pool volume was 1.80 m3 (± 4.03 SD) ranging from 0.012 m3 to 22.03 m3. Sampling was conducted once in each rock pool.
Figure 1 a, Pacific Coast of El Salvador with insets; b, locality El Zonte, Chiltiupán La Libertad, and c, Rocky shore locations where rock pools were sampled.
Three measurements of width (W), length (L), and depth (D) and one of perimeter (P) were recorded in each rock pool before collecting fish. Volume was estimated by the formula V = A*D, where V = rock pool volume, A = surface area of the rock pool (W*L) estimated by the average width (W), length (L) and average rock pool depth (D). The relationship between perimeter (P) and volume (V) was estimated by dividing the perimeter by the volume and the relationship between the surface area (A) and volume by dividing the area by the volume. Herein, these metrics will be referred to as perimeter-volume ratio (P/V) and area-volume ratio (A/V), respectively. Rock pool roughness was calculated by dividing the linear distance (LD) between 2 random points of the rock pool by the distance between the same points considering all the surface irregularities (RD) with the formula 1+ (LD/RD), which gave a value between 0 and 1, expressed as a rock percentage. The algae, pebbles, sand, and rock on the rock pool substrata were measured using quadrats of 40 × 40 cm containing 100 sub squares of 4 × 4 cm. The number of sub squares occupied by each substratum category was counted and expressed as a percentage.
A subset of 15 out of 18 rock pools where fish occurred, were randomly selected and drained using hoses and buckets. Fishes were collected using hand nets, searching in crevices, algae, and under rocks, and then returned to adjacent pools or subtidal areas after total length was measured (mm). Some specimens were collected and preserved in 70% ethanol and donated to the Museo de Historia Natural de El Salvador (MUHNES, Natural History Museum of El Salvador) ichthyology collection under catalogue numbers MUHNES 40-1022 to 40-1024. Species were categorized by rock pool utilization following Griffiths (2003), under the categories: a) permanent residents (R), occurring as larvae, juveniles, and adults possessing morphological, anatomical, or physiological adaptations for this environment; b) opportunists (O), frequently occurring as juveniles with few morphological adaptations for intertidal life, and c) transients (T), occurring frequently as juveniles or adults without adaptations for intertidal environment; these fish may simply be trapped in rock pools when the tide recedes. Behavioural affinities of each species were categorized using the criteria established by Griffiths (2003), based on observations that described the fish as: a) solitary (S), species swimming alone; not forming schools; b) aggregating (A), species swimming in schools; c) cryptic (C), species attached to the substrata with camouflaged coloration or displaying secretive behaviour, hiding in crevices, algae, and under rocks; d) territorial (T), species showing aggressive behaviour toward conspecifics or heterospecifics for a particular resource. Behavioural affinities were not mutually exclusive categories and individuals of some species showed more than 1 behaviour.
Life history stages were determined using the minimum length of post larval fish for each species and records of maximum length, then dividing the length range into 3 equal size classes to represent juvenile, sub adult, and adult. Since there is no evidence of marked differences in the biology or behaviour of sub adult and adult fish of the species studied, fishes in sub adult stages were pooled in the category adult. We acknowledge that this method would not be accurate enough due to intrinsic traits of each species and environmental factors affecting them that could reduce or enlarge slot sizes and aging but provide appropriate information for our analyses. Although reading growth marks on hard structures such as scales, bones, or otoliths and others or direct inspection of gonads are ideal for age and maturity estimation, they are logistically more complicated, expensive, and time consuming (Das, 1994). Additionally, applying these methods requires the collection of a fair number of specimens that we could not achieve due to dispositions presented in the permits for this research.
Fish with sizes lower than the minimum length recorded for juveniles or with anatomical or morphological traits typical of larvae (lack of colour, or pigmentation of larvae, spines or rays typical of fishes in larval stages) were labelled as such. We considered this method an adequate indication of fish life history stages as it was successful in numerous previous studies of rock pool fishes (Ghanbarifardi & Malek, 2009; González-Murcia et al., 2016; Grossman, 1982).
Rock pool structural variables and habitat complexity were used to assess the occurrence, abundance, species richness, and length distribution of rock pool fishes with principal components analysis (PCA) performed in the R package Vegan (Oksanen et al., 2016). Rock pool width, depth, length, perimeter, volume, height, roughness, relationship of the surface area-volume ratio and perimeter-volume ratio, and habitat structure (rocks, sand, pebbles, and algae) were included in the PCA. All environmental variables were tested for normality, and transformations (log, log10, square root, 1/x, arcsine) were applied to fulfil normality assumptions where necessary. Then, the variables were standardized (z-scores) (Borcard et al., 2011). To select the number of interpretable axes in the PCA the Broken Stick Model was computed, which randomly divides a stick of one-unit length into the same number of components as there are PCA axes. The components are arranged in decreasing order and compared to the eigenvalues of each component of the PCA analysis. The PCA components whose eigenvalues were larger than the length of the corresponding piece of the Broken Stick Model were interpreted. A step-wise process was conducted to aid in the exclusion of highly collinear explanatory variables, using Cook’s distance and Variance Inflation Factor (VIF), (Oksanen et al., 2016).
Volume, roughness, height, rock, pebbles, and A/V had VIF values below 3.5 and were used as explanatory variables in generalized linear models (GLMs). Presence or absence of fish was set as the response variable under a binomial distribution (Table 1). In this model rock pool volume and roughness were highly correlated, but with no evidence that changes in roughness are determined by changes in volume, we constructed individual models for each variable (Table 1). Either fish abundance or species richness were set as response variables and tested using values from 42 rock pools. These explanatory variables were standardized and scaled (mean and SD) to aid in comparisons and model contributions. For both response variables a Poisson Negative Binomial distribution was used to account for the high frequency of absences (zeros) in the rock pools. Fish total length, recorded for all species in 15 rock pools, was used as the response variable in a linear model with volume, roughness, height, rock, pebbles, and A/V as explanatory variables and a Gaussian distribution. The same process was applied independently for each of the 2 most abundant species (Table 1). Model selection was based on the Akaike information criteria (AIC) applying the dredge function of the MuMIn package in R (Oksanen et al., 2016), and models were validated with graphical tools, goodness of fit, and over dispersion tests in R (Oksanen et al., 2016; Zuur et al., 2009). Final models for each dependent variable had a maximum of 2 explanatory variables and their interactions (Table 1).
Table 1 Final models to test for presence and absences of fish, fish abundance, fish richness, total length for all fish, and the 2 most abundant species.
| Model name | Distribution | Response variable | Explanatory variables |
| Fish occurrence | Binomial | Presence/absence | (1) volume; (2) roughness |
| Fish abundance | Poisson negative binomial | Fish abundance | Volume + roughness + volume*roughness |
| Species richness | Poisson negative binomial | Fish species richness | Volume + roughness + volume*roughness |
| Total length all species | Gaussian | Total length all species | Volume |
| Total length B. ramosus | Gaussian | Total length B. ramosus | Volume |
| Total length A. concolor | Gaussian | Total length A. concolor | Volume |
Results
The variables of the rock pools presented wide variation in their physical attributes, structural traits, and habitat complexity (Table 2). For instance, 23 (50%) of the rock pools had depth, width, and length values lower than 13.3, 36.7, and 56.7 cm, respectively. Therefore, the majority of the rock pools had volumes lower than 0.3 m3. The surface area and perimeters of the rock pools and the values of the A/V and P/V showed that most of the rock pools (60%) presented a small surface area and perimeter in relation to their volume. A total of 38 (84%) rock pools were located at intertidal heights higher than 100 cm and had low values of surface roughness, implying limited refuge for fishes. The substratum was mainly rock (≥ 40%; 24 rock pools, 53%) or sand (≥ 40%; 20 rock pools, 44%), with mostly few pebbles (≤ 20%; 37 rock pools, 82%) and little algal cover (≤ 10%; 41 rock pools, 91%).
Table 2 Physical attributes, structural traits, and habitat complexity of 45 rock pools in El Zonte, Chiltiupán, La Libertad, 2018.
| Variable | Average ± SD | Minimum | Median | Maximum |
| Depth (cm) | 18.7 ± 16.4 | 2.0 | 13.3 | 71.0 |
| Width (cm) | 55.8 ± 44.1 | 15.6 | 36.7 | 221.0 |
| Length (cm) | 82.46 ± 69.0 | 15.0 | 56.7 | 293.3 |
| Volume (m3) | 1.80 ± 4.03 | 0.1 | 0.3 | 22.9 |
| Perimeter (cm) | 348.9 ± 324.4 | 65.0 | 260.0 | 1630.0 |
| A/V | 1.2 ± 1.3 | 0.1 | 0.8 | 5.0 |
| P/V | 14.6 ± 17.3 | 0.7 | 7.3 | 77.3 |
| Height (cm) | 122.31 ± 22.11 | 30.0 | 131.0 | 145.0 |
| Roughness (%) | 9.21 ± 6.77 | 0.0 | 9.091 | 24.81 |
| Sand (%) | 39.8 ± 29.7 | 0.0 | 35.0 | 95.0 |
| Rock (%) | 47.0 ± 27.8 | 5.0 | 40.0 | 100.0 |
| Pebbles (%) | 9.3 ± 13.3 | 0.0 | 5.0 | 50.0 |
| Algae (%) | 3.8 ± 7.4 | 0.0 | 0.0 | 40.0 |
A total of 309 fish representing 9 species, 8 genera, and 8 families were observed (Table 3). Pomacentridae had the highest species richness (2 species) and Gobiidae the highest abundance (160 fish, 52%). The most abundant fish were the Panamic frillfin B. ramosus (Ginsburg 1947) (160 individuals, 52%) and the Petaca A. concolor (Gill 1862) (88 individuals, 28%), comprising 80% of the total abundance. Resident fish were more abundant (193 individuals, 63%) than opportunists (116 individuals, 37%) and no transient fish were recorded. Juvenile fish were more abundant (223 individuals, 72%) than adults (86 individuals, 28%) and larvae were not recorded (Table 3).
Table 3 Abundance, ontogenetic stage, behavioural affinity, residence status, length range, total length mean and standard deviation (SD), and percentage of occurrence of the intertidal fishes of El Zonte, Chiltiupán, La Libertad, 2018. na = Abundance; ontogenetic stageb: A = adult, J = juvenile, L = larvae; behavioural affinityc: S = solitary, C = cryptic, A = aggregating, T = territorial; residential statusd: R = residents, O = opportunists, T = transients; SDe = standard deviation.
| Family | Species | na | Ontogenetic stageb |
Behavioural affinityc |
Residence statusd | Length range (mm) |
Mean ± SDe | Occurrence (%) |
| Blennidae | Ophioblennius steindachneri | 2 | A | S-C | O | 60-65 | 62.50 ± 03.54 | 13 |
| Gobiesocidae | Tomicodon zebra | 16 | J-A | S-C | R | 9-28 | 21.81 ± 05.32 | 13 |
| Gobiidae | Bathygobius ramosus | 160 | J-A | S-C-T | R | 10-102 | 43.56 ± 23.46 | 87 |
| Labrisomidae | Malacoctenus sudensis | 14 | A | S-C | R | 28-75 | 57.71 ± 16.58 | 33 |
| Mugilidae | Mugil curema | 17 | J | S-G | O | 34-75 | 52.65 ± 11.34 | 33 |
| Pomacentridae | Abudefduf concolor | 88 | J | S-A | O | 14-62 | 35.53 ± 12.09 | 87 |
| Pomacentridae | Abudefduf troschelii | 6 | J | S-A | O | 35-42 | 39.00 ± 02.97 | 7 |
| Serranidae | Epinephelus labriformis | 5 | J | S | O | 50-90 | 68.60 ± 14.38 | 20 |
| Holocentridae | Neoniphon suborbitalis | 1 | J | S | O | 60 | 60.00 ± ---- | 7 |
Fish occurred in only 18 (40%) of the 45 sampled rock pools. The first 2 components of the PCA explained 62.6% of the variation and rock pools tended to aggregate in 3 groups (Fig. 2). The first group included rock pools with volumes below 0.56 m3, surface roughness ranging from 0 to 9%, and a wide range of A/V ranging from 0.38 to 5 (Fig. 2, top left panel). These rock pools were located over 1 m height in the intertidal zone and the substrata had low rock cover (< 40%) and pebbles (< 35) but had high cover of sand. Rock pools in the second group had similar characteristics to the first group with volumes below 0.33 m3, surface roughness ranging from 0 to 11%, and A/V values ranging from 0.38 to 5 (Fig. 2, bottom left panel). These rock pools were distributed from 0.9-1.35 m height in the intertidal zone and mainly differed from those in the first group because of the high values of rock cover (> 55%) and low values of pebbles (< 5%). The third group was characterized by rock pools with larger dimensions (width, length, surface area, perimeter, and mainly depth) and consequently, volume that ranged from 0.32 m3 to 22.09 m3, surface roughness ranging from 4-24%, and A/V ranging from 0.14 to 2.14 (Fig. 2, top and bottom right panels). These rock pools were located in a wide range of intertidal heights from 0.3 to 1.42 m height, cover of rock varied from 25-75% and pebbles constituted between 0-50% of the substratum. Fish did not occur in the first and second group but were present in the third group of rock pools.
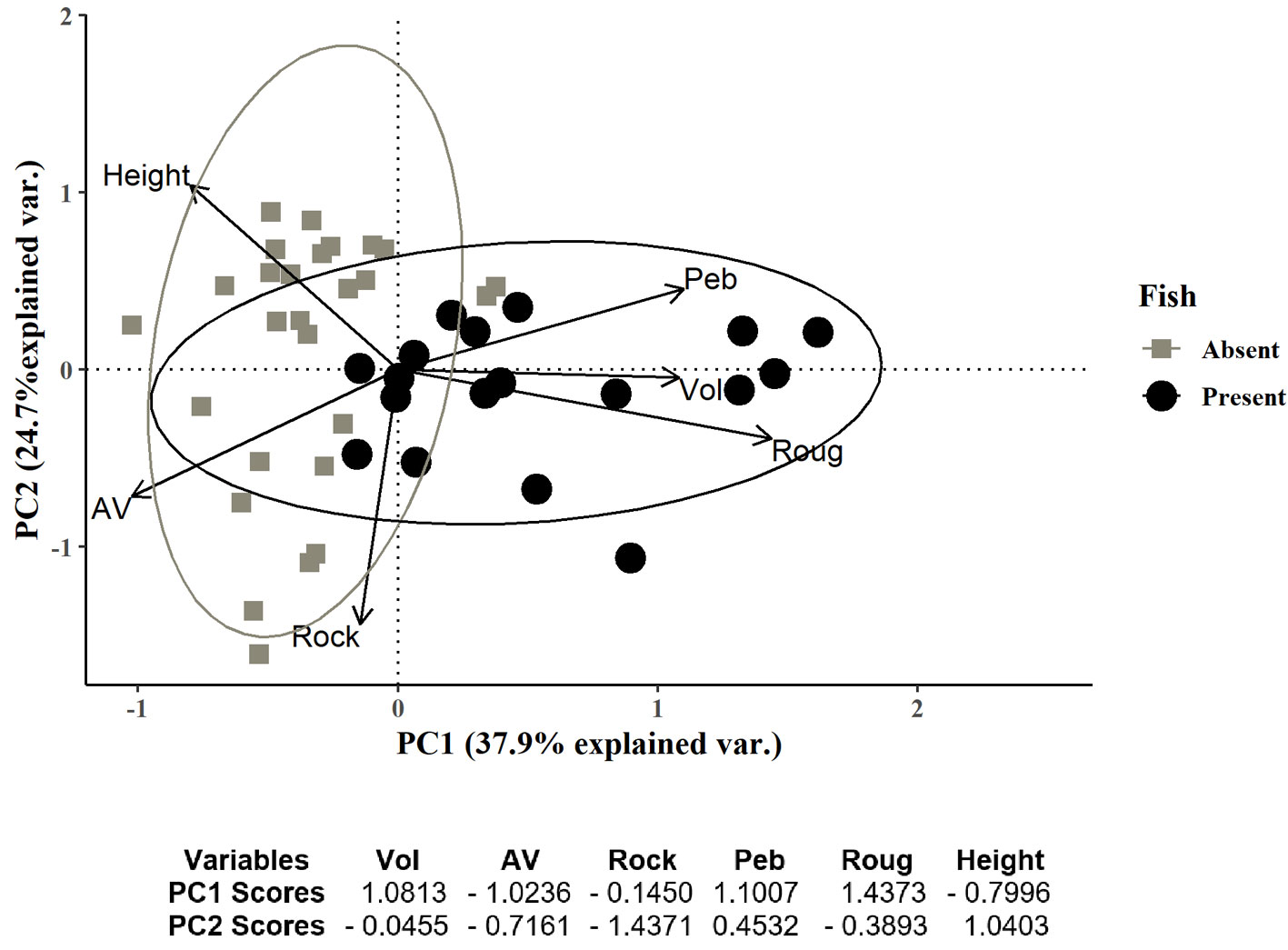
Figure 2 Principal component analysis (PCA) of the environmental variables measured in 45 rock pools of El Zonte, Chiltiupán, La Libertad, 2018. Vector legends: volume (Vol), rock pool intertidal height (Height), percentage of surface roughness (Roug), surface area volume ratio (A/V), rock cover (Rock) and pebbles cover (Peb). Fish occurrence in rock pools is represented as presence (black circles) and absence (grey squares).
Fish occurrence was determined by rock pool volume and roughness (Table 4). Fishes only occurred in rock pools with volumes larger than 0.29 m3 and roughness higher than 4.7% (Fig. 3). The model for fish occurrence with volume as the explanatory variable accounted for 69% of the variance in fish occurrence, while the model including surface roughness explained 49% of the variance. We found that the odds ratio of fish presence to fish absence changed by a factor 6.58 for every one-unit increase in volume, and by a factor 0.42 for a one-unit increase in surface roughness. The probability of fish occurrence changed from absence (0) to presence (1), at a volume of 0.59m3 and at 10.85% roughness (Fig. 3).
Table 4 Results of GLM for the significance of the terms for fish occurrence in 45 rock pools, and abundance and species richness in 42 rock pools (see methods).
| DF | Deviance residuals | DF | Residuals deviance | Pr (> Chi) | |
| Fish occurrence (fish~volume) | |||||
| Null | 44 | 60.571 | |||
| Volume | 1 | 42.017 | 43 | 18.554 | < 0.000 |
| Fish occurrence (fish~roughness) | |||||
| Null | 44 | 60.571 | |||
| Roughness | 1 | 30.283 | 43 | 30.288 | < 0.000 |
| Fish abundance (abundance~Log (volume + 1)*roughness) | |||||
| Null | 41 | 132.207 | |||
| Volume | 1 | 78.129 | 40 | 54.078 | < 0.000 |
| Roughness | 1 | 10.437 | 39 | 43.641 | < 0.000 |
| Volume*roughness | 1 | 17.327 | 38 | 26.314 | < 0.000 |
| Fish richness (species richness~Log (volume + 1)*roughness) | |||||
| Null | 41 | 108.469 | |||
| Volume | 1 | 83.329 | 40 | 25.14 | < 0.000 |
| Roughness | 1 | 3.733 | 39 | 21.407 | < 0.053 |
| Volume*roughness | 1 | 8.413 | 38 | 12.994 | < 0.003 |
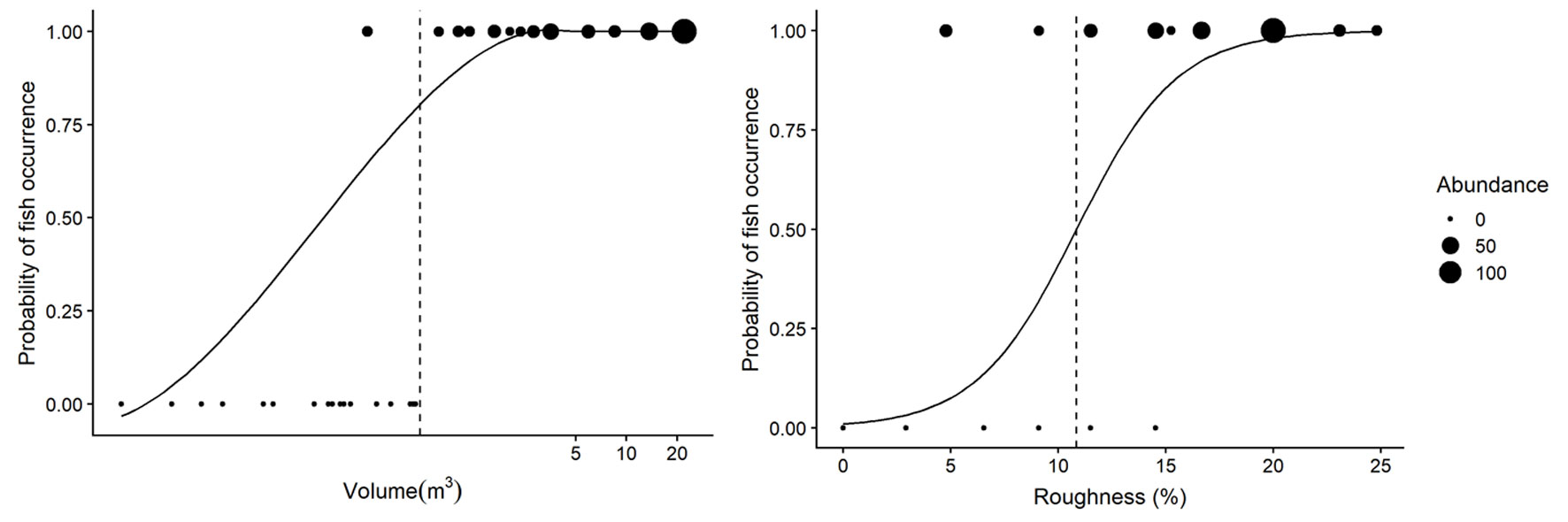
Figure 3 The effect of volume (left panel) and roughness (right panel) on fish occurrence in rock pools. Fish occur in rock pools with volume over of 0.29m3 or a roughness above 4.7%, while the likelihood of fish being present increases in pools greater than 0.59m3 and a roughness higher than 10.85% indicated by the dashed vertical line. The x axes in the left panel has been log transformed to aid in visual interpretation. The size of the dots represents the total fish abundance in each rock pool.
Rock pool volume and roughness and the interaction of these terms had significant impacts on fish abundance and species richness (Table 4). Abundance increased 16.5 times with an increment of one-unit of volume (1 m3) and 1.14 times with an increment of one-unit roughness. However, the contribution of surface roughness to abundance was reduced when volume increased. For species richness, an increment of one-unit of volume (1 m3) in rock pools increased fish richness by 1.65; similarly, a change of one-unit of surface roughness in rock pools increased fish abundance 0.7 times. Nonetheless, the interaction of both terms indicates that the influence of surface roughness on species richness decreased when volume increased. Total length of all fishes (R2 = 0.1209), and the most abundant species B. ramosus (R2 = 0.0784), and A. concolor (R2 = 0.05246) were related to rock pool volume (Fig. 4, Table 5). However, rock pool volume explained only low levels of variance and small fish did also occur in larger pools.
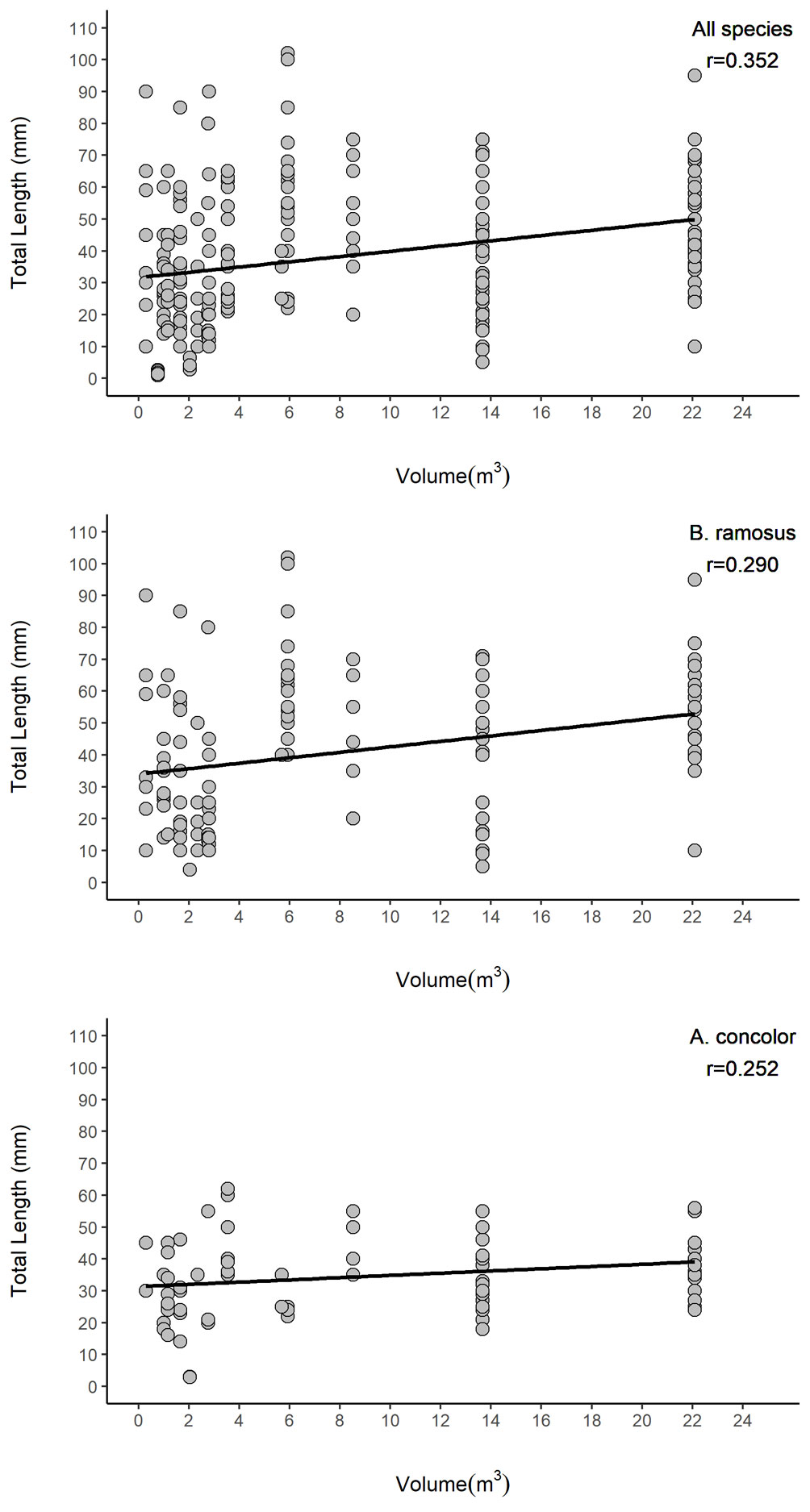
Figure 4 Total length (mm) of fishes relative to the volume of rock pools of El Zonte, Chiltiupán La Libertad, El Salvador.
Table 5 Results of GLM for total length of all the fishes and the 2 most abundant species B. ramosus and A. concolor using data from 15 rock pools (see methods).
| DF | Sum of squares | Mean sum of squares | F value | Pr (> F) | |
| Total length for all species (TL ~ volume) | |||||
| Volume | 1 | 17,079 | 17,078.7 | 43.368 | < 0.000 |
| Residuals | 307 | 120,899 | 393.8 | ||
| Total length B. ramosus (TL ~ volume) | |||||
| Volume | 1 | 7,373 | 7,373.1 | 14.54 | < 0.000 |
| Residuals | 158 | 80,118 | 507.1 | ||
| Total length A. concolor (TL ~ volume) | |||||
| Volume | 1 | 805.2 | 805.15 | 5.8166 | < 0.018 |
| Residuals | 86 | 11,904.4 | 138.42 | ||
Discussion
The occurrence, abundance, and species richness of rock pools fishes in El Zonte are influenced by volume and surface roughness. We detected that the occurrence of fishes was limited in pools with volumes below 0.29 m3 and surface roughness lower than 4.7% (Fig. 3). Rock pools below those thresholds could undergo drastic changes in environmental variables during low tide, offering limited space for fish that actively swim and shelter there (Fig. 2, groups I and II). Rock pools unoccupied by fishes have been observed in other areas, but few studies have quantified rock pool features that could explain the exclusion of fishes. Malard et al. (2016) suggested that rock pools had to be at least 10 cm deep with a minimum volume of 40 L (~ 0.04 m3) to accommodate fish. In other intertidal environments fishes occupied rock pools of 0.12 m3 or even as low as 0.087 m3 (Castellanos-Galindo et al., 2005; González-Murcia et al., 2012). We suggest that the interaction of volume and surface roughness can ameliorate the conditions that make low volume rock pools unfavourable for fish to inhabit because they have crevices and holes that provide shelter for fish and that are less affected by environmental changes than rock pools with low surface roughness (Fig. 3, Table 4). Moreover, we consider that rock pools of smaller volumes or lower surface roughness closer to the low intertidal mark could still be potential habitat for fishes because a low exposure time could reduce the extent at which changes in environmental variables make rock pools unsuitable for fishes to occur.
The scattered distribution observed in the fish assemblage of El Zonte could be the result of environmental filtering over spatial scales. The reduction in volume and surface roughness was coupled with a decrease in habitat diversity. Rock pools with volumes over 0.29 m3 provided suitable environment and were frequently composed of diverse substrata, such as pebbles and algae, increasing habitat heterogeneity (Fig. 3). Larger and structurally more complex rock pools are associated with greater habitat area, prey resources, and refuges, but also intraspecific competition (Faria, 2001). Usually, the number of species and individuals diverge significantly among rock pools as a response to variations in their volume and the type of substrate cover (Cunha et al., 2007; Macieira & Joyeux, 2011; Mahon & Mahon, 1994). Our results indicated a similar pattern. Volume and surface roughness were the main drivers of fish abundance and richness in El Zonte rock pools. However, the effect of roughness on abundance tended to decrease in large rock pools. Griffiths et al. (2006) stated that structural complexity has limited effects on fish abundance and richness and asserted that volume had a stronger influence on fish abundance compared to roughness. In contrast, Castellanos-Galindo et al. (2005) did not detect a relationship between fish species composition and volume. Thus, within and between sites differences in fish assemblages could be the result of local conditions and the interaction with environmental factors or limited by local diversity and their preferences and tolerance to the conditions in available habitats.
The fish community in the El Zonte region is characterized by low species richness. Interestingly, 2 species with markedly different behaviours occurred in small pools, the resident B. ramosus and the opportunist A. concolor. These species are amongst the most abundant in rock pools of the tropical eastern Pacific (Castellanos-Galindo et al., 2005; González-Murcia et al., 2012, 2016). B. ramosus is well adapted to the intertidal zone, displaying territorial behaviour and inhabiting the bottom and crevices, while A. concolor forms schools, consuming pelagic plankton. Other species with similar ecomorphological traits did not occur in rock pools below 0.77 m3. Whether ecological, physiological, or behavioural traits allow B. ramosus and A. concolor to inhabit small rock pools that could represent suboptimal environments needs to be investigated. The intertidal zone of El Zonte has a steep slope and most of the rock pools are located higher up the intertidal platform. Few species can tolerate the environmental conditions in the high intertidal rock pools in El Salvador (González-Murcia et al., 2016). These rock pools are submerged during high tide only, temporally limiting the colonization of subtidal species. Additional studies could determine if juveniles of opportunist species occur in El Zonte rock pools only at specific periods of time.
Small size fishes occurred in all rock pools regardless of volume, but larger fishes were more common in larger rock pools. Segregation patterns in fish size classes have been attributed to physiological and behavioural factors. Hernández et al. (2002), reported that small species, such as Graus nigra, can cope better at high temperatures and drastic intertidal environmental variations than large fish. Faria (2001) suggested that underlying intraspecific interactions, such as territoriality, preclude smaller fish from larger pools, where larger fish are dominant. In contrast, we observed that small fish occurred in rock pools of all volumes and were more abundant in large rock pools. Hence, competition is not likely to explain the segregation of fish in our study. We consider that physiological constrains or lack of suitable shelter could affect large fish in small rock pools. A similar pattern has been described by Malard et al. (2016) for Bathygobius cocosensis, where most size classes occurred in all pools; small fish were not excluded from lower tide pools and larger adults were able to occupy pools in the high intertidal zone.
The intertidal rock pools of El Zonte present severe changes in habitat structure and substratum homogeneity along a spatial gradient. As in other highly dynamic environments (Córdova-Tapia et al., 2018; González-Murcia & Álvarez, 2018; Miyazono et al., 2010; Ortega et al., 2015), these conditions modify the structure of fish assemblages. Understanding patterns of occurrence of species has wide applications in conservation. Our results highlight that interaction among variables can generate suitable environments for some species or restrict them within a threshold. Moreover, high presence of residential, juvenile fish indicates that intertidal rock pools serve as essential areas for juvenile growth. Overall, the intertidal rock pool fish community of El Zonte is composed of 9 species that include mainly permanent resident fishes. In El Zonte, fishes do not occur in rock pools with volumes below 0.29 m3 and roughness lower than 4.7%. We found that changes in abundance and species richness were mainly related to volume and substrata roughness.
We observed that large fish are more likely to occur in larger rock pools, whereas small fish can occupy a wide range of rock pools irrespective of volume. Future conservation efforts should consider the importance of biotic and abiotic factors, such as surface roughness and rock pool volume in providing the most advantageous conditions to enhance the diversity of fish communities.











 text new page (beta)
text new page (beta)


