The genus Echeveria DC. (Crassulaceae) is endemic to the Americas. Echeveria species have marked preference for rocky outcrops such as steep slopes, canyons and rocks with low depth and humidity. This topological preference is attributed to their succulence in their organs, an adaptation to withstand prolonged water deficit (Reyes-Santiago et al. 2011). The genus is one of the most diverse in the Crassulaceae Family (Pilbeam 2008); it includes about 154 species of which 85 % are present in Mexico, mainly in the states of Oaxaca, Puebla and Hidalgo (Reyes-Santiago et al. 2015). Because of the complexity of the genus, it has been divided into 17 series. The Gibbiflorae Series contains widely distributed species, prominent hybridization events and morphological variation. An example is Echeveria aff. gigantea Rose & Purpus, which have a wide distribution in the states of Oaxaca, Puebla and Michoacán (Mexico), and inhabits a wide range of arid and mild environments, within rocky soils and dense shrubs. Due its distribution, this species displays great morphological variability, making its taxonomic identification difficult (Reyes-Santiago et al. 2011).
The qualitative and quantitative features of leaf and stem anatomy in Crassulaceae have been useful to describe or define taxonomic groups in Crassula (Tolken 1977), Kalanchoe (Abdel-Raouf 2012, Chernetskyy 2012, Hyakutake & Souza 1972, Moreira et al. 2012) and Sedum (Ardelean et al. 2009, Wu et al. 2013). The leaf anatomy characterization has also helped to understand the relationships between the internal structure of the leaf and the physiological adaptations to their xerophytic habitat such as Aeonium, Aichryson, Monanthes, Greenovia (Caballero-Ruano & Jiménez-Parrondo 1978), Crassula (Jones 2011, Karwowska et al. 2015, Moteetee & Nagendran 1997, Rost 1969), Kalanchoe (Chernetskyy & Weryszko-Chmielewska 2008), Rhodiola (Costica et al. 2007) and Sedum (Ardelean et al. 2009). We evaluated leaf anatomical and morphological characteristics in order to characterize the variation in Echeveria aff. gigantea accessions. We also aimed to detect possible correlations between anatomical features and organ shape, as well as to discuss whether these morpho-anatomical attributes allowed the group to colonize a variety of environments.
Materials and methods
Biological material. Seven accessions of Echeveria aff. gigantea were selected. E. gibbiflora was also studied as a comparison group because it was used to define the Series Gibbiflorae, preliminary analyses show that both species belong to the same clade (unpublished data), and the species have sympatric distribution. Plants were originally collected from the wild and were assigned accession numbers (Table 1). Prior to this study, plants were kept under greenhouse conditions for more than five years, at the National Collection of the Crassulaceae Family (Jardín Botánico, UNAM; Figure 1A-I). Selected leaves were taken from the middle part of the rosette, which were mature and healthy. Three leaves were used to measure leaf area. For the histological analyses, the middle and basal region of a leaf was dissected (Figure 1J). Samples were fixed in FAA (Formaldehyde-Acetic Acid-Alcohol-Water) for 24 hours, dehydrated in Tert-Butyl alcohol and embedded in paraffin. 10-15μm-thick transversal sections were obtained with an American Optical 820 rotary microtome. Sections were stained with safranin-fast green and mounted as permanent slides. To study the epidermal architecture, the foliar epidermis was mechanically removed to obtain semi-permanent slides (Sandoval-Zapotitla et al. 2005).
Table 1 List of Echeveria species, Gibbiflorae accessions used in this study.
| Accession | Location of origin | Altitude (m a.s.l.) |
Annual precipitation (mm) |
Average annual temperature (°C) |
|---|---|---|---|---|
|
E. aff. gigantea JE3914 |
San Pedro Nopala, Oaxaca | 2,555 | 842.1 | 18.1 |
|
E. aff. gigantea JE5151 |
St. MaríaTexcatitlan, Oaxaca | 2,125 | 842.1 | 18.1 |
| E. aff. gigantea JE5599 |
Tlaxiaco, Oaxaca | 2,123 | 1,010.8 | 16.7 |
| E. aff. gigantea JE5609 | San Isidro Chicahuaxtla, Oaxaca | 2,465 | 2,479.0 | 17.8 |
| E. aff. gigantea JE5692 | Zinacantepec-San Luis de los Pinos, Puebla | 2,035 | 378.5 | 23 |
|
E. gibbiflora JE6589 |
Turundeo and Los Calvos, Michoacán | 1,914 | 847.7 | 19 |
| E. aff. gigantea JE6693 | Zapotitlán, Puebla | 1,640 | 450.0 | 21.5 |
| E. aff. gigantea JE6787 | Zinacantepec-San Luis de los Pinos, Puebla | 2,035 | 378.5 | 23 |
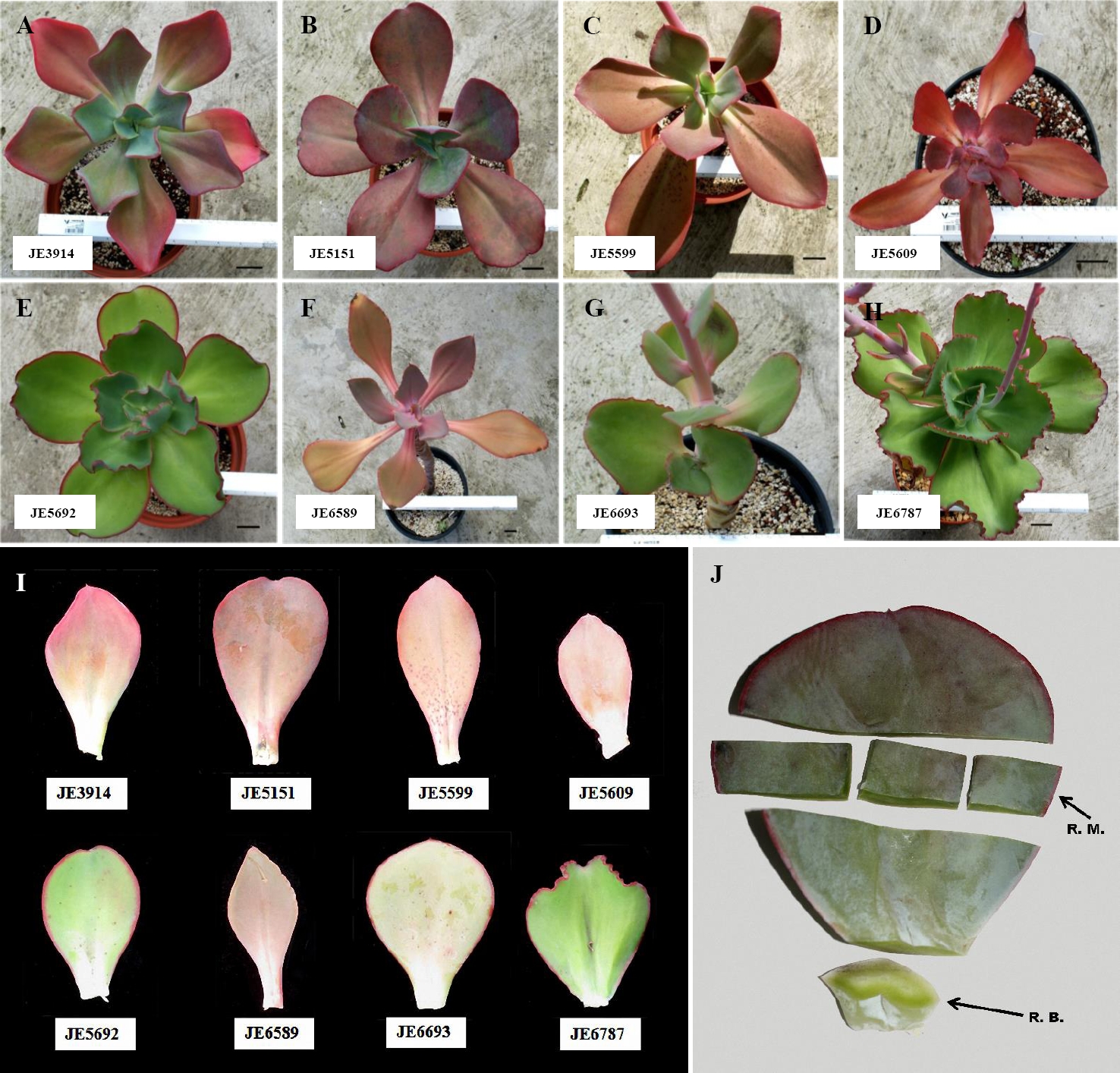
Figure 1 Echeveria accessions. A-E) and G-H) Whole plants of. E. aff. gigantea accessions. F) E. gibbiflora Lindl. I) Variation of leaves between analyzed accessions. Bar = 5 cm. J) Leaf subdivisions on this study; middle region (R.M.), for cross-sections and removal of epidermis; basal region (R.B.), for cross sections.
Anatomical and morphological analysis. Slides were analyzed with a Carl Zeiss-Axioskop photomicroscope. Photomicrographs were captured with a Sony Exwave HAD video camera and digitalized with Pinnacle Studio Plus v.11. We evaluated 32 anatomical characteristics (20 quantitative and 12 qualitative) of the dermal, fundamental and vascular tissues, in addition to the leaf area in each of the accessions (Table 2). To reflect the abundance of the stomata we used the Salisbury stomatal index (Willmer 1983). For each of the 20 quantitative characteristics, 20 measurements were performed on each accession with the ImageJ v.1.48 image analyzer (Supplementary files 1 and 2).
Table 2 List of discrete and continuous anatomical characters analyzed in eight Gibbiflorae accessions.
| Character ID | Character |
|---|---|
| A01 | Leaf area (cm2) |
| Middle region | |
| A02 | Adaxial occlusive cell length (µm) |
| A03 | Adaxialstomatal index |
| A04 | Adaxial epidermal cell area (µm2) |
| A05 | Abaxial occlusive cell length (µm) |
| A06 | Abaxialstomatal index |
| A07 | Abaxial epidermal cell area (µm2) |
| A08 | Stomata position |
| A09 | Outer periclinal wall thickness of adaxial epidermal cells (µm) |
| A10 | Outer periclinal wall thickness of abaxial epidermal cells (µm) |
| A11 | Adaxial epidermal cell width (µm) |
| A12 | Adaxial epidermal cell height (µm) |
| A13 | Abaxial epidermal cell width (µm) |
| A14 | Abaxial epidermal cell height (µm) |
| A15 | Adaxial hypodermal cell area (µm2) |
| A16 | Abaxial hypodermal cell area (µm2) |
| A17 | Hypodermal cell shape |
| A18 | Hypodermal cell wall thickness (µm) |
| A19 | Type of vascular bundles |
| A20 | Vessel diameter in the mid vein (µm) |
| A21 | Number of strata of colenquimatous sheath |
| Basal region | |
| A22 | Adaxial epidermal cell width (µm) |
| A23 | Adaxial epidermal cell height (µm) |
| A24 | Outer periclinal wall thickness of adaxial epidermal cells (µm) |
| A25 | Abaxial epidermal cell width (µm) |
| A26 | Abaxial epidermal cell height (µm) |
| A27 | Outer periclinal wall thickness of abaxial epidermal cells (µm) |
| A28 | Adaxial hypodermal cell area (µm2) |
| A29 | Abaxial hypodermal cell area (µm2) |
| A30 | Hypodermal cell shape |
| A31 | Hypodermal cell wall thickness (µm) |
| A32 | Vascular bundle size |
Shape analysis. Leaves were flattened, avoiding damaging the tissue as much as possible. We obtained digital photographs and adjusted them to a resolution of 600 ppi. We fitted a point model template of 35 points to each of the leaf shapes: two points at the basal end of the blade, one point at the apex, and 16 points evenly distributed at each side of the blade (Figure 7A). A shape analysis was done using the Shape Model Toolbox in Matlab environment, with procrustes for size, translation and rotation. Based on principal component analyzes (PCA), accessions were characterized for quantitative comparisons. (Langlade et al. 2005, Rosas et al. 2010).
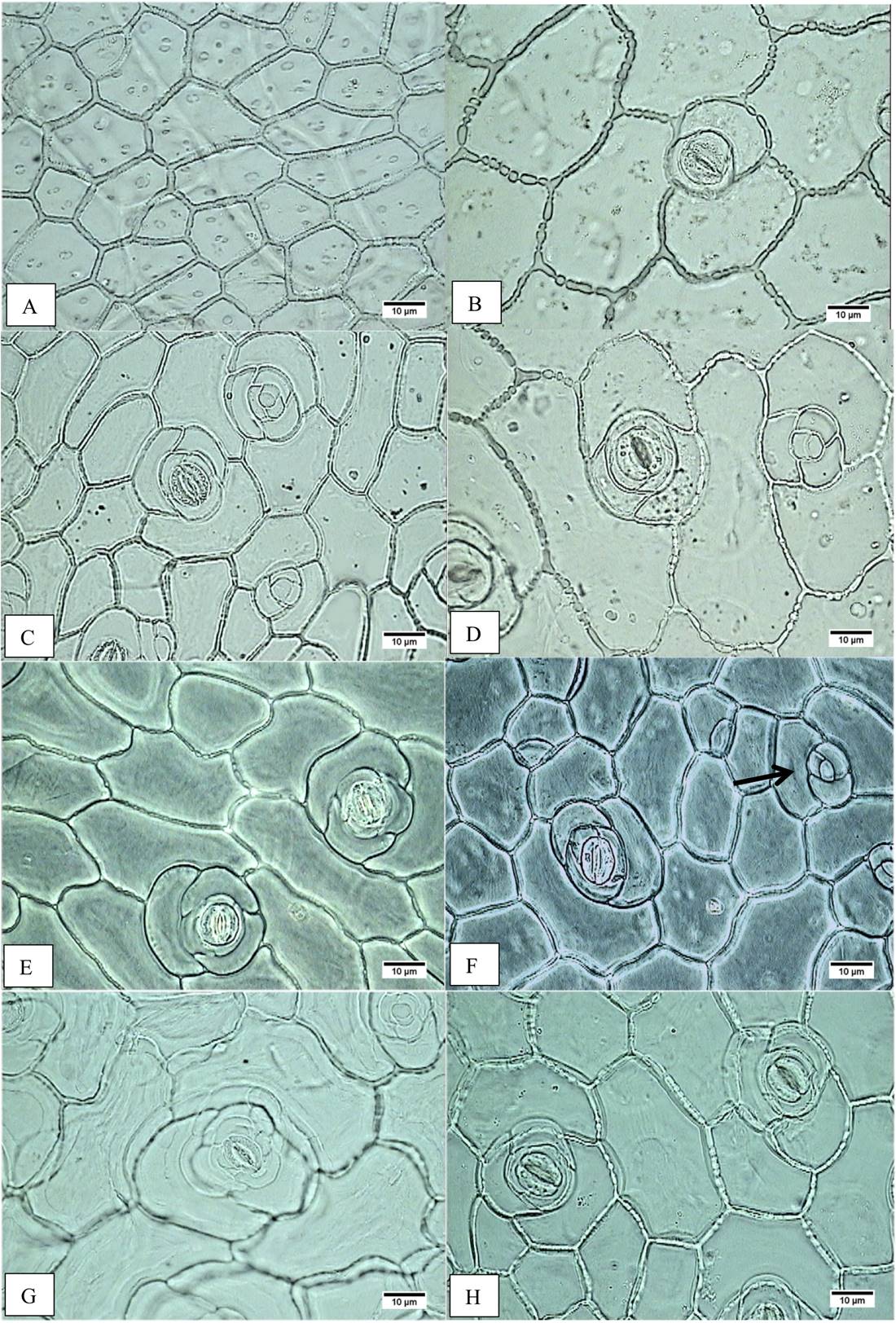
Figure 2 Epidermal cell area. A) Accession JE5599, adaxial epidermis, minor cell area. B) Accession JE6693, adaxial epidermis, major cell area. C) Accession JE6787, abaxial epidermis, minor cell area. D) Accession JE6693, abaxial epidermis, major cell area. Occlusive cell length. E) Accession JE5692, adaxial epidermis, shorter cell length. F) Accession JE5609, adaxial epidermis, longer cell length; cells in rosette (arrow). G) Accession JE5692, abaxial epidermis, shorter cell length. H) Accession JE5151, abaxial epidermis, longer cell length.
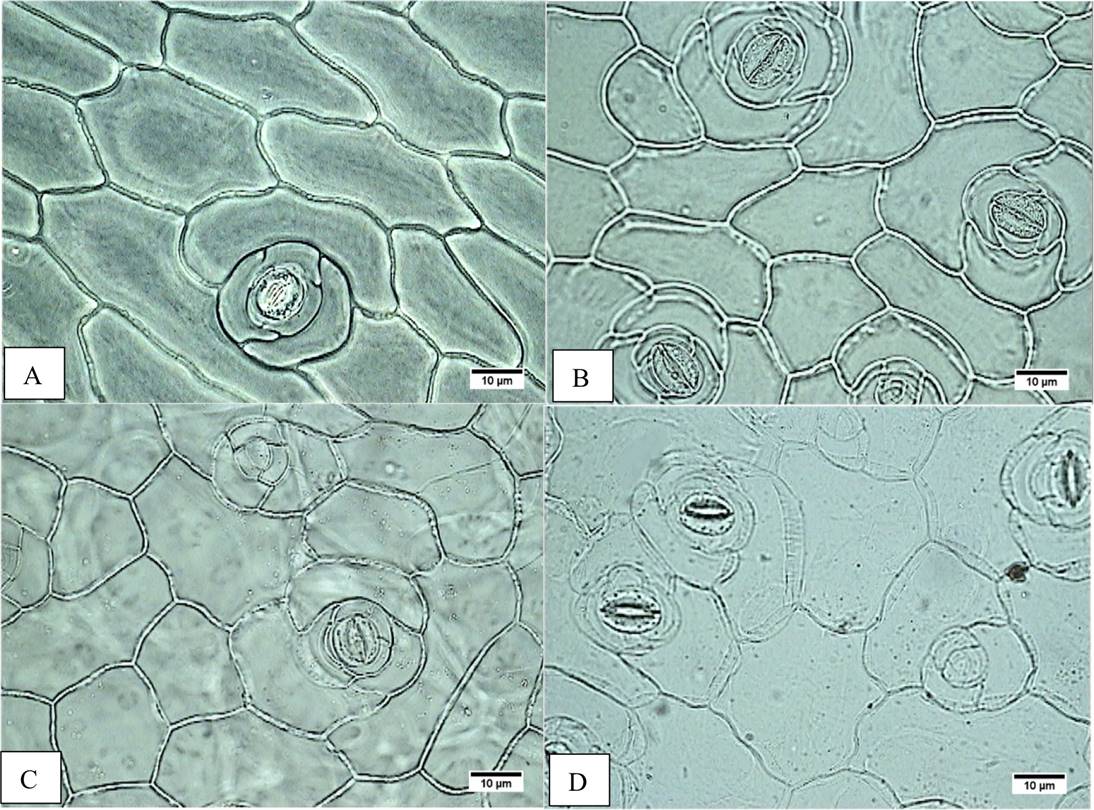
Figure 3 Stomata frequency. A) Accession JE5609, adaxial epidermis, lower frequency. B) Accession JE3914, adaxial epidermis, major frequency. C) Accession JE5599, abaxial epidermis, lower frequency. D) Accession JE5609 epidermal abaxial, major frequency.
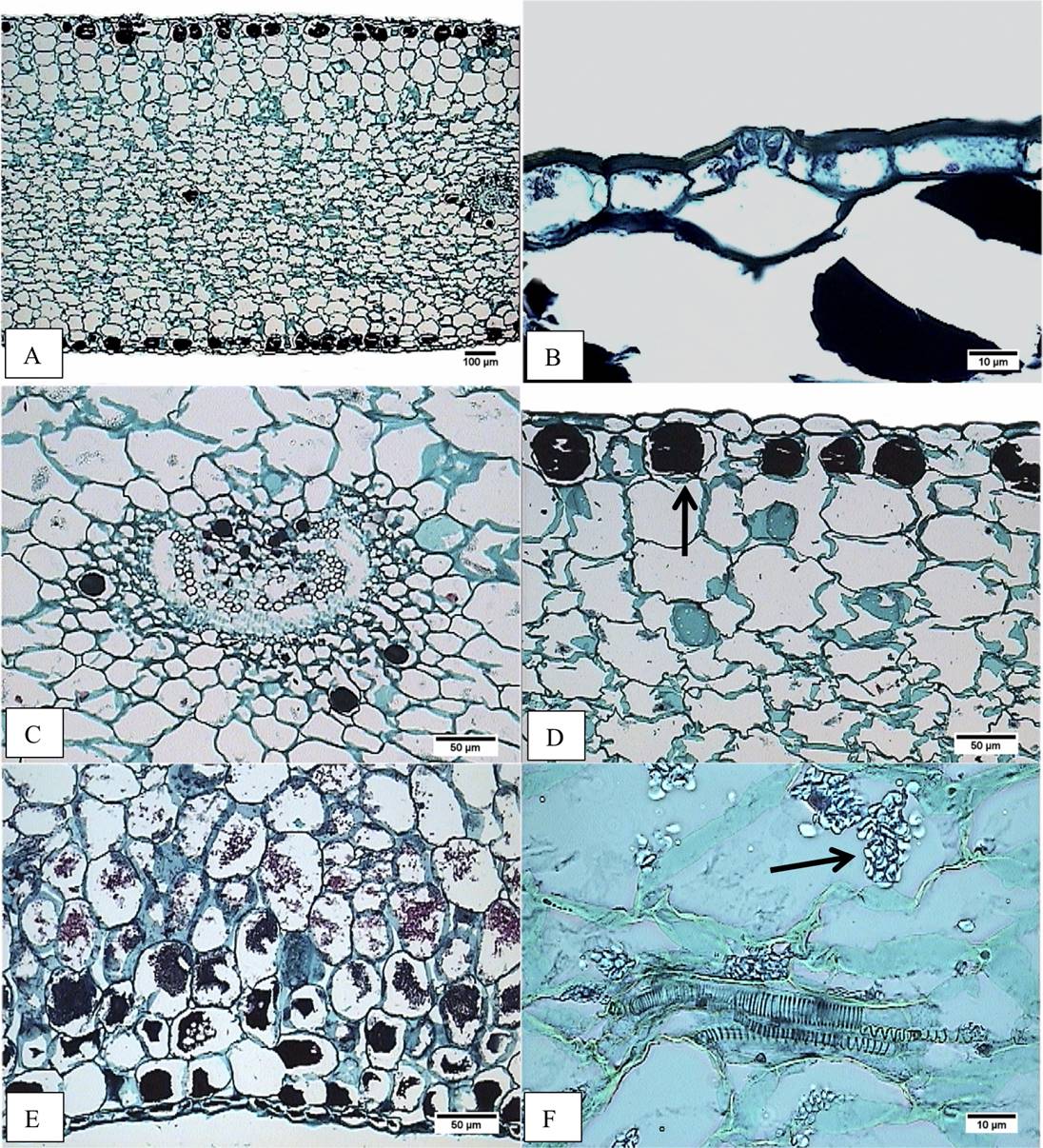
Figure 4 Cross-section of the middle region of the leaf blade. A) Accession JE6693. Heterogeneous mesophyll. B) Accession JE5692. Stoma level. C) Accession JE5692. Amphicribal vascular bundle. D) Accession JE6693. Hypodermis with tannins (arrow). E) Accession JE3914. Tannins in parenchyma, abaxial side. F) JE3914. Vascular bundle with tracheids and starch in parenchyma (arrow).
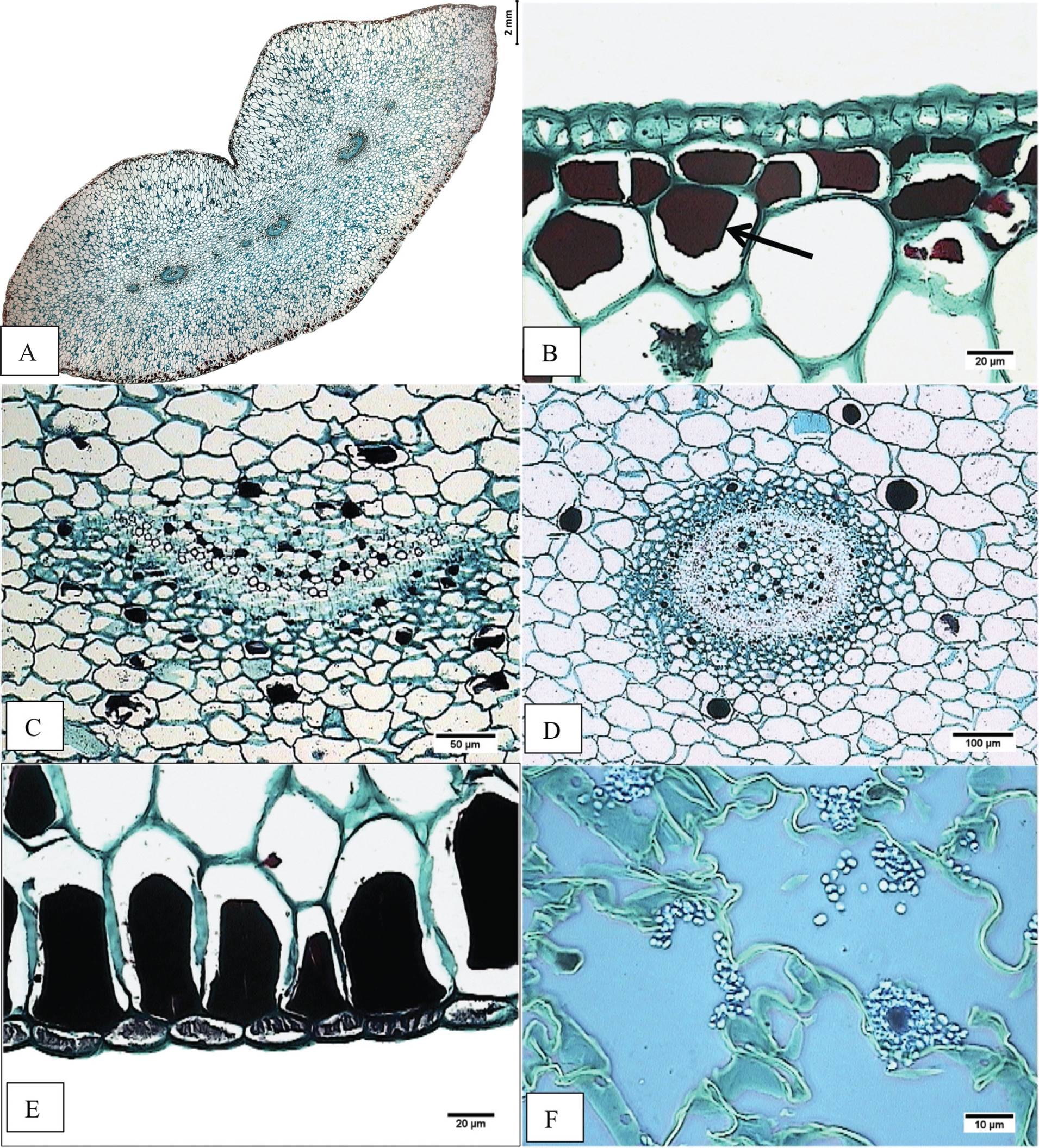
Figure 5 Cross-section of basal region of the leaf blade. A) Accession JE5151. Panoramic view. B) Accession JE3914. Epidermis, adaxial hypodermis with tannins (arrow). C) Accession JE5609. Vascular collateral bundle surrounded by tannins. D) Accession JE6589. Amphicribal vascular bundle. E) Accession JE6589. Tannins as cellular contents in abaxial hypodermis. F) Accession JE6693. Starch in mesophyll (arrow).
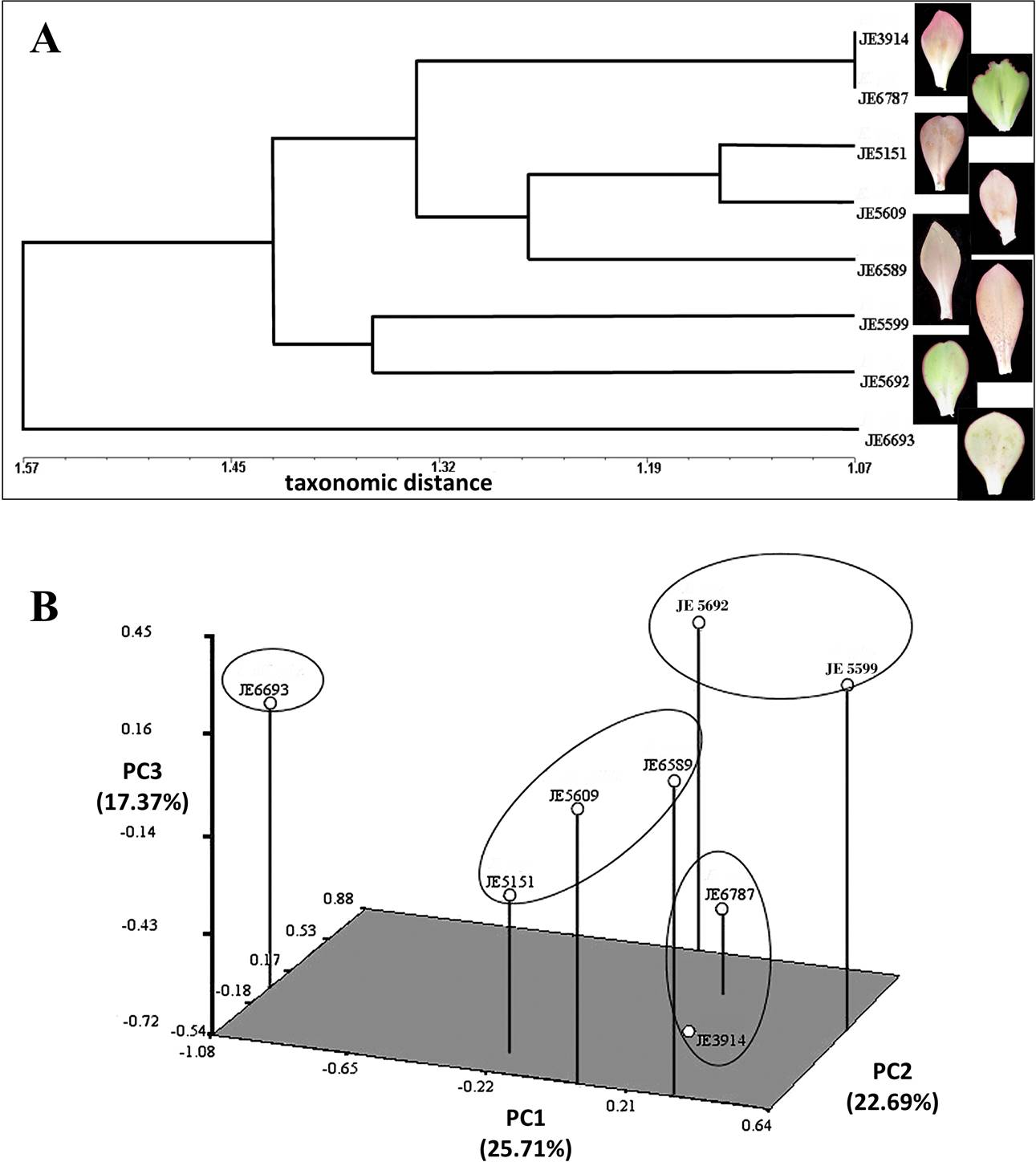
Figure 6 Multivariate analysis of quantitative anatomical characters. A) Scatter plot of points in three-dimensional space of cluster analysis by Average Taxonomic Distance, based on 32-standardized character averages (continuous and discrete) from the accessions. B) Grouping by similarity, evaluated from the 32 characters (continuous and discrete).
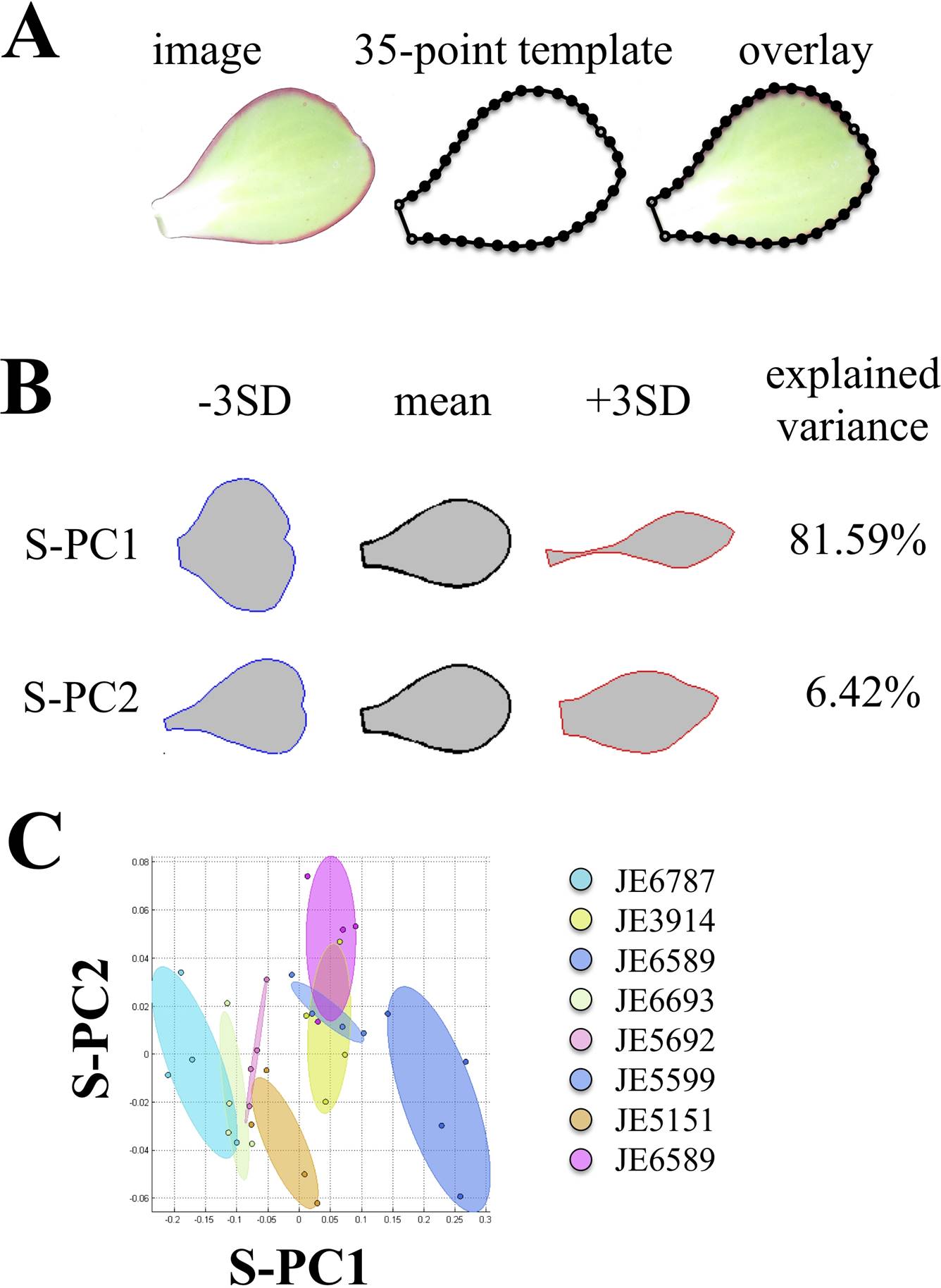
Figure 7 Quantitative variation of leaf shape using geometric morphometrics. A) 35-point model construction. Open circles: primary points. Filled circles: secondary points. B) Shape model results, capturing > 85 % of the variation, prior removal of size component. ± SD Standard deviation. S-PC: Shape Principal Components.
Statistical analysis. The mean and standard deviation were calculated for the quantitative characters. Means were compared with one-way ANOVA, with 8 levels (accessions) and post-hoc Tukey test (p < 0.05). A cluster analysis was performed to determine the similarity between taxa. A Principal Component Analysis (PCA) was also performed to identify the subset of characters that determine spatial distribution of taxa. For both multivariate analyses we used the original quantitative and qualitative characters coded as binary data and applied a linear standardization method to the data. For the cluster analysis, a similarity matrix was performed through the Taxonomic Distance Index and the average linkage (UPGMA). Analyzes were performed with the statistical packages JMP v.7 (SAS Institute, Inc.) and NTSYSpc v.2.21 respectively. Taking together the 20 quantitative characters, and the two most relevant PCs from the geometric morphometric analysis, we performed a pair-wise Pearson correlation analysis in R, and highlighted significant correlations between anatomy and shape.
Results
To characterize the extent of anatomical variation in E. aff. gigantea, we performed 10-15 µm permanent anatomical sections, and assessed 32 traits. 12 qualitative and 20 quantitative traits characterized from seven accessions of E. aff. gigantea, and the related species E. gibbiflora for comparison are shown in Table 1.
On the leaf, the middle and basal leaf area ranges from 42.68 to 97.31 cm2 on the E. aff. gigantea accessions, whereas it is 47.33 cm2 in E. gibbiflora. In surface view (Figure 2A-H), the dermal tissue the adaxial epidermal cells are polygonal and range from 197.93 to 644.47 µm2 in area in E. aff. gigantea, compared to 346.45 µm2 in E. gibbiflora. The anticlinal walls are straight and 2.5 µm thick in all accessions. The leaves are amphistomatic and the stomata are anisocytic with three adjacent cells to the guard cells and are randomly oriented. The latter are 30.75 to 40.13 µm in length in E. aff. gigantea accessions, compared to 31.88 µm in E. gibbiflora. Adaxial stomatal frequency ranges from 0.90 to 2.75 in E. aff. gigantea accessions, compared to 1.80 in E. gibbiflora (Figure 3). Some cells are arranged in rosettes among the ordinary epidermal cells (Figure 2F).
In surface view, the abaxial epidermal cells also are polygonal ranging in size from 196.62 µm2 to 702.24 µm2, as compared to 290.69 µm2 in E. gibbiflora. Cells have straight anticlinal walls 2.5 µm thick. Stomata are similar to those observed in the adaxial surface, with guard cells of 28.50 µm to 36.75 µm long in E. aff. gigantea, compared to 32.25 µm long in E. gibbiflora. Stomatal frequency ranges from 3.15 to 6.50 in E. aff. gigantea accessions, compared to 6.15 in E. gibbiflora (Figure 3). In the adaxial epidermis, some cells arranged in rosettes (Figure 3C, D). In general this indicates that epidermal traits are not useful to differentiate the range of variation observed in E. aff. gigantea accessions, from the studied E. gibbiflora accession.
In cross-section the epidermis is simple (Figure 4A,B, 4D,E); the size of adaxial epidermal cells is similar in both, the middle and the basal region of the leaf: for E. aff. gigantea it ranges from 53.90 to 106.79 µm in width, and 33.64 to 55.63 µm in height; meanwhile cell size in E. gibbiflora shows differences in both the middle (65.96 µm width and 45.33 µm height) and the basal region (85.88 µm width and 37.20 µm height). The outer periclinal wall of the epidermal cells is convex to slightly flat, about 5 µm thick in four accessions of E. aff. gigantea and E. gibbiflora, but in other E. aff. gigantea accessions (JE3914, JE5692 and JE6693) is thicker (7.5 µm). Stomata have outer cuticular ledges. The guard cells are at the same level as the rest of epidermal cells (Figure 4B), except in the JE6693 E. aff. gigantea accession where the guard cells are slightly sunken in the adaxial epidermis.
Regarding the fundamental tissue, the hypodermis has 1 to 3 layers, whose cells have an oblong shape (Figure 4A, D), except in E. aff. gigantea (JE5692 and JE6787) whose cells are isodiametric. The adaxial hypodermal cells area is 8,060.51 to 17,013.21 µm2 in E. aff. gigantea accessions compared to 6,351.31 µm2 in E. gibbiflora. The abaxial hypodermal cells area is 8,409.80 to 17,288.61 µm2 in E. aff. gigantea, compared to 5,705.33 µm2 in E. gibbiflora. In the basal region (Figure 5B, 5E), the adaxial hypodermal cell area is consistently wider (8,040.28 to 23,028.41 µm2) in E. aff. gigantea accessions than in E. gibbiflora (21,579.25 µm2). Nevertheless, the abaxial hypodermal cell area in E. aff. gigantea accessions (9,280.70 to 21,631.34 µm2) does contain what is observed in E. gibbiflora (9,461.82 µm2). The hypodermal cells have thin walls in all E. aff. gigantea accessions and E. gibbiflora and are similar in thickness to the walls of the mesophyll cells, except in E. aff. gigantea accessions JE3914, JE5599 and JE6787, with thicker walls. The mesophyll has oblong spongy parenchyma cells, with intercellular spaces near the epidermis (Figure 4A). The summary of 20 quantitative anatomical traits is shown in Appendix 1.
In regards to the vasculature, vascular bundles are usually collateral (Figure 4C), but in E. aff. gigantea accessions JE3914 and JE6787, vascular bundles were amphicribral, and those at the basal region are larger and amphicribral (Figure 5D). The main vascular bundles are usually located in the middle region of mesophyll, while the higher order bundles are dispersed. Vascular bundles have different sizes; two sizes in some accessions (JE5151, JE5609, JE5599, JE5692, and JE6693), four sizes in other accessions including E. gibbiflora (JE3914, JE6787 and JE6589). Vascular bundles are distributed within several layers of the fundamental tissue. There is a collenchymatous sheath surrounding the vascular bundles, except in E. aff. gigantea accession JE5609.
Starch and tannins are in parenchyma cells (Figure 4, 5). Starch is scarce in E. aff. gigantea accessions JE5599 and JE5692, but abundant in the rest of the accessions. Starch is always present in the mesophyll, while the location of tannins is variable throughout the parenchyma cells. Tannins are abundant in all accessions, are distributed in epidermis, hypodermis and around vascular bundles, except in E. aff. gigantea accessions JE6693 and JE6787. In JE6693 tannins were present only in the hypodermis, while in JE6787 tannins were present in epidermal cells and around vascular bundles (Figure 4C). This indicates that the distribution and amount of tannins might be useful to distinguish some of the accessions in E. aff. gigantea.
To identify the quantitative variation within E. aff. gigantea accessions, we performed a 1-way Analysis of Variance (ANOVA), including the 7 accessions of E. aff. gigantea and E. gibbiflora (related species) as one factor, with 8 levels in each of the traits (Appendix 1). The ANOVA showed that 7 traits have no significant differences. These are, at the middle region of the leaf blade, the height of adaxial epidermal cells (A12), the area of the abaxial hypodermal cells (A16), and the diameter of vessels in the midrib (A20). At the basal region of the leaf blade, the width (A22) and height (A23) of the adaxial epidermal cells, the width of abaxial epidermal cells (A25), and the area of abaxial hypodermal cells (A29) also have no significant differences. The result suggests that these 7 traits are robust among the analyzed accessions. Meanwhile, 13 of the traits showed significant differences in at least one of the levels. Among them, the area and width of adaxial epidermal cells (A04), plus the height of the abaxial epidermal cells (A14), which were the three most variable characteristics in our studied accessions (Appendix 1). It should be noted that none of the characteristics showed significant differences between the E. gibbiflora (JE6589) and any of the E. aff. gigantea accessions, demonstrating the large overlap in anatomical characters between the two species. Moreover, one of the accessions in E. aff. gigantea (JE6693), showed larger significant values in four traits (i.e. area and width of the cells in both adaxial and abaxial epidermis [A04, A07, A11, and A13]), when compared to the rest of the E. aff. gigantea accessions (Appendix 1). Except for the height of abaxial epidermal cells and the area of adaxial hypodermic cells, the other characters of the basal region are homogenous. From this we infer that the middle region of the leaf is highly variable among the accessions of E. aff. gigantea, and probably not suitable for identifying diagnostic characteristics.
We performed a multivariate analysis to observe how the accessions cluster according to their anatomical features. A conglomerate analysis showed the conformation of two groups (Figure 6A). Additionally, one of the E. aff. gigantea accessions (JE6693) has a position external to the rest of the accessions. These two groups are related by a distance of 1.42. The largest group is defined by a distance of 1.34 and includes E. aff. gigantea accessions JE3914, JE5151, JE5609, JE6787, and E. gibbiflora (JE6589). This group is divided into two subgroups: the first defined at a distance of 1.07, and includes two accessions of E. aff. gigantea (JE3914 and JE6787). At the level of the middle region of the blade, these accessions share similar values of abaxial occlusive cell length (A05), abaxial epidermal cell area (A07), adaxial and abaxial epidermal cell width (A11 and A13), adaxial hypodermal cell area (A15) and adaxial-abaxial epidermal cell width (A22 and A25). They also share higher adaxial epidermal cell height (A23) in the basal region. The second subgroup is defined at a distance of 1.26, which contains two E. aff. gigantea accessions (JE5151 and JE5609) and the E. gibbiflora accession (JE6589). The first two form a subset at a distance of 1.15, and share similar features in the middle region, such as abaxial epidermal cell area (A07), adaxial epidermal cell width (A11) and adaxial hypodermal cell area (A15). These two E. aff. gigantea accessions joined with the E. gibbiflora accession (JE6589), at a distance of 1.26, because of the similarities on the middle region of the leaf, such as the abaxial epidermal cell area (A07), the adaxial and abaxial epidermal cell width (A11 and A13), the adaxial and abaxial epidermal cell height in the basal region (A23 and A26).
The second group is defined by a distance of 1.36 and includes two accessions of E. aff. gigantea (JE5599 and JE5692). They share similarities on the adaxial occlusive cells length (A02), stomatal index on the abaxial surface (A06), width of adaxial and abaxial epidermal cells (A11 and A13), vessel diameter in the mid-vein in the middle region (A20), and width of adaxial epidermal cells in the basal region (A22). Finally, the E. aff. gigantea accession JE6693 detaches from the two main branches (Figure 6A) at a distance of 1.57. Characters unique to JE6693 are adaxial and abaxial epidermal cell area (A04 and A07), and adaxial and abaxial epidermal cell width (A11 and A13), all of them from the middle region.
To understand the main trends of overall variation among all the traits within and between accessions, we performed the multivariate approach Principal Component Analysis (PCA). The first three components explained 65.77 % of the variation. PC1 explained 25.71 %, PC2 22.69 %, and PC3 17.37 % (Table 3). According to the Principal Component loads, PC1 is explained by the adaxial and abaxial epidermal cell area (A04 and A07), the abaxial epidermal cell width at the middle portion (A14), and the abaxial epidermal cell width at the basal portion (A25). On the other hand, PC2 is explained by the adaxial hypodermic cells area (A15), the hypodermal cells shape (A17), the outer periclinal wall thickness of the abaxial epidermal cells (A27), and the hypodermal cell wall thickness at the basal portion (A31). PC3 is explained by the adaxial stomatal index (A03), the type of vascular bundles (A19), the number of strata of colenquimatous sheath (A21), and the adaxial epidermal cell width at the basal portion (A22) (Table 3). This is visualized in a three-dimensional plot with the projection of the OTUs (Operational Taxonomic Units) in the PC1, PC2 and PC3 respectively (Figure 6B). In this plot the distances between accessions is an estimate of their similarity. This analysis confirms the clustering of the six E. aff. gigantea accessions JE3914, JE5151, JE5599, JE5609, JE5692, and JE6787, grouping together with E. gibbiflora (JE6589). Meanwhile we observed that the adaxial and abaxial epidermis cell area (A04 and A07 respectively) have the largest PC1 loads (Table 3), and distinguish JE6693 from the rest of the E. aff. gigantea accessions (Figure 6B).
Table 3 Principal component loads per character. Highest loads are specified in bold. Character IDs are shown in Table 2.
| Character ID | PC 1 (25.71 %) |
PC 2 (22.69 %) |
PC 3 (17.37 %) |
|---|---|---|---|
| A01 | 0.1789 | 0.5922 | 0.2644 |
| A02 | -0.4891 | -0.5269 | -0.2785 |
| A03 | -0.1916 | 0.2321 | -0.6809 |
| A04 | -0.9245 | 0.0496 | -0.0484 |
| A05 | -0.3951 | -0.7360 | -0.1972 |
| A06 | 0.3180 | -0.5163 | -0.3181 |
| A07 | -0.8986 | -0.1103 | 0.3645 |
| A08 | -0.2585 | 0.5494 | 0.6493 |
| A09 | -0.4938 | 0.5618 | -0.1268 |
| A10 | -0.3819 | 0.7490 | -0.4068 |
| A11 | -0.7609 | -0.1344 | 0.5532 |
| A12 | -0.6171 | -0.0073 | -0.0638 |
| A13 | -0.7863 | -0.2360 | 0.4229 |
| A14 | -0.6731 | -0.2949 | -0.2521 |
| A15 | -0.2830 | 0.8434 | -0.1975 |
| A16 | -0.5172 | 0.6965 | -0.2691 |
| A17 | 0.0900 | 0.8081 | -0.0551 |
| A18 | 0.5611 | 0.2943 | -0.4742 |
| A19 | 0.2376 | 0.2898 | -0.8629 |
| A20 | 0.4724 | 0.2576 | 0.1600 |
| A21 | 0.0881 | 0.0506 | -0.9117 |
| A22 | 0.0757 | 0.5127 | 0.8065 |
| A23 | -0.4425 | 0.5568 | -0.4372 |
| A24 | 0.6523 | 0.3182 | -0.2133 |
| A25 | -0.5869 | 0.0448 | -0.2424 |
| A26 | -0.7935 | -0.0670 | -0.4750 |
| A27 | 0.4840 | 0.0729 | 0.1025 |
| A28 | -0.3319 | 0.2026 | 0.3608 |
| A29 | -0.6035 | 0.5088 | -0.0786 |
| A30 | -0.0276 | 0.7482 | 0.3573 |
| A31 | 0.4291 | 0.7579 | 0.2484 |
| A32 | 0.2424 | -0.4570 | 0.2623 |
In order to obtain insights into the shape variation on leaves of the analyzed accessions, we dissected 4 leaves from each of the accessions. Using the point data (Figure 7A), we performed procrusted analyses, including size, as the accessions display large variation of this trait. The procrusted data points were subject of Principal Component Analysis, which gave two main Principal Components, capturing 88.1 % of the variation (Figure 7B). The first Shape Principal Component (S-PC1) seems to capture the variation regarding the roundness-sharpness of the leaf (Figure 7B). The second Shape Principal Component (S-PC2) seems to capture whether the leaf apex is acute or emarginate, as well as the width of the leaf base is cuneate or spatulate (Figure 7B). Other Shape Principal Components captured very little variation and were mainly related to slight leaf orientations when plants were photographed. Together, S-PC1 and S-PC2 can be used as morphological traits to evaluate the leaf shapes in each of the accessions (Figure 7C). Moreover, it is worth underscoring that we removed the size effect in this analysis.
To better understand the relationships between anatomical characteristics and their potential relationship with shape characteristics, we performed a pairwise-correlation analysis (Appendix 2). As expected, several anatomical characters showed significant correlations, mainly those that were related to aspects of the epidermis. As an example, there is a correlation between the abaxial epidermis cell area (A07) and the width of the adaxial epidermis cells (A11) (Pearson correlation coefficient = 0.95, p < 0.0001), reinforcing that these two characteristics are part of the same tissue system: the epidermis. Another example is the hypodermal cell area on the abaxial side of the lamina (A16), which was correlated to the same character but at the base of the leaf (A29, Pearson correlation coefficient = -0.92, p = 0.001). This also indicated that our sampling method and measurement of character is reproducible regardless of the leaf area (middle or basal), also in addition to showing the tight and robust control of cell size along the leaf regions.
On the other hand, we observed unexpected correlations for characters that are not directly connected. For instance, the cell area on the adaxial epidermis and the vessel diameter on the mid vein show a negative correlation (Pearson correlation coefficient = -0.73, p = 0.039), suggesting developmental and physiological constraints yet to be understood.
We also analyzed the correlations between anatomy characteristics and leaf shape characteristics (S-PC1 and S-PC2). There were three significant correlations between the anatomy and the shape of the leaf (Appendix 2). The first two were related to the S-PC1, which explained the roundness-sharpness of the lamina (Figure 7B). This characteristic was negatively correlated to the area of hypodermal abaxial cells on the lamina (A16, Pearson correlation coefficient = -0.91, p = 0.001) and area of hypodermal abaxial cells at the leaf base (A29, Pearson correlation coefficient = -0.81, p = 0.013). This indicates that, in the analyzed accessions, rounder leaves have larger hypodermal cells at the lamina and the base. S-PC2 explained the shape of the apex and the base of the leaf (Figure 7B), which was negatively correlated to the height of adaxial epidermal cells on the lamina (A12, Pearson correlation coefficient = -0.81, p = 0.014). In other words, plants with a sharper apical end, but wide basal end, have shorter epidermal cells. Together, these results suggest that cell shape and size at the tissue level might have an impact on the whole organ leaf morphology within these accessions.
Discussion
From the analysis of variance of the 20 quantitative characters in the accessions, it is clear that some features are robust among the regions of the leaf or the accessions, while other characters are highly variable. 65 % of these characters are heterogeneous among the accessions of E. aff. gigantea, while 35 % are robust. Since the analyzed accessions are morphotypes of the same species, and plants were kept in a greenhouse for more than five years, we can expect a greater percentage of homogeneous characters; however, our results show a different picture. This suggests that some of the anatomical traits are genetically determined among morphotypes in E. aff. gigantea accessions.
Based on the geographical origin of the accessions, we made some observations. The accessions JE5692 and JE6787 of E. aff. gigantea correspond to samples from locations less than 5 km apart and both of them grow along the Zongolica Mountain Range, characterized by xerophytic shrubs and oak trees. In consequence, the accessions have similar morphologies, including the shape of the rosette, the pigmentation of the leaves, crenulated leaves, and the structure of the flower. Nevertheless, 50 % of the anatomical features are different between the two accessions, to such an extent that the multivariate analysis shows them to be in different branches of the tree (Figure 6A). Meanwhile, there are other traits that do not change. In other words, these traits are robust despite other differences. Our observations raise questions pertaining to the origin of the variation between accessions, adaptions, and the role of the environment shaping the characters.
On the accession JE6693 another peculiarity was observed. Multivariate analysis showed the largest anatomical differences when compared to the rest of the accessions, mapping to a separate branch in the phenogram (Figure 6A). This could be explained by the history of the site of origin. The location was a pre-Columbian archaeological site previously occupied by the Popoloca civilization, and currently a very dry environment. It is possible that the accession was introduced by the Popoloca civilization and the plant survived, once the city was abandoned (Castellón-Huerta 2006, Rivas-Castro 2003). The anatomical features in our study suggest that this accession does not belong to the E. aff. gigantea species, or that anatomical features have been modified because of anthropogenic management.
Regarding stomata, it was found that the occlusive cells of the adaxial epidermis tend to be longer than those of the abaxial epidermis. This is frequently seen in some other angiosperms (Dickison 2000); nevertheless, E. aff. gigantea accession JE5599 has shorter occlusive cells and low stomata frequency in the adaxial epidermis. This accession grows at the western end of the state of Oaxaca, in a Pinus-Quercus forest, in a shaded environment, with high annual precipitation (1,010.8 mm) and low annual temperature (16.7 °C), however located in a xerophytic enclave devoid of moisture, thus explaining their anatomical features. On the other hand, the E. aff. gigantea accession JE5692, on the abaxial side has shorter occlusive cells. It grows in the southern part of the state of Puebla, in altered rocky dry sites, in a semi-dry micro-environment, with a very low annual precipitation (378.5 mm) and high annual temperature (23 °C). This suggests that short size of the occlusive cells and the low stomata frequency could be adaptive to prevent excessive water loss (Willmer & Fricker 1996) in these accessions.
Caballero-Ruano & Jiménez-Parrondo (1978) report that some other species of Crassulaceae, such as Aeonium, Aichryson, Monanthes and Greenvia have glandular trichomes, and non-glandular trichomes as in Kalanchoe pumila (Chernetskyy & Weryszko-Chmielewska 2008). In the analyzed Echeveria accessions, no trichomes were observed, however to verify their absence as adiagnostic taxonomic feature, it is suggested to further characterize other species. In our studied Echeveria accessions, leaves are amphistomatic and the stomas are anisocitic. This was previously observed in several species of Kalanchoe (Chernetskyy & Weryszko-Chmielewska 2008, Czernecki 2006, Inamdar & Patel 1970, Sharma & Dunn 1968) and Sedum (Ardelean et al. 2009). Stomata placed on both sides of the leaf are typical of xerophytic plants (Chernetskyy & Weryszko-Chmielewska 2008, Rotondi et al. 2003).
Amphistomic leaves could be adaptive in both species with thick leaves, and species with high photosynthetic rates (Parkhurst 1978, Mott et al. 1982). This could be due to ecotype variations among different individuals, or adaptations of single leaves to differential light stress (Mott & Michaelson 1991). Although our studied accessions came from the field, and remained in a greenhouse for two years, they seemed to have maintained the amphitomy; however, the stomatal frequency is greater on the abaxial epidermis. This difference is consistent with what has been reported in leaves of Ambrosia cordifolia (A. Gray) W.W. Payne (Compositae), where they cultivated plants under regimes of high and moderate light intensity (Mott & Michaelson 1991). On the other hand, there seems to be a positive correlation between altitude and stomatal frequency in couple of accessions of E. aff. gigantea (JE5609 and JE3914), given that both have high abaxial stomatal frequency and location of origin higher than 2,400 m a.s.l. This same positive correlation has been observed in the Asteraceae species Oyedaea verbesinoides DC. (García & Lapp 2005).
Nevertheless, stomata frequency is higher in the abaxial epidermis. This same difference occurs in Aichryson (Caballero-Ruano & Jiménez-Parrondo 1978), Kalanchoe fedtschenkoi (Sharma & Dunn 1968) and Kalanchoe pumila (Chernetskyy & Weryszko-Chmielewska 2008), whereas in other taxa such as Aeonium, Monanthes and Greenvia, the abundance of stomata is similar in both epidermis; in these five genera the stomas are also anisocytic, with external cuticular ridges (Caballero-Ruano & Jiménez-Parrondo 1978, Chernetskyy & Weryszko-Chmielewska 2008).
On the epidermis, the presence of rosette-shaped cells was observed. We report this for the first time in Echeveria. Chernetskyy & Weryszko-Chmielewska (2008) mentioned that the epidermis of Kalanchoe pumila contains stomata in various developmental stages, so we infer that possibly the rosette-shaped cells in Echeveria might be developing stomata as well.
In all of the accessions, large epidermal cell size was observed. E. aff. gigantea (JE6693), which is located outside the conglomerate (Figure 6B), is distinguished by having the largest anatomical estimates between the analyzed accessions. The presence of large epidermal cells could translate into a larger evapotranspiration area, which might allow for the decrease of the temperature at the surface and towards the inside of the leaf, and therefore might provide tolerance to high temperatures and high insolation. It is known that attributes of the epidermal cells, together with stomata distribution can facilitate the decrease of the inner temperature of the leaf by 10 to 15 °C lower than the surrounding air temperature (Willmer & Fricker 1996, Taiz & Zeiger 2010). It should be noted that this accession comes from an altitude of 1,640 m a.s.l., which is a xerophyte scrub, with a relatively high annual temperature (21.5 °C) and low annual rainfall (450 mm). Thus, we suggest that E. aff. gigantea accession JE6693 might not resemble the rest of the E. aff. gigantea accessions because of the rather extreme environment that it inhabits. Alternatively, this accession might belong to another taxonomic group.
The presence of hypodermis is a constant in our studied accessions, however E. aff. gigantea JE5692 has a significantly larger hypodermic cell area. The presence of large cells allows for larger water storage (Dickison 2000, Sandoval-Zapotitla et al. 2010). This accession comes from a semi-dry environment, with high annual temperature (23 °C) and low annual precipitation (378.5 mm), which could explain the large size of hypodermic cells in the accession JE5692.
Our study shows that vasculature in Echeveria is poorly developed, which was also observed in Kalanchoe pumila and plants of Sedum (Chernetskyy & Weryszko-Chmielewska 2008, Ardelean et al. 2009). The position of the vascular bundles in the mesophyll follows a pattern: the larger vascular bundles are located in the central part of the mesophyll, and the smaller bundles are located towards the ends. A similar pattern has been reported for several species of Aichryson and for Monanthes brachycaulon (Caballero-Ruano & Jiménez-Parrondo 1978). Interestingly, the E. aff. gigantea accession JE6693, and other Crassulaceae have vascular bundles scattered in the mesophyll. It is suggested that a broadly branched vascular system, even with small vascular bundles, allows for faster and more efficient transmission of water to all cells of the mesophyll (Caballero-Ruano & Jiménez-Parrondo 1978). Hence, broadly branched vasculature might give an adaptive advantage, as the accession JE6693 inhabits a warm xerophyte scrub on disturbed limestone soils with low precipitation and high temperature.
The presence and abundance of the taniniferous compounds constitute a mechanism for the plant to avoid foliar desiccation, as suggested for Quercus and Pistacia (Fahn 1969). All the studied Echeveria accessions have tannins, although there were differences in the amounts and distribution within the tissues. These were observed in epidermis, hypodermis, mesophyll and closely associated with the vascular system; these phenolic compounds are also present in other groups of the Crassulaceae, and their location is related to certain functional aspects (Rost 1969, Caballero-Ruano & Jiménez-Parrondo 1978, Chernetskyy & Weryszko-Chmielewska 2008). Tannins have been important to distinguish species in Saxifragaceae (Stern et al. 1970); however, they are absent in some species of Monanthes, and this may be because they live in more humid places (Caballero-Ruano & Jiménez-Parrondo 1978). We speculate that tannins might be useful to distinguish accessions within the E. aff. gigantea species.
Amphicribal vascular bundles are uncommon in Angiosperm leaves, but common in Pteridophytes, the medullary bundles of Menmbryanthemum (Aizoaceae), Begonia (Begoniaceae) and Rumex (Polygonaceae). They are also observed in flowers and fruits of Angiosperms. The E. aff. gigantea accessions JE3914 and JE6787 have amphicribal vascular bundles, visible in the middle region of the leaf. Nevertheless Caballero-Ruano & Jiménez-Parrondo (1978) report that in other Crassulaceae such as Aeonium canariense, A. lindleyi and A. haworthii, the vascular bundles in the middle leaf region are collateral, whereas at the basal region they are amphicribal, suggesting that dimorphic vascular bundles are more common in Crassulaceae than previously believed. A more complete survey of vascular bundles in the family is ideal to determine their developmental bases, spatial distribution, and their relationship to organ shape.
The morphometric differences found between the different accessions of E. aff. gigantea suggest the potential presence of genetic variation and/or phenotypic plasticity, which could occur simultaneously (Schlichting & Piggliucci 1998). In E. aff. gigantea, characters such as height of adaxial epidermal cells, area of abaxial hypodermic cells, diameter of vessels in the middle vein, width and height of adaxial epidermal cells, width of abaxial epidermal cells, and area of abaxial hypodermic cells, are characters whose variation does not show significant differences between the accessions. This potential robustness might be genetically determined. These characters are more robust at the base than at the middle of the leaf. We found that a set of characters are highly variable. However, a third alternative is that our observed robustness or variability is not due to natural variation or phenotypic plasticity, but rather the age of the plant, as was previously seen in Yucca capensis seen in (Arteaga et al. 2015).
In conclusion, E. aff. gigantea is a species characterized by its wide morphological variation. Here we presented a detailed analysis of the anatomy on some accessions within the species. Although most of the characteristics analyzed were highly variable, several robust characteristics were also found. Leaves in these accessions displayed xeromorphic microstructure, with thickening of the periclinal wall on epidermal cells, slightly sunken stomata, multi-layered hypodermis with thickened cell walls, aquifer mesophyll, and presence of abundant tannins. All of these features reflect environmental and soil water restrictions, and allow the accessions to be highly tolerant to drought and high temperatures. This also explains why these accessions are found in sites with contrasting environmental conditions. The presence of organized rosette cells in the epidermis and the presence of hypodermis in the genus are reported for the first time. Some of the most remarkable features are the epidermis, hypodermis, type of vascular bundles and the number of strata of the collenchyma sheath in the vascular bundles. Moreover, we found anatomical traits correlated to the whole organ leaf shape, suggesting developmental relationships yet to be investigated. Finally, it is proposed to take into account these results for future anatomical studies in the genus Echeveria and to explore their taxonomic relevance.











 text new page (beta)
text new page (beta)


