Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.13 n.2 Texcoco Feb./Mar. 2022 Epub Aug 01, 2022
https://doi.org/10.29312/remexca.v13i2.2656
Articles
Diesel degradation by residual substrate of Agaricus bisporus at the microcosm level
1CONACYT-Instituto de Ciencias-Posgrado en Ciencias Ambientales-Instituto de Ciencias-Benemérita Universidad Autónoma de Puebla. Puebla, Puebla, México. AP. 1622. CP. 72570. (amg2510@hotmail.com; terjimensal@yahoo.com.mx).
2Centro de Agroecología-ICUAP-Benemérita Universidad Autónoma de Puebla. Edificio VAL 1, carretera a San Baltazar Tetela-San Pedro Zacachimalpa km 1.7, Puebla, México. CP. 72960.
In Mexico there are large areas of soils contaminated by hydrocarbons, causing economic and social damage to agricultural production, in this sense, the need to seek economic alternatives that allow contributing to the recovery of affected agricultural soils arises. The present work aimed to determine the biodegradation of diesel in an agricultural soil using residual substrates (RS) of Agaricus bisporus. Soil contaminated with 7 039 ppm of diesel was used with different doses of RS, incubated for 28 days at 37 ºC. CO2 production, diesel biodegradation, initial and final population of fungi, as well as specific enzymatic activity of initial and final laccases were determined. In all treatments, removal increased significantly (p= 0.001) at 37 °C, as well as CO2 production rates. Treatment T4 had the highest percentage of diesel biodegradation (68.747%) and a final cumulative production of 6.144 x 10-4 mmol CO2 m-3. The activity of laccases and tolerant fungal populations decreased in all treatments; in addition, the bacteria increased from 7.6 to 8.9 log CFU gss -1. Therefore, the diesel biodegradation activity is attributed to bioaugmentation and biostimulation by the residual substrate of A. bisporus.
Keywords: bioaugmentation; biodegradation; biostimulation; enzymatic activity
En México existen extensas áreas de suelos contaminados por hidrocarburos, provocando un perjuicio económico y social para la producción agrícola, en este sentido, surge la necesidad de buscar alternativas económicas que permitan contribuir con la recuperación de suelos agrícolas afectados. El presente trabajo tuvo como objetivo determinar la biodegradación de diésel en un suelo agrícola utilizando sustratos residuales (SR) de Agaricus bisporus. Se utilizó suelo contaminado a 7 039 ppm de diésel con diferentes dosis de SR, incubados por 28 días a 37 ºC. Se determinó la producción de CO2, biodegradación de diésel, población inicial y final de hongos, así como actividad enzimática específica de lacasas inicial y final. En todos los tratamientos aumentó la remoción significativamente (p= 0.001) a 37 oC, así como las tasas de producción de CO2. El tratamiento T4 presentó el mayor porcentaje de biodegradación de diésel (68.747%) y una producción acumulativa final de 6.144 x 10-4 mmol CO2 m-3. La actividad de lacasas y poblaciones de hongos tolerantes disminuyeron en todos los tratamientos; además las bacterias aumentaron de 7.6 a 8.9 log UFC gss -1. Por lo que la actividad de biodegradación de diésel se atribuye a una bioaumentación y bioestimulación por parte del sustrato residual de A. bisporus.
Palabras clave: actividad enzimática; biodegradación; bioaumentación; bioestimulación
Introduction
In Mexico, Agriculture is one of the main primary activities that contributes 2.3% of the gross domestic product (GDP) (INEGI, 2020). The municipality of Acatzingo, Puebla-Mexico, is one of the main agricultural producers of crops such as: corn, beans, alfalfa, cabbage, lettuce, nopal, tomato, prickly pear and carrot mainly (SIAP, 2021). However, these agricultural areas have been affected by the intensive use of chemical fertilizers and pesticides, as well as by spills of diesel, gasoline or crude oil (Cavazos-Arroyo et al., 2014). Environmental deterioration with respect to soil degradation generates a cost of 0.41% of GDP (INEGI, 2018). Diesel is a pollutant that causes health problems due to the presence of polyaromatic hydrocarbon (PAH) compounds, which are considered genotoxic, mutagenic and carcinogenic (Schulte and Hauser, 2012). For all this, the need to recover agricultural soils contaminated with diesel in this region arises, because it is the main source of subsistence for peasants.
The ‘mushroom’ Agaricus bisporus contributes 15% (34x106 t) of the world’s production of edible fungi and is the fourth most cultivated species, mainly for its flavor and nutritional, functional and medicinal properties (Royse et al., 2017). In Mexico, the production of A. bisporus was 59 349 tonnes, representing 93.7% of the national production of fungi, where it maintains the leadership in Latin America (Martínez-Carrera et al., 2016). During the growth and fruiting of A. bisporus, approximately 44% cellulose, 29% xylan and 8% lignin are degraded; however, there are 20 to 30% of the polysaccharides present in the substrate that remain undegraded; this calculation does not consider the conversion of substrate into vegetative mycelium (Kapu et al., 2012; Vos et al., 2017), thus, a significant part of the substrate can be used as a residual or post-harvest substrate (RS).
On average, a producing plant generates 5 kg of residual substrates (Lau et al., 2003) for each kg of fungi harvested, obtaining up to 24 t of residual substrate per month (Singh et al., 2011). The residual substrate has been studied as animal feed (Kim et al., 2011; Li et al., 2018), for the purification and extraction of enzymes, for applications of production of biogas, biofuel or bioremediation (Phan and Sabaratnam, 2012; Wan and Li, 2012), as it presents high concentrations of nutrients, microorganisms and enzymes (Ball and Jackson, 1995; Chiu et al., 1998; González-Matute et al., 2011; Kapu et al., 2012). Therefore, this research aimed to evaluate the residual substrate of A. bisporus in the bioremediation of an agricultural soil contaminated with diesel at the microcosm level, allowing to offer a biotechnological alternative for the restoration of agricultural soils impacted with hydrocarbons.
Materials and methods
A sampling of uncontaminated agricultural soil was carried out in Acatzingo, Puebla, Mexico with the following geographical coordinates 18º 57’ 01” north latitude and 97º 43’ 40” west longitude. The residual substrate of Agaricus bisporus used in this study was obtained from local producers from the city of Puebla, Mexico.
Physical, chemical and microbiological characterization of the soil and residual substrate of A. bisporus
The physical and chemical characterization of the agricultural soil and the residual substrate of A. bisporus was carried out as described in NOM-021-SEMARNAT-2000 (DOF, 2002). For the microbiological characterization of the soil and the RS, serial dilutions and the plate-counting technique were performed in differential culture media for the quantification of populations of bacteria, fungi, actinomycetes and bacteria tolerant to diesel expressed as log10 CFU gss -1 (colony-forming units/gram of dry soil).
The mesophilic bacterial population was quantified in nutritive agar medium (Bioxon, Mexico), the fungi in the potato dextrose agar (PDA) medium, the actinomycetes in Czapek Dox Agar and the diesel-tolerant bacteria in the basal medium; the latter with the following preparation (g L-1): NH4NO3, 1; K2HPO4, 1; KH2PO4, 1; MgSO47H2O, 0.409; CaCl2, 0.02; FeCl3, 0.00005; bacteriological agar, 6.5; pH 7, supplemented with 100 μl of sterile diesel distributed on the gelled agar (Mauricio-Gutiérrez et al., 2014). Mesophilic bacteria, tolerant bacteria and actinomycetes were incubated at 30 °C for 72 h, fungi were incubated at 25 °C for 72 h.
Diesel biodegradation at microcosm level using RS of A. bisporus
The soil used was sterilized and intentionally contaminated with 7 039 ppm of diesel and weathered for three months. A completely randomized experimental design was used in a microcosm system to evaluate the participation of microbial populations and laccase (Lac) activity present in the study system with four treatments with different soil: substrate ratios on a dry basis (T1 (95: 5), T2 (90: 10), T3 (85: 15) and T4 (80:20)) and the contaminated soil (T01) and RS (T02) were used as controls.
For each treatment, serological bottles of 120 ml were used, with 30 g of the soil-substrate mixture and were adjusted to the C: N: P ratio to 100: 10: 1 using sterile solutions of NH4SO4 1N and K2HPO4 1N. Moisture was maintained between 23.8-25.6% ±5.23. The atmosphere of the bottles was exchanged every third day with an airflow (1.8 ml air/sec); through a sterile membrane (0.22 μm), the amount of CO2 produced was previously evaluated. All treatments were incubated for 28 days at two temperatures (25 and 37 ºC).
The percentage of diesel biodegradation (%), actual density by means of the NOM-021-SEMARNAT-2000, initial and final activity of laccases (Lac), initial and final population of diesel-tolerant bacteria and fungi (DOF, 2002) were determined. All treatments were carried out in triplicate. The percentage of biodegradation was determined according to the following equation: percentage of biodegradation= [(A - B)/A]*100, A= initial concentration of diesel; B= final concentration of diesel
Basal respiration
The CO2 produced by the soil for 24 h was quantified and absorbed by 5 ml of NaOH 0.1 N, calculated through titration with HCl 0.1 N using phenolphthalein, the excess of carbonates was previously precipitated with 5 ml of BaCl2 0.5 N (Rivera-Espinoza and Dendooven, 2004).
Diesel quantification
The initial and residual diesel was quantified based on the EPA Method 8015 C (Nonhalogenated Organics by Gas Chromatography) using a GC-MS with an HP-5 capillary column (30 m x 0.25 mm x 0.25 μm), whose initial flow was 1 ml min-1. The conditions of analysis of the furnace were: initial temperature 50 ºC, maximum temperature 300 ºC.
Enzyme quantification
An extraction was carried out at the beginning and end of the experiment of the sample of the RS of A. bisporus and of the different treatments in soil: substrate ratios (5 g) with 20 ml of sodium acetate buffer (50 mM, pH 5) for 2 h at 80 rev min-1 and at 4 °C, then filtered through Whatman No. 1 paper. The extracts were stored at 4 °C for the determination of Laccases (Isikhuemhen and Mikiashvilli, 2009). The activity was determined by oxidation of 2.2’-azinobis, 3-ethylbenzothiazoline-6-sulphonate (ABTS) at 25 °C and read at 420 nm (ε420= 36 000 mM-1 cm-1), enzymatic activity was reported as specific activity (U g soil-1) (Bollag and Leonowicz, 1984; Gayosso-Canales et al., 2011).
Microbiological analysis
The initial and final population of diesel-tolerant bacteria and fungi was quantified by direct plate counting. The determination of bacteria was performed for treatments incubated at 37 °C grown in basal medium mentioned above. The determination of fungi was for treatments incubated at 25 °C and the basal medium with the following chemical composition (g L-1) was used: (NH4)2SO4, 7; K2HPO4, 1; KH2PO4, 1; MgSO47H2O, 0.409; dextrose, 0.1; Sol. E 100x, 100 μl; rose bengal, 0.05; streptomycin sulfate, 0.05; pH 5.5. Diesel previously sterilized by filtration was used as a source of carbon, adding 100 μl on the petri dish (Mauricio-Gutiérrez et al., 2014). The microorganisms were reported as log 10 CFU gss -1 (colony-forming units/gram of dry soil).
Results and discussion
The residual substrate (RS) derived from the edible fungus industry represents an environmental problem (Phan and Sabaratnam, 2012), in Europe 3.5 x 106 t are generated per year (García-Delgado et al., 2013). Mexico contributes to the generation of this waste, since it is the largest producer of edible fungi in Latin America, with A. bisporus being one of the main cultivated fungi (Martínez-Carrera et al., 2016). For these reasons, RSs have been studied for different applications such as bioremediation of soils contaminated with PAH (Cerniglia and Sutherland, 2010; Stabnikova et al., 2010; Pardo-Giménez et al., 2010; Upadhyay and Singh, 2011; Phan and Sabaratnam, 2012; García-Delgado et al., 2013).
Physical, chemical and microbiological characterization of soil and RS
According to the physical and chemical characteristics (Table 1) of the agricultural soil of Acatzingo, Puebla, Mexico, it was classified as loam-sandy, with low values of moisture (17.35%), organic matter (2.04%), total nitrogen (0.032%) and phosphorus (0.003%) compared to the parameters of the RS (190.32%, 57.62%, 1.46% and 0.79% respectively). In addition, it presented a moderately alkaline pH value (pH= 8.01). The RSs of Agaricus bisporus are characterized by high concentrations of nutrients, these properties have been used as biostimulant agents for the bioremediation of xenobiotic since they have high doses of nitrogen (2.16%), phosphorus (0.69%) and carbon (54.3%) (Corral-Bobadilla et al., 2019).
Table 1 Physical, chemical and microbiological characterization of soil and RS of A. bisporus.
| Parameters | Soil | Residual substrate |
| pH | 8.01 | 7.26 |
| EC (S cm-1) | 205 | Nd |
| Texture (%) [clay, sil, sand] | 17.4, 18, 64.6 | Nd |
| Moisture (%) | 17.355 | 190.328 |
| Total organic matter (%) | 2.04 | 57.625 |
| Total nitrogen (%) | 0.032 | 1.46 |
| Available phosphorus (%) | 0.003 | 0.791 |
| Actual density (g ml-1) | 2.464 | Nd |
| Bacteria (log CFU gss -1) | 6.021 | 7.708 |
| Fungi (log CFU gss -1) | 3.455 | 5.554 |
| Actinomycetes (log CFU gss -1) | 2.895 | 3.559 |
| Hydrocarbonoclastic bacteria (log CFU gss -1) | 5.037 | 3.828 |
Nd= not determined; gss= grams of dry soil.
With respect to the quantification of cultivable microorganisms in Table 1, the bacterial population had the highest values in the soil (6.021 log CFU gss -1) and the RS (7.708 log CFU gss -1). The size of the other microbial populations varied between the samples, with the agricultural soil where there was a greater number of hydrocarbonoclastic bacteria (5.037 log CFU gss -1), followed by the fungal population (3.455 log CFU gss -1) and finally actinomycetes (2.895 log CFU gss -1). However, in the RS there was a greater number of fungi (5.554 log CFU gss -1) and the groups of actinomycetes and hydrocarbonoclastic bacteria remained at the same order of magnitude (3.559 and 3.828 log CFU gss -1). The soil and RS harbor a native microbial population, the latter have been used as sources of bioaugmentation for bioremediation processes (Wang et al., 2016; Leong et al., 2022).
Diesel biodegradation at microcosm level using RS of A. bisporus
Diesel biodegradation experiments at the microcosm level (Table 2) showed a higher biodegradation at 37 ºC; treatment T4 was the one that obtained the highest removal (68.747%) followed by T4 at 25 ºC (61.261%) and T3 at 37 ºC (60.14%). On the contrary, the treatments with the lowest percentage of removal were T01 (contaminated soil) at 25 ºC with a removal of 20.603%, followed by T1 (27.034%) and T2 (29.791%) at 25 ºC, presenting highly significant statistical differences (p= 0.001).
Table 2 Diesel biodegradation and production of cumulative CO2 using RS of A. bisporus.
| Treatment | Biodegradation | mmol CO2 m-3 day* | |||
| 25 °C | 37 °C | 25 °C | 37 °C | ||
| (%) | (x10-4) | ||||
| T1 | 27.034 c | 40.966 bc | 4.319 ±0.287 c | 5.608 ±0.533 b | |
| T2 | 29.791 c | 48.408 b | 4.343 ±0.266 c | 5.685 ±0.564 b | |
| T3 | 37.177 b | 60.14 a | 4.808 ±0.574 b | 6.014 ±0.214 a | |
| T4 | 61.261 a | 68.747 a | 5.785 ±0.46 a | 6.144 ±0.429 a | |
| T01 | 20.603 d | 37.357 c | 4.902 ±0.334 b | 5.432 ±0.346 b | |
| T02 | Nd | Nd | 6.173 ±0.245 a | 5.767 ±0 b | |
*= ±standard deviation; Nd= not detected. Different letters in the column indicate statistically significant differences according to the Tukey test (p= 0.05).
The cumulative and quantified CO2 generation in the study system indicated that treatments incubated at 37 ºC produced a higher concentration (mmol CO2 m-3 day) (Table 2). The treatments that obtained the highest CO2 production were T02 (RS) (6.173 x 10-4 mmol CO2 m-3 day) at 25 ºC, T4 (6.144 x 10-4 mmol CO2 m-3 day) and T3 (6.014 x 10-4 mmol CO2 m-3 day) at 37 ºC. And the treatments: T1 (4.319 x 10-4 mmol CO2 m-3 day) at 25 ºC, T2 (4.343 x 10-4 mmol CO2 m-3 day), T3 (4.808 x 10-4 mmol CO2 m-3 day) and T01 (contaminated soil) (4.902 x 10-4 mmol CO2 m-3 day) at 25 ºC presented a lower CO2 production rate. Margesin et al. (2007) positively correlated hydrocarbon degradation with microbial activity and biomass, results similar to those obtained in the present investigation.
A 4:1 ratio of soil: substrate (T4) biodegraded 68.747% of diesel in 28 days, which allows treating 6.92 t m-3 of soil, as mentioned by Sasek et al. (2003), who added RS of A. bisporus to a 4:1 ratio (soil: substrate) and obtained a degradation of 68.8% of PAH (polyaromatic hydrocarbons) at the end of 154 days in 2.8 m3 of soil. While Mohammadi-Sichani et al. (2019) reported a degradation of 71.5% in three months of TPH (total petroleum hydrocarbons) in soil, applying 10% of RS of A. bisporus. In addition, the actual density of each treatment was determined in order to assess the RS of A. bisporus as a texturing agent for application in bioremediation of soils contaminated with hydrocarbons.
The results obtained indicated that there is no statistically significant difference in the actual density between the treatments, where the values are in the range of 2.36 to 2.536 g mL-1 (Table 3). The percentages of biodegradation obtained can be due to the physical and chemical properties that RS provides to the soil such as: texture, water retention capacity, porosity and contribution of essential nutrients such as nitrogen and phosphorus (Table 1), which allow stimulating degradative microbial activity, helping in soil bioremediation (Stabnikova et al., 2010; García-Delgado et al., 2013).
Table 3 Actual density and amount of contaminated soil treated by RS of A. bisporus applied.
| Treatment | Actual density* | Treated soil/residual substrate |
| (g ml-1) | (t m-1) | |
| T1 | 2.536 a | 32.85 |
| T2 | 2.413 a | 15.56 |
| T3 | 2.43 a | 9.76 |
| T4 | 2.36 a | 6.92 |
| T01 | 2.334 a | Na |
| T02 | 1.729 b | Na |
Different letters in the column indicate statistically significant differences according to the Tukey test (p= 0.05). Na= not applicable.
The population dynamics of diesel-tolerant bacteria in the study system indicated a highly significant statistical increase (p= 0.001) at the end of the experiment for all treatments: T1 (7.83 log CFU gss -1), T2 (8.9 log CFU gss -1), T3 (7.7 log CFU gss-1) and T4 (7.6 log CFU gss -1). However, the population count of tolerant fungi showed a highly significant statistical decrease (p= 0.001) with population sizes of 4.8 to 5.6 log CFU gss -1 (Figure 1).
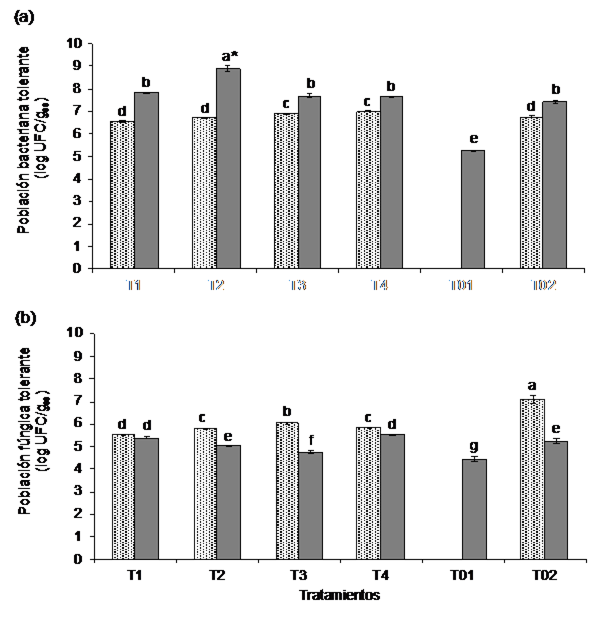
Figure 1 a) dynamics of the bacterial population of the treatments incubated at 37 °C; and b) of the fungal population of the treatments incubated at 25 °C tolerant to diesel. At the beginning (light bars) and end (dark bars) of the experiment with different soil: RS of A. bisporus ratios on a dry basis. Each value represents the average of triplicates. Different letters indicate statistically significant differences between treatments (p= 0.05) according to the Tukey test.
The quantification of Lac decreased significantly (p= 0) in the treatments incubated at 25 °C, quantifying specific enzymatic activities from 0.083 to 0.48 U g-1 (Figure 2). Treatment T4 (80:20) had the highest enzymatic activity (0.48 U g-1) unlike the control group T02 (RS) of A. bisporus (2.46 U g-1). In the treatments incubated at 37 °C, Lac activity was not detected according to the established methodology.
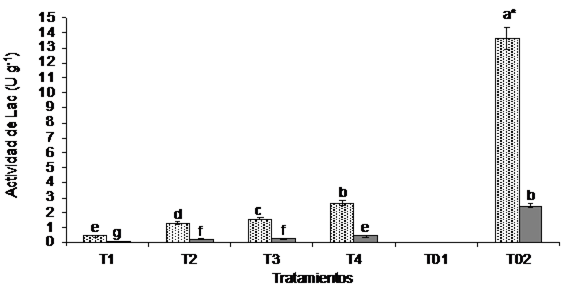
Figure 2 Laccases activity at the microcosm level with different soil: RS of A. bisporus ratios on a dry basis. Each value represents the average of triplicates of the treatments at the beginning (light bars) and end (dark bars) of the experiment. *= different letters indicate statistically significant differences between treatments (p= 0.05) according to the Tukey test.
The group of microorganisms studied in the biodegradation of diesel were the tolerant bacteria native to the RS and the soil, since they presented an effectiveness in the biodegradation and contributed to the bioremediation process (Gallego et al., 2001). On the contrary, treatments incubated at 25 ºC showed a decrease in the population of fungi since they were affected by the conditions of the microcosm (environment) and the type of contaminant used, as well as in the production of enzymes such as Lac. Bento et al. (2005) mention that microorganisms in a hostile environment decrease their metabolic activity and limit the growth of their population.
Although there are different reports of extracellular enzymes secreted by A. bisporus in RS that can be used in bioremediation processes (García-Delgado et al., 2013; Chatterjee et al., 2017). It is important to mention that the control treatment T01 (soil) presented a diesel biodegradation of 37.357%, this value may be associated with the loss due to volatilization since it can be 35-40% in soil (Rhykerd et al., 1999; Saviozzi et al., 2009) and the presence of reproductive structures of the native microbiota that resisted soil sterilization (Sylvia et al., 1999), since in the present study, T01 (soil) presented populations of bacteria and fungi of 5.3 and 4.4 log CFU gss -1.
The RS of A. bisporus was characterized as a biostimulant agent for presenting organic nutrients, it also increased the porosity of the contaminated soil, improving oxygen diffusion and water retention capacity. It also presented bioaugmentation characteristics since it provided microbial load as a degrading source of diesel with a potential effect on the biodegradation of hydrocarbons. As established by Chiu et al. (1998), where they mention that RSs have the advantage of degrading xenobiotic compounds by the consortium of enzymes of various microorganisms, in addition to being relatively rich in nutrients that stimulate these microorganisms in the secretion of enzymes without having any nutritional limitation.
In this context, there are several studies where different RSs are evaluated, such as: activated sludge, sugarcane bagasse, sugarcane filter cake and corn residues for purposes of bioremediation of soils contaminated with diesel with biodegradation percentages of 61 to 90% in a time of 15 to 109 days, results similar to those obtained in the present study (Gallego et al., 2001; Molina-Barahona et al., 2004; Rivera-Espinoza and Dendooven, 2004; Bento et al., 2005; García-Torres et al., 2011).
Conclusions
This research identified an alternative use the RS of A. bisporus for the bioremediation of agricultural soils contaminated with diesel, attributing this biodegradation to the native bacterial activity of RS and soil; in addition, the RS was an important source of nutrients. Therefore, further studies on the microorganisms and biodegradation routes involved are required, for small-scale and industrial application under real conditions in field trials.
Acknowledgements
The authors thank SEP-PRODEP. This work was partially supported by the catedra-CONACYT program and SEP-PRODEP.
REFERENCES
Ball, A. and Jackson, A. 1995. The recovery of lignocellulose-degrading enzymes from spent mushroom compost. Bioresour Technol. 54(3):311-314. [ Links ]
Bento, F.; Camargo, F.; Okeke, B. and Frankenberger, W. 2005. Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour. Technol. 96(9):1049-1055. Doi: 10.1016/j.biortech. 2004.09.008. [ Links ]
Bollag, J. and Leonowicz, A. 1984. Comparative studies of extracellular fungal laccases. Appl. Environ. Microbiol. 48(4):849-854. [ Links ]
Cavazos-Arroyo, J.; Pérez-Armendáriz, B. y Mauricio-Gutiérrez, A. 2014. Afectaciones y consecuencias de los derrames de hidrocarburos en suelos agrícolas de Acatzingo Puebla, México. Agric. Soc. Des. 11(4):539-550. [ Links ]
Cerniglia, C. E. and Sutherland, J. B. 2010. Degradation of polycyclic aromatic hydrocarbons by fungi. In: Handbook of hydrocarbon and lipid microbiology. Timmis, K.N. (ed.). 1. Springer. Berlin, Heidelberg. 2079-2110 pp. Doi: 10.1007/978-3-540-77587-4-151. [ Links ]
Chatterjee, S.; Sarma, M.; Deb, U.; Steinhauser, G.; Walther, C. and Gupta, D. 2017. Mushrooms: from nutrition to mycoremediation. Environ. Sci. Pollut. Res. 24(24):19480-19493. Doi: 10.1007/s11356-017-9826-3. [ Links ]
Chiu, S. W.; Ching, M. L.; Fong, K. and Moore, D. 1998. Spent oyster mushrooms substrate performs better than many mushroom mycelia in removing the biocide pentachlorophenol. Mycol Res. 102(12):1553-1562. Doi: 10.1017/S0953756298007588. [ Links ]
Corral-Bobadilla, M.; González-Marcos, A.; Vergara-González, E. P. and Alba-Elías, F. 2019. Bioremediation of wastewater to remove heavy metals using the spent mushroom substrate of Abaricus bisporus. Water. 11(454):1-15. Doi: 10.3390/w11030454. [ Links ]
DOF. 2002. Official Journal of the Federation. NOM-021-RECNAT-2000, Official mexican standard, which establishes the specifications of fertility, salinity and soil classification, studies, sampling and analysis. [ Links ]
Gallego, L. R.; Loredo, J.; Llamas, J. F.; Vázquez, F. and Sánchez, J. 2001. Bioremediation of diesel-contaminated soils: evaluation of potential in situ techniques by study of bacterial degradation. Biodegradation. 12(5):325-335. Doi: 10.1023/A:1014397732435. [ Links ]
García-Delgado, C.; Jiménez-Ayuso, N.; Frutos, I.; Gárate, A. and Eymar, E. 2013. Cadmium and lead bioavailability and their effects on polycyclic aromatic hydrocarbons biodegradation by spent mushroom substrate. Environ. Sci. Pollut. Res. 20(12):8690-8699. Doi: 10.1007/s11356-013-1829-0. [ Links ]
García-Torres, R.; Ríos-Leal, E.; Martínez-Toledo, A., Ramos-Morales, F. R.; Cruz-Sánchez, J. S. y Cuevas-Díaz, M. 2011. Uso de cachaza y bagazo de caña de azúcar en la remoción de hidrocarburos en suelo contaminado. Rev. Intern. Contamin. Amb. 27(1):31-39. [ Links ]
Gayosso-Canales, M.; Esparza-García, F. J.; Bermúdez-Cruz, R. M.; Tomasini, A.; Ruíz-Aguilar, G. M. and Rodríguez-Vázquez, R. 2011. Application of 2III7-3 fractional factorial experimental design to enhance enzymatic activities of Pleurotus ostreatus with high concentrations of polychlorinated by phenyls. J. Environ. Sci. Health A. 46(3):298-305. Doi: 10.1080/10934529.2011.539095. [ Links ]
González-Matute, R.; Figlas, D. and Curvetto, N. 2011. Agaricus blazei production on non-composted substrates based on sunflower seed hulls and spent oyster mushroom substrate. World J. Microbiol. Biotechnol. 27(6):1331-1339. Doi: 10.1007/s11274-010-0582-5. [ Links ]
INEGI. 2018. Sistema de cuentas nacionales de México. Cuentas económicas y ecológicas de México. 2003-2017. [ Links ]
INEGI. 2020. PIB y cuentas nacionales. Producto interno bruto trimestral. Cifras desestacionalizadas por grupo de actividades económicas, base. [ Links ]
Isikhuemhen, O. S. and Mikiashvilli, N. A. 2009. Lignocellulolytic enzyme activity, substrate utilization, and mushroom yield by Pleurotus ostreatus cultivated on substrate containing anaerobic digester solids. J. Ind. Microbiol. Biotechnol. 36(1):1353-1362. Doi: 10.1007/s10295-009-0620-1. [ Links ]
Kapu, N. S.; Mannign, M.; Hurley, T. B.; Voigt, J.; Cosgrove, D. J. and Romaine C. 2012. Surfactant-assisted pretreatment and enzymatic hydrolysis of spent mushroom compost for the production of sugars. Biores Technol. 114(1):399-405. Doi: 10.1016/j.biortech.2012.02.139. [ Links ]
Kim, M.; Lee, H.; Park, J.; Kang, S. and Choi, Y. 2011. Recycling of fermented sawdust-based oyster mushroom spent substrate as a feed supplement for postweaning calves. Asian-aust J. Anim. Sci. 24(4):493-499. Doi: 10.5713/ajas.2011.10333. [ Links ]
Lau, K. L.; Tsang, Y. Y. and Chiu, S. 2003. Use of spent mushroom compost to bioremediate PAH-contaminated samples. Chemosphere. 52(9):1539-1546. Doi: 10.1016/S0045-6535(03)00493-4. [ Links ]
Leong, Y. K.; Ma, T. W.; Chang, J. S. and Yang, F. C. 2022. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): a review. Boresour. Techol. 344:126-157. Doi: 10.1016/j.biortech.2021.126157. [ Links ]
Li, S.; Li, D.; Li, J.; Li, Y.; Li, G.; Zang, B. and Li, Y. 2018. Effec of spent mushroom substrate as a bulking agent on gaseous emissions and compost quality during pig manure composting. Environ. Sci. Pollut. Res. 25(13):12398-12406. Doi: 10.1007/s11356-018-1450-3. [ Links ]
Margesin, R.; Hämmerle, M. and Tscherko, D. 2007. Microbial activity and community composition during bioremediation of diesel-oil-contaminated soil: effects of hydrocarbon concentration, fertilizers and incubation time. Microbial. Ecol. 53(2):259-269. Doi: 10.1007/s00248-006-9136-7. [ Links ]
Martínez-Carrera, D.; Larqué-Saavedra, A.; Tovar, P. A.; Torres, N.; Meneses, M. E.; Sobal, C. M.; Morales, A. P.; Bonilla, Q. M.; Escudero, U. H.; Tello-Salgado, I.; Bernabé-González, T.; Martínez, S. W. y Mayett, Y. 2016. Contribución de los hongos comestibles, funcionales y medicinales a la construcción de un paradigma sobre la producción, la dieta, la salud y la cultura en el sistema agroalimentario de México. In: Martínez-Carrera, D. y Ramírez- Juárez, J. (Eds.). Ciencia, Tecnología e Innovación en el Sistema Agroalimentario de México. (Ed.) del Colegio de Posgraduados-AMC-CONACYT-UPAEP-IMINAP. San Luis Huexotla, Texcoco, Estado de México. 581-640 pp. [ Links ]
Mauricio-Gutiérrez, A.; Jiménez-Salgado, T.; Tapia-Hernández, A.; Cavazos-Arroyo, J. and Pérez-Armendáriz, B. 2014. Biodegradation of hydrocarbons exploiting spent substrate from Pleurotus ostreatus in agricultural soil. Afr. J. Biotechnol. 13(33):3385-3393. Doi: 10.5897/AJB2014.13964. [ Links ]
Mohammadi-Sichani, M.; Assadi, M. M.; Farazmand, A.; Kianirad, M.; Ahadi, A. M. and Hadian-Ghahderijani, H. 2019. Ability of Agaricus bisporus, Pleurotus ostreatus and Ganoderma lucidum compost in biodegradationof petroleum hydrocarbon-contaminated soil. Inter. J. Environ. Sci. Technol. 16(5):2313-2320. Doi: 10.1007/s13762-017-1636-0. [ Links ]
Molina-Barahona, L.; Rodríguez-Vázquez, R.; Hernández-Velasco, M.; Vega-Jarquín, C.; Zapata-Pérez, O.; Mendoza-Cantú, A. and Albores, A. 2004. Diesel removal from contaminated soils by biostimulation and supplementation with crop residues. Appl. Soil Ecol. 27(2):165-175. Doi: 10.1016/j.apsoil2004.04.002. [ Links ]
Pardo‑Giménez, A.; Cunha-Zied, D. and Pardo‑González, J. 2010. Utilización de compost agotado de champiñón como capa de coberturas en nuevos ciclos de producción. Pesqui. Agropecu. Bras. 45(10):1164-1171. doi: 10.1590/S0100-204X2010001000016. [ Links ]
Phan, C. W. and Sabaratnam, V. 2012. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 96(4):863-873. Doi: 10.1007/s00253-012-4446-9. [ Links ]
Rhykerd, R. L.; Crews, B.; Mclnnes, K. and Weaver, R. 1999. Impact of bulking agents, forced aeration, and tillage on remediation of oil-contaminated soil. Biores. Technol. 67(3):279-285. [ Links ]
Rivera-Espinoza, Y. and Dendooven, L. 2004. Dynamics of carbon, nitrogen and hydrocarbons in diesel-contaminated soil amended with biosolids and maize. Chemosphere . 54(3):379-386. Doi: 10.1016/S0045-6535(03)00653-2. [ Links ]
Royse, D. J.; Baars, J. and Tan. Q. 2017. Current overview of mushroom production in the world. In: Diego, C. Z. and Pardo-Giménez, A. (Ed.). Edible and medicinal mushrooms: Technology and Applications. John Wiley y Sons LtD, Hoboken. 5-13 pp. [ Links ]
Sasek, V.; Bhatt, M.; Cajthaml, T.; Malachová, K. and Lednická, D. 2003. Compost-mediated removal of polycyclic aromatic hydrocarbons from contaminated soil. Arch. Environ. Contam. Toxicol. 44(3):336-342. Doi: 10.1007/s00244-002-2037-y. [ Links ]
Saviozzi, A.; Cardelli, R. and Cozzolino, M. 2009. Bioremediation with compost of a diesel contaminated soil: monitoring by dehydrogenase activity and basal respiration. Compost Sci. Util. 17(1):55-60. Doi: 10.1080/1065657X.2009.10702400. [ Links ]
Schulte, P. A. and Hauser, J. E. 2012. The use of biomarkers in occupational health research, practice, and policy. Toxicol. Letters. 213(1):91-99. Doi: 10.1016/j.toxlet.2011.03.027. [ Links ]
SIAP. 2021. Anuario Estadístico de la Producción Agrícola. http://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119. [ Links ]
Sylvia, D. M.; Fuhrmann, J. J.; Hartel, P. G. and Zuberer, D. A. 1999. Principles and applications of soil microbiology. Upper Saddle River, New Jersey. ISBN-13:978-0130941176. [ Links ]
Singh, A. D.; Vikineswary, S.; Abdullah, N. and Sekaran, M. 2011. Enzymes from spent mushroom substrate of Pleurotus sajor-caju for the decolourisation and detoxification of textile dyes. World J. Microbiol. Biotechol. 27(3):535-545. Doi: 10.1007/s11274-010-0487-3. [ Links ]
Stabnikova, O.; Wang, J. Y. and Ivanov, V. 2010. Value-added biotechnological products from organic wastes. In: Wang, L.; Ivanov, V. and Tay, J. H. (Ed.) environmental biotechnology. Handbook of environmental engineering, vol 10. Humana Press, Totowa, NJ. 343-394 pp. Doi: 10.1007/978-1-60327-140-0-8. [ Links ]
Upadhyay, R. and Singh, M. 2011. Production of edible mushrooms. In: hofrichter M. (Ed.) industrial applications. The mycota (a comprehensive treatise on fungi as experimental systems for basic and Aplied Research), vol 10. Springer, Berlin, Heidelberg. 79-97 pp. Doi: 10.1007/978-3-642-11458-8-4. [ Links ]
Vos, A. M.; Heijboer, A.; Boschker, H. S.; Bonnet, B. and Lugones, L. 2017. Microbial biomass in compost during colonization of Agaricus bisporus. AMB Express. 7(12):1-7. Doi: 10.1186/s13568-016-0304-y. [ Links ]
Wan, C. and Li, Y. 2012. Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 30(6):1447-1457. Doi: 10.1016/j.biotechadv.2012.03.003. [ Links ]
Wang, C.; Yu, D.; Shi, W.; Jiao, K.; Wu, B. and Xu, H. 2016. Application of spent mushroom (Lentinula edodes) substrate and acclimated sewage sludge on the bioremediation of polycyclic aromatic hydrocarbon polluted soil. RSC Advances. 6(43):37274-37285. Doi: 10.1039/C6RA05457A. [ Links ]
Received: January 01, 2022; Accepted: March 01, 2022











 text in
text in 


