Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
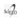 Similars in
SciELO
Similars in
SciELO
Share
Revista ALCONPAT
On-line version ISSN 2007-6835
Rev. ALCONPAT vol.5 n.3 Mérida Sep./Dec. 2015
Articles
Study on the hydration of Portland cement paste replaced with blast furnace slag, fly ash, and metakaolin: effect on the usage of two superplasticizer additives
1Universidad Autónoma de Nuevo León, Facultad de Ingeniería Mecánica y Eléctrica, Programa Doctoral en Ingeniería de Materiales, Ave. Universidad s/n, Ciudad Universitaria, San Nicolás de los Garza, Nuevo León, México, CP 66450.
2Corporación Mexicana de Investigación en Materiales, Ciencia y Tecnología No 790 Col. Saltillo 400, Saltillo, Coahuila. México, C.P. 25290.
This research was focused on assessing the impact that different replacement materials and two superplasticizers on the development of the mechanical properties, phases formation and heat evolution of portland cement pastes, with replacement levels up to 60%. For this purpose, fly ash, ground granulated blast furnace slag, metakaolin and silica fume were used. The mixtures were manufactured with a water/solid of 0.4, 0.3% of superplasticizer and cured up to 60 days. Results showed that with the use of replacement materials, the calcium hydroxide content was reduced, due to the pozzolanic reaction, and the compressive strength was increased.
Keywords: Reactivity; Supplementary cementitious materials
Este trabajo de investigación se enfocó en la evaluación del efecto que tienen diferentes materiales de reemplazo y dos aditivos superplastificantes en el desarrollo de las propiedades mecánicas, formación de fases y en la evolución del calor de hidratación de pastas de cemento portland reemplazadas hasta un 60%. Para esto se empleó ceniza volante, escoria granulada de alto horno, metacaolín y humo de sílice. Las mezclas fueron fabricadas con una relación agua/sólidos de 0.4, 0.3% de aditivo superplastificante e hidratadas durante 60 días. En base a los resultados se confirmó que con el uso de materiales de reemplazo, se redujo la cantidad de hidróxido de calcio, debido a la reacción puzolánica y se incrementó la resistencia a la compresión.
Palabras clave: Reactividad; materiales cementosos suplementarios
Esta pesquisa teve como objetivo a avaliação do efeito de diferentes adições e de dois aditivos superplastificantes no desenvolvimento das propriedades mecânicas, formação de fases e na evolução do calor de hidratação de pastas de cimento com substituição de até 60%. Para tanto foi empregada cinza volante, escória granulada de alto forno, metacaulim e sílica ativa. Os traços foram elaborados com uma relação água/sólidos de 0,4, com 0,3% de aditivo superplastificante e curadas durante 60 dias. Com base nos resultados obtidos, confirmou-se que com o uso de adições, a quantidade de hidróxido de cálcio foi reduzida, devido à reação pozolânica e elevou-se a resistência à compressão.
Palavras chave: Reatividade; materiais cimentícios complementares
1. INTRODUCTION
Currently, a great part of scientific and technological investigation is focused towards sustainable development. This involves the development of new materials characterized as eco-friendly, reducing the environmental impact of the processes involved in their acquisition, transport, production, implementation, and recycling. One of the industrial sectors most concerned with reducing this impact is construction, in particular the cement industry. The production process for cement requires a great quantity of thermic and electrical energy; furthermore, the process demands a large quantity of non-renewable raw materials, such as the use of limestone, clays and fossil fuels necessary for the production of clinker. This process emits large quantities of CO2 (827 kg CO2/t of clinker) and other gasses (such as SO2 and NOx) that cause harmful environmental impact and which contribute to global warming. The production of Portland cement (CP) emits 5 to 8% of the global CO2 [Cembureau (2014); Damtoft et al. (2008); Schneider et al. (2011)]; thus, the increase in the demand for cement and the environmental impact the elaboration process carries with it have made it necessary to opt for alternative materials and technologies. In recent years, supplementary cementitious materials (MCS) such as blast furnace slag from the steel-making process (EGAH), fly ash (CV) from the burning of coal in power plants, as well as natural or artificial pozzolans, and limestone are used for the production of compound cements. It is possible to implement different types of materials that partially replace Portland cement; however, the effect that each one has individually on the hydration process of the cement is still being researched and the effects that two or more constituents have on the reactions is still under constant investigation [Juenger and Siddique (2015)]. The replacement level, the chemical composition, the amorphous fraction, the pozzolanic or hydraulic activity, the size of the particulates, and the morphology of each material are some of the variables to be taken into account when designing compound cement pastes with more than one replacement material [Juenger and Siddique (2015)]. It is important to note that the development of the mechanical properties of compound cement pastes is directly related to the effect of the replacement materials in the hydration process of the cement pastes. For a compound cement, the hydration mechanism involves the participation of various species in the reaction in a solid state. Additionally in binary cements, Bentz et al. (2011) found that the MCS, in addition to altering the distribution of the particles of the cementitious material, also increase degassing. Yun et al. (2013) found that for mortars with 60% EGAH, the mechanical properties increased with the amorphous fraction of the same. Sharfuddin et al. (2008) reported that the addition of silica fume (HS) reduced the permeability of the concrete due to the reduction in porosity and the density of the matrix caused by the pozzolanic reaction. However, the combined use of CV and HS caused greater porosity in the cementing matrix, probably caused by the rapid reaction of the HS. In this manner, the HS must be maintained at a maximum of 10% weight of the cementing material. The concurrent addition of HS and EGAH reduced the resistance of the concrete. Furthermore, according to Ali et al. (2014), the type of additives modified the morphology of the ettringite and therefore the reaction heat. Ping et al. (2013) analyzed the content of calcium hydroxide (CH) in cements with 40% CV and 20% EGAH, reporting a 65% decrease when compared to pure CP due to the pozzolanic and hydraulic reactions. Schöler et al. (2015) concluded that the types of hydrates observed in the systems with a 50% substitution of MCS are similar to those formed in pure CP, with a reduction in the quantity of CH and an increase in the C-S-H and the AFm phases. However, if the CV has a relatively small amorphous fraction, its reactivity will diminish and thus the quantity of products formed and the compression strength. It is worth highlighting that the authors mention additions of up to 30% CV do not significantly reduce compression strength. Janotka et al. (2010) investigated the effect of different types of MK in the mechanical properties of partially replaced pastes and reported that by increasing the replacement level of MK, the compression strength of the cement pastes decreased. Snelson et al. (2008) utilized MK and CV as replacement materials for CP in order to investigate the effect in the progress of released heat. The hydration process of the CP pastes replaced with CV changed with the level of replacement, given that an increase in the CP replacement level decreased the hydration heat. The replacement materials such as CV, MK, and HS, better the performance of the CP pastes, mortars and cements, but tend to reduce the workability of the mixtures. The most common reason is that the fine particles of the replacement materials have a greater surface area and therefore the amount of water to create a mixture increases. Various authors [Mansour et al. (2010); Esteves et al. (2010)] have shown that by adding HS, CV, and MK the required water increased, thus, in order to better the workability, they employed chemical superplasticizers (SP) additives.
This article contributes investigation on the development of sustainable technologies and materials for the construction sector, with the assessment of the behavior of supplementary materials in cement paste and the analysis of the results, with the purpose of contributing parameters that make it feasible to partially replace Portland cement. It is worth mentioning that this article contributes in an important manner to the understanding of the effect of various materials on the kinetic hydration of cement, which currently is under investigation by various groups around the world.
2. MATERIALS AND METHODS
The starting materials used were: (a) Portland cement obtained from CEMEX Mexico; (b) granulated blast furnace slag (from AHMSA, with 97% amorphous fraction); (c) type F fly ash (Comisión Federal de Electricidad (Federal Electricity Commission) - Mexico); (d) metakaolin (obtained through the calcination of kaolin mineral at 700ºC, with a particle size of less than 75 microns); (e) silica fumes (decondensed through mechanical grinding); and, (f) two polycarboxylate-ether based superplasticizer additives. The chemical composition and surface area are shown in Table 1. The DRX patterns for the raw material are shown in Figure 1. Initially, the materials were homogenized into powder over the course of 5 minutes so that the particles would have a homogeneous distribution; subsequently, a quarter of water with additive was added. When the first agglomerates formed, they were mixed at the second velocity and the rest of the water was added slowly over the course of 3 minutes. After having obtained an adequate workability, the mixture was left to mix for one more minute and then poured into the molds. Afterwards, in order to let out the air that was generated during the mixing of the materials, the cubic 5cm molds were vibrated for a minute and were subsequently set up at 25ºC with 100% humidity during the hardening and treatment. After 24 hours, the test pieces were taken out of the molds and placed in a saturated solution of calcium hydroxide (with the purpose of avoiding lixiviation) for the treatment process at 1, 3, 7, 14, 28, and 60 days. Different systems of cement pastes were prepared, pure and substituted with 10% fly ash, different levels of granulated blast furnace slag of 25% to 45%, with metakaolin being used from 5 to 15%, and 5% and 10% silica fumes being used. The mixtures were manufactured with an a/s ratio of 0.4 and 0.3% of superplasticizer additive.
Table 1 Elemental composition in percentage of oxides of the starting materials and surface area (BET) of the materials used
| CP | EGAH | CV | MK | |
| SiO 2 | 18.69 | 38.01 | 61.17 | 56.97 |
| Al 2 O 3 | 4.73 | 9.98 | 25.14 | 35.46 |
| Fe 2 O 3 | 2.17 | 1.85 | 4.56 | 1.02 |
| CaO | 63.46 | 34.32 | 2.42 | 1.45 |
| MgO | 1.78 | 10.04 | 0.85 | 0.03 |
| SO 3 | 4.21 | 2.18 | 0.18 | 1.01 |
| Na 2 O | 0.27 | 0.49 | 0.27 | 0.13 |
| K 2 O | 0.67 | 0.58 | 1.41 | 0.54 |
| TiO 2 | 0.21 | 1.21 | 0.99 | 1.08 |
| P 2 O 5 | 0.13 | 0.00 | 0.00 | 0.20 |
| Mn 2 O 3 | 0.07 | 1.03 | 0.01 | 0.00 |
| LOI | 3.73 | 0.34 | 3.02 | 2.17 |
| Total | 100.12 | 100.13 | 100.02 | 100.06 |
| BET* (m 2 /kg) | 511 | 491 | 432 | 573 |
* Surface area
The samples were characterized through their compression strength (RC); subsequently, solid fractions of the samples were submerged in acetone and dried in a vacuum at 50ºC for 24 hours, with the purpose of stopping the hydration reactions and analyzing them through X-ray diffraction (DRX) and isothermal calorimetry through conduction (CIC).
3. RESULTS AND DISCUSSION
3.1. Compression strength
Figure 2 shows the results of all the systems analyzed in this study. In Figure 2 A, it can be observed that the use of additives SP1 and SP2 bettered the development of the RC for all treatment time periods, resulting in a RC at 1 and 60 days of approximately 49 and 76 MPa for the system manufactured with SP1 and of 44 and 76 MPa for the paste with SP2. The polycarboxylate-based superplasticizers are efficiently absorbed on the surface of the CP particles causing a highly observed dispersion in the functional ether groups, providing as a result an increase in workability and compression strength. The CP paste manufactured with the SP1 additive showed slightly higher resistances than that made with SP2. This result is possibly due to the presence of the functional ether group that appears in the FTIR spectrum of the SP1 additive. Winnefeld et al. (2007) reported a compression strength between 38.1 to 54.6 MPa at 7 days into the treatment, indicating that at early stages the RC increases with the increase of longitude and density of the lateral chain of the functional ether groups of the superplasticizer. Figure 2 B shows the results of the RC developed by the binary systems, where it is observed that at 60 days, 10CV pastes with SP1 and SP2 developed a compression strength above the binary systems of 45EGAH and 15 MK. Golapan (1993), Isaia et al. (2003), and Slanicka S (1999) have reported that the RC increase of cement paste partially replaced with CV is due to the size and morphology of the fly ash particles. Small and spherical particles fill the gaps and produce a dense matrix, further increasing the pozzolanic activity of the material which causes an increase in the RC. It is worth mentioning that this pozzolanic activity is not immediate, rather it can take up to 3 to 7 days; thus, improvement in the RC is seen at intermediate treatment stages. This effect is also seen in cement pastes with 10 and 15% CV [Gutteridge et al. (1990)]. The 15MK pastes with both additives showed superior RC to CP and other binary systems at a period of 1 day of treatment. This behavior is attributed to the fine size of MK. It has been published [Mansour et al. (2010); Caldarone et al. (1994); Wild et al. (1996)] that the use of MK contributes to the development of mechanical resistances at early stages due to the fine particle size that densifies the microstructure of the pastes, and due to its strong pozzolanic activity. Khatib et al. (1996) analyzed the development of resistances for cement pastes replaced with 10% MK at different points in time during the treatment and obtained the maximum RC at 14 days, which indicated that the pozzolanic activity of MK reaches its maximum reaction point in this timeframe. It has also been reported [Mansour et al. (2010)] that in order to disperse the MK in a cement paste the use of superplasticizers is needed, which improves the workability, rheology and mechanical properties of the pastes. The CP paste replaced with MK using SP2 showed superior RC, approximately 10% when compared to 15MK paste with SP1. The factors that could have affected this behavior were the a/s ratio, the SP quality and the dispersion effect caused by the additive.
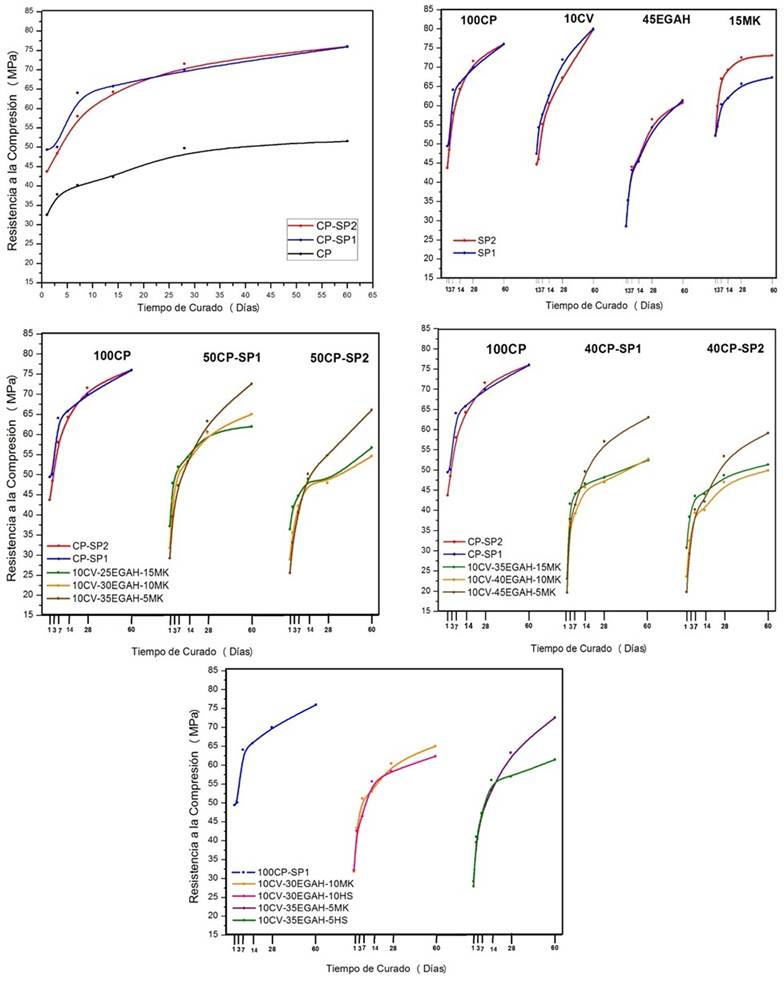
Figure 2 Compression strength of the systems with SP1 and SP2 additives: A) CP pastes, B) Binary systems, C) CP pastes 50% replaced with CV, EGAH and MK, D) CP pastes 60% replaced with EGAH, CV and MK, E) CP pastes 50% replaced with CV, EGAH, MK and its HS comparative using SP1 additive.
The development of the RC for the 45EGAH binary systems, at 1 and 60 days of treatment, was found to be below the RC of 100CP pastes and 10CV and 15MK systems; this behavior was attributed to the fact that under these conditions the a/s ratio, level of EGAH replacement and SP dosage, the fluidity of the paste of the 45EGAH binary systems was increased causing an excess of water in the mixture and consequently a change in the hydration process and on the mechanical properties of the system.
The results for the RC tests in the quaternary cements with both superplasticizers are shown in Figures 2 C and D. It is understood that the 10CV-25EGAH-15MK system was the one that developed the greatest RC at 1 day of treatment (approximately 37 and 36 MPa for both SP). However, these resistances were found to be below the RC of CP pastes at the same stage of treatment. The 10CV-35EGAH-5MK paste for the two superplasticizer additives developed the best RC at 60 days of treatment, being greater for SP1 (73 MPa). This behavior was related to the one presented by the binary systems with 10CV and 15Mk, given that the effect of the pozzolanic activity of MK in the early development of RC was evident. On the other hand, the pozzolanic activity of the CV and the larger ratio of EGAH benefitted the development of RC at 60 days of treatment. Furthermore, it can be observed that the pastes with SP1 developed RC above those manufactured with SP2. The factors that could have had an impact on this behavior were the a/s ratio, the dosage of the superplasticizer, and the dispersion caused by the additives. On the other hand, the development of RC in the CP pastes replaced 60% with CV, EGAH, and MK show a similar tendency to the RC results of the CP pastes with 50% replacement; however, the RC development at early stages was smaller. Moreover, the 10CV-35EGAH-15MK with both additives developed a better RC at 1 day of treatment in comparison with the 10CV-45EGAH-5MK paste, which showed the best RC at 60 days of treatment. Due to the fact that the 10CV-35EGAH-5MK paste manufactured with SP1 showed the highest RC values at 60 days of treatment, the effect of replacing the 5% MK for 5% HS was compared. In addition, there was consideration to study the same effect on the 10CV-30EGAH-10MK paste.
The tendency of the results in Figure 2 E indicates that pastes 10CV-30EGAH-10MK and 10CV-35EGAH-5MK showed a greater RC than the pastes with 10CV-30EGAH-10HS and 10CV-35EGAH-5HS at 60 days of treatment, with the resistance being greater in the pastes where MK ((73MPa) was used. Roy (2001) indicated that HS only increases resistance at early stages, while RC at later stages decreases with the increase of CV replacement. However, in this investigation, the CV replacement was maintained at a set point. Furthermore, the best resistances were in the pastes with a greater replacement of EGAH and a treatment time of 60 days, which corresponds to the study presented by Gesoglu et al. (2003), who concluded that the addition of 30% EGAH and 10% HS betters the RC at 28 days of treatment.
3.2. X-ray Diffraction
Representative samples of the cement pastes replaced at 50% and 60%, manufactured with the two SP and treated for 28 days, were analyzed, observing the following phases:
* - Portlandite (CH). C - Calcite (CaCO3), α - Quartz (α-SiO2), * - Alite (C3S), ▲- Ettringite (Ca6Al2(SO4)3 (OH)12.26H2O), S - Stratlingite (Ca2Al2SiO7.8H2O,), β - Belite (Ca2SiO4- C2S,), ● - Hydrotalcite ((Mg0.667Al0.33)(OH)2(CO3)0.167(H2O)0.5).
In Figure 3 A, the results of the DRX analysis for the CP paste manufactured with SP1 (CP-SP1) are shown and in Figure 3 B those manufactured with SP2 (CP-SP2) at 28 days of hydration, where it was possible to observe that for both the portlandite (CH) reflection characteristics were shown in an angular position 2θ of 18.08º and 34.3º. Also in the CP-SP1 system, ettringite was identified with characteristic reflections in angular positions 2θ of 9.147º, 23º and 51.784º. The ettringite is directly related to the quantity of C3A and the availability of SO4 2- ions in the liquid phase (Meredith et al., 2004). A study on the effect of the polycarboxylate-based superplasticizer additives in the hydration process of C3A showed that the molecules of the superplasticizer additive are preferably absorbed in the surface of the C3A phase [Plank et al. (2006)]. However, during initial hydration of the C3A, the molecules of the superplasticizer additive can form complex organometallics by interlocking between the layers of the hydrated phases of the C3A. The interlocking of the polycarboxylate molecules is not a desired process, as it decreases the dispersion effect of the cement particles. Plank et al. (2010) carried out a study on the interlocking mechanism of polycarboxylate between the layers of hydrated C3A phases and the function of the sulfate ions present in the cement. They reported that a high concentration of sulfate ions in the aqueous solution favors the interlocking mechanism of the sulfate ions between the layers of the hydrated C3A phases giving rise to the formation of AFt and AFm• phases.
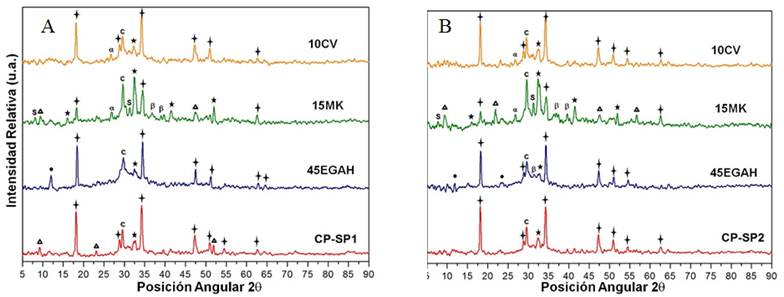
Figure 3 Diffraction pattern of the binary systems at 28 days of treatment, manufactured with (A) SP1 additive, and (B) SP2 additive.
Figure 3 also shows the DRX patterns for the binary systems 10CV, 15MK, and 45EGAH manufactured with additives SP1 and SP2. In this figure one can observe that the 15MK paste showed a greater pozzolanic activity due to the reflection intensity of the CH at 34.3º from an angular position 2θ decreasing in a larger proportion with regard to the other pastes; this was associated with a greater CH consumption. It was also possible to identify the stratlingite-Ca2Al2SiO7.8H2O (angular position 2θ at 7.045º and 31.08º), phase from the AFm phase family, a characteristic of the hydration products of cement pastes replaced with MK (Janotka et al., 2010) and the hydration of EGAH (Martinez Alvarado, 2009). The ettringite phase was also identified, whose intensity was slightly greater in the 15MK paste with SP2 additive. The formation of ettringite is evidently due to the preferential reaction of the MK aluminates with the SO4 2- ions of CP [Talero, (2005)]. In the same patterns, the presence of C3S and β-C2S was also observed, indicating the incomplete reaction of the phases of Portland cement, at least up to the first 28 days of treatment. Another of the identified phases was hydrotalcite (angular position 2θ at 11.81º), which was observed in the 45EGAH pastes with both additives. The reflective intensity of the characteristic peak of this phase was slightly greater in the 45EGAH paste with additive SP1. The formation of this phase is attributed to the hydration of EGAH through the activation caused by the presence of CH. Haha et al. (2011) reported that for an alkaline activated slag, hydrotalcite is observed intermingled with the C-S-H and MgO that contained the slag as one of the products of the reaction.
Figure 4A shows the diffraction patterns of the CP pastes and the CP pastes replaced 50% with CV, MK, and EGAH and manufactured with SP1 at 28 days of treatment. Based on the results, it is possible to consider a high consumption of calcium hydroxide caused by the pozzolanic reaction of CV and MK and by the hydration of the EGAH. Crystalline phases were also identified, which are characteristics of the hydration products of MK and EGAH, such as stratlingite and hydrotalcite, respectively. Furthermore, the analysis of the CP pastes replaced with CV, MK, and EGAH indicated that paste 10CV-35EGAH-5MK showed reflections characteristic of stratlingite (angular position 2θ at 11.65º and 34.88º) and hydrotalcite, and in paste 10CV-30EGAH-10MK only stratlingite was present. Based on these results, it is possible to consider that with a replacement level of 15% MK in the Portland cement pastes replaced with CV, EGAH and MK, the formation of stratlingite is favored due to the pozzolanic activity of MK at early stages. It is also suggested that the formation of hydrotalcite is due to the activation of EGAH with CH and is favored at 28 days of treatment in the system with greater EGAH replacement.
Figure 4 Diffraction pattern for the CP pastes replaced 50% with CV, EGAH, and MK using additive a) SP1 and b) SP2 at 28 days of treatment.
Figure 4 B shows the diffraction patterns for CP pastes and CP pastes with 50% replacement with CV, MK, and EGAH manufactured with SP2 at 28 days of treatment. According to the analysis of this information, SP2 had a slightly different effect in the hydration process of these cement pastes when compared to SP1. Whereas in the diffraction patterns of the CP pastes, reflections characteristic of weak intensity ettringite were identified, which was not observed in the CP paste manufactured with SP2. In pastes 10CV-25EGAH-15K, 10CV-30EGAH-10MK, and 10CV-35EGAH-5MK, the reflections characteristic of stratlingite were identified, but not those for ettringite and hydrotalcite. These results suggest that the SP2 was preferably absorbed in the C3A phase and the reaction of the sulfate, calcium, and aluminum ions in the aqueous solution promote the formation of ettringite during the initial hydration. Moreover, the interaction of the SP2 polycarboxylate molecules with the amorphous silica-alumina fraction favors the formation of stratlingite during the initial hydration of the cement pastes. In pastes 10CV-30EGAH-1OMK and 10CV-35EGAH-15MK, the presence of hydrotalcite was observed. According to these results, the SP2 additive favors the formation of the stratlingite phase and a greater consumption of CH in the pastes with a larger MK content at 28 days of treatment.
Figure 5 shows the diffraction patterns of the CP pastes 60% replaced with CV, EGAH, and MK at 28 days of treatment. In general, the tendency of the X-ray diffraction results for the system with 60% CV, EGAH, and MK manufactured with SP1 can be described as follows: The reflective intensity of the peak characteristic of the CH phase in an angular position 2θ at 34.3º decreased in greater proportion in pastes 10CV-35EGAH-15MK and 10CV-40EGAH-10MK at 1 and 28 days of treatment, which contained a greater percentage of MK replacement, demonstrating the strong pozzolanic activity of MK. Stratlingite, ettringite, and hydrotalcite were identified at 1 and 28 days of treatment. Stratlingite showed a weaker intensity in paste 10CV-45EGAH-5MK. By using the SP1 additive and comparing the results of 50 and 60% replacement, the presence of the hydrotalcite phase could be observed in the latter. Carrying out the same comparison, but for pastes with SP2, it can be observed that ettringite was not identified at 28 days of treatment and no stratlingite reflections were present in pastes 10CV-40EGAH-10MK and 10CV-45EGAH-5MK at 28 days of treatment.
3.3. Conduction Isothermal Calorimetry
Initially, superplasticizer additives were tested in the CP pastes. Subsequently, based on the replacement percentage and the RC results, systems 10CV-35EGAH-5MK and 10CV-45EGAH-5MK were selected and manufactured with both additives. According to the RC results, the pastes with SP1 additive developed the best RC, therefore another paste was selected for this additive, paste 10CV-30EGAH-10MK. Finally, a comparison between the effects of adding HS instead of MK in the pastes manufactured with the SP1 additive was carried out. Figure 6 shows the results of the isothermal calorimetry analysis of the CP pastes with and without additives at 25ºC.
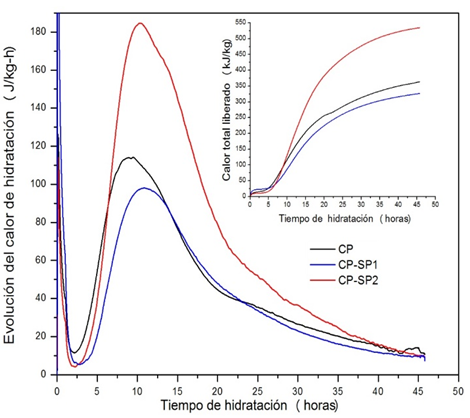
Figure 6 Progression curves for hydration heat and total heat released from the CP pastes without additives and those with additives SP1 and SP2.
It can observed that the main peak of the evolution curve of the hydration heat of the CP paste without additives, due to the hydration of the C3S, corresponded to 9 hours of hydration time. By comparing this curve with those obtained for the CP with both additives, it is possible to observe a displacement of the same at the main hydration peak, which corresponded to 11 hours, showing a delay in the hydration process. It has been reported that the superplasticizer additives delay the hydration of silicates (especially C3S). Lothenbach et al. (2007) found that the polycarboxylate based superplasticizers delay the dissolution of C3S, and therefore the formation of portlandite and C-S-H. Furthermore, a difference was observed in the start of the acceleration period between the CP pastes without additives and those that contained SP1 and SP2. The initial hydration time for the acceleration period for the CP pastes was 2.5 hours, whereas for pastes with SP1 it was 4 hours and for those with SP2 3 hours. This difference was attributed to the fact that the introduction period in the presence of both additives was longer, providing more time for the hydration reaction of C3S in order to form C-S-H. It is also observed that the progression of the hydration heat (J/Kg-h) and the release of total heat (Q/Kg) of the CP paste with SP2 was greater than that of the CP paste without additive and that with SP1. The acceleration period due to the nucleation and growth of the C-S-H gel and CH for the pastes with SP2 was more exothermic.
From these results it is possible to infer that the superplasticizer additives change the hydration mechanism of the CP pastes; however, each additive also showed a different behavior in pure cement. In this way, additive SP1 prolonged the induction period, which can be related to the dispersion effect caused by the observed aversion to the presence of the ether functional groups of the polycarboxylate-ether based superplasticizers, associated with the presence of the vinyl ether functional group shown by SP1. Additive SP2 caused the induction period to be shorter than that of the CP paste with SP1, which can be attributed to the lesser absorption of the polycarboxylate molecules on the surface of the cement particles. The SP2 additive showed two additional absorption bands characteristic of carboxylate salts. It has been reported that high density and a long lateral chain polycarboxylate-based superplasticizer additives decrease the delay of the induction period due to the polymer molecules being absorbed in a smaller ratio in the cement particle. It is possible that the rapid progression of the hydration heat during the acceleration period of the hydration process of the CP paste with SP2 is related to the low absorption of the polymer molecules on the surface of the cement particles, due to a larger spread of Ca+ ions and water from the liquid phase to the solid phase, which could have generated more sites of nucleation and growth of the C-S-H gel. Mollah et al. (2000) proposed three different mechanisms in order to explain the retardant effect of the polycarboxylate-based superplasticizers on the hydration of the cement, of which it is possible to associate the effect of SP1 and SP2 to two of them: (a) the molecules of the superplasticizer are absorbed on the surface of the cement particle and hinder the spread of water and calcium ions on the cement-solution interphase; however, the absorption in C3S is less than in C3A; (b) the spreading action of the superplasticizer changes the growth kinetic and morphology of the hydrated phases. According to the DRX results, one of the hydration products formed in the CP paste with SP1 at 28 days of treatment was ettringite; this phase could have been formed during the induction period, due to the fact that the absorption of the polycarboxylate-ether molecules of SP1 reduced the spread of Ca+ ions and water from the liquid phase to the hydrated calcium silicate phase, and it is possible that the hydrated calcium aluminate phase and the SO42- ions of the aqueous solution were favored.
Figure 7 compares the progression curves of the hydration heat for pastes 10CV-35EGAH-5MK and 10CV-45EGAH-5MK manufactured with SP1. The progression of the hydration heat for pastes 10CV-35EGAH-5MK and 10CV-45EGAH-5MK was less than that for the CP pastes without additives and those with SP1. It has been reported [Langan et al. (2002); Snelson et al. (2008)] that the progression of the hydration heat of cement pastes with EGAH, CV, and MK was less than that of the CP paste and are related to the higher water requirement of the pozzolanic materials and to the lesser availability of Ca+ ions for the growth of CH and C-S-H.
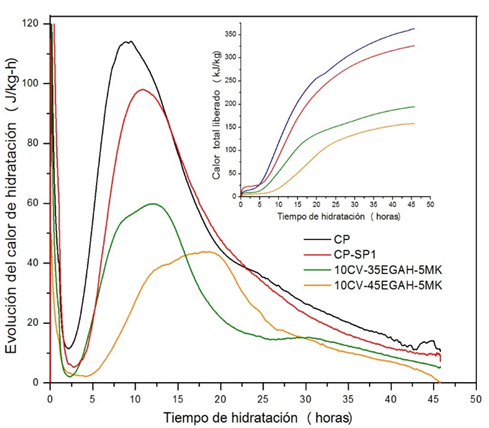
Figure 7 Progression curves for hydration heat and total heat released from the CP pastes without additives, with SP1, and pastes replaced with CV, EGAH, and MK manufactured with additive SP1.
Another difference of the pastes 10CV-35EGAH-5MK and 10CV-45EGAH-5MK with regard to the CP paste is the formation of an additional peak in the hydration heat progression curve during the acceleration period. Talero and Rahhal (2009) reported a third peak in the hydration heat progression curves of the CP pastes replaced with 20% MK, to which they associated the pozzolanic activity of Al2O3 r- of MK. Escalante (1996) also reported the appearance of a third peak in the hydration heat progression curve due to the contribution of the EGAH to the hydration process at temperatures of 10 to 60ºC. It is also possible to relate this increase in progression of the hydration heat with the formation of hydration products such as stratlingite and hydrotalcite, favored by the activation of the EGAH the formation of hydrotalcite was favored, whereas the pozzolanic activity of MK favored the formation of stratlingite and based on the RC results, it was also higher for paste 10CV-35EGAH-5M manufactured with SP1.
The delay in the hydration process of pastes 10CV-35EGAH-5MK and 10CV-45EGAH-5MK with regard to the CP pastes without additives and those with SP1, can be due to a slow dissolution of the C3S for the production of C-S-H and CH. However, this effect was more significant for the paste 10CV-45EGAH-5MK, given that its main peak was observed at 12.5 hours, in comparison with the paste 10CV-35EGAH-5MK that occurred at 8 hours. This difference is likely due to the greater EGAH content and the retardant effect of the CV. According to Hwang and Shen (1991), the increase in the quantity of EGAH reduces the hydration heat produced by C3S and C3A. Langan et al. (2002) found that during the first minutes of hydration for CP pastes with 10 and 20% CV, the ion concentration in the solution for the first couple of hours decreases, which is why the nucleation and growth of the C-S-H- and CH is delayed.
The results of the isothermal calorimetry at 25ºC for pastes 10CV-35EGAH-5MK and 10CV-45EGAH-5MK with SP2 as an additive are shown in Figure 8. The progress of the hydration heat for these pastes was less (60 and 50 J/Kg-h, respectively) than that of the CP pastes with SP1 (190 J/Kg-h) and without it (115 J/Kg-h), due to the aforementioned. These curves also showed a third peak due to the MK pozzolanic activity and the hydration of the EGAH. Furthermore, the delay of the hydration process during the induction period was less for these systems, which can be related to the lesser absorption of the SP2 molecules on the surface of the cement particles.
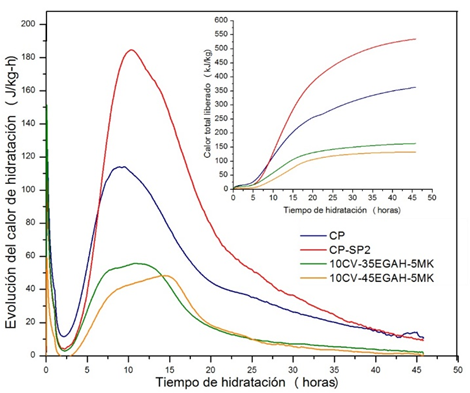
Figure 8 Progression curves for hydration heat and total heat released by the CP paste without additives, with SP2, and pastes replaced with CV, EGAH, and MK manufactured with SP2
The results of the isothermal calorimetry analysis for the CP paste with SP1 are shown in Figure 9, comparing the behavior of an additional system manufactured with 5 and 10% HS. It can be observed that the hydration reactions of pastes 10CV-35EGAH-5HS and 10CV-30EGAH-10HS develop less hydration heat than paste 10CV-35EGAH-5MKS. This relates to the RC results in the presence of MK it generated the best values at early stages. The decrease in the hydration heat can be due to: (1) the precipitation of the reaction products in the CP pastes with MK was less exothermal than in the CP pastes, and (2) due to the smaller quantity of cement utilized it is possible for there to be a smaller quantity of hydration products formed in the initial stages [Janotka et al. (2010)]. The total heat released by the paste 10CV-30EGAH-5HS was greater than that of the paste 10CV-35EGAH-5MK, and it can be associated to the fact that during the hydration of paste 10CV-30EGAH-5HS the deceleration period was prolonged favoring the hydration process of the CP and that both the pozzolanic reaction of the CV and HS as well as the activation of the EGAH were more exothermal.
4. CONCLUSIONS
The addition of 10% CV improved the development of RC in the binary system. The optimal replacement level of EGAH in pastes that also contained CV and MK was 35% and 45%. This replacement percentage favored the development of RC at stages of 60 days of treatment, being greater in the system with 10CV-35EGAH-5MK. Additive SP1 favored the development of RC of cement paste 10CV-35EGAH-5MK compound system. Additive SP2 favored the development of RC of the binary system of CP paste replaced with 15% MK.
The pozzolanic activity of MK was favored in cement pastes with a greater level of MK replacement at 1 day of treatment, which favored the development of RC.
The hydration process of the EGAH led to the formation of hydrotalcite, being favored in pastes with a greater EGAH replacement. The hydration of MK and the pozzolanic reaction with the CH of the hydration of the CP favored the formation of stratlingite in CP pastes with 15% MK and in CP pastes replaced with CV, EGAH, and MK.
In the hydration heat curve of the CP pastes, the superplasticizer additives delayed the nucleation and growth of the C-S-H- gel. In the hydration heat curve of the CP pastes, SP2 increased the release of heat of the main peak, being a more exothermal process. Additive SP1 bettered the hydration process of the systems with CV, EGAH and MK, with high EGAH and low MK replacements, showing greater hydration heat in the main peak of the hydration heat progression curve. The heat released by the hydration of the EGAH in system 10CV-35EGAH-5MK modified the hydration heat progression curve of the CP, showing an additional peak, which was prolonged at a greater EGAH replacement level. This implied a more exothermal hydration process for the paste with SP1. The hydration heat due to the pozzolanic activity of MK was superior to that shown by the HS, given that the main peak of the hydration curve of system 10CV-35EGAH-5MK was above that of the system 10CV-35GAH-5HS.
In general, the use of high quantities of pozzolanic and hydraulic materials in the formation of the systems presented here produces an increase in the mechanical properties of the systems, as well as in the hydration reactions, bettering the compression strength.
Acknowledgements
The financial support of CONACYT - Mexico for the realization of this project is greatly appreciated.
REFERENCES
Ali M., Gözde I.N.S., Kambiz R., 2014. Comparison of fly ash, silica fume and metakaolin from mechanical properties and durability performance of mortar mixtures view point., Construction and Building Materials, 70: 17-25. [ Links ]
Bentz D.P., Hansen A.S., Guynn J. M. (2011) "Optimization of cement and fly ash particle sizes to produce sustainable concretes", Cement and Concrete Composites, 33, pp. 824-831. [ Links ]
Caldarone M. A., Gruber K. A., Burg R.G. (1994) "High-reactivity metakaolin: a new generation minerals admixture"; Concrete International: design and construction, pp. 37-40. [ Links ]
CEMBUREAU (2014), The European Cement Association Activity Report. [ Links ]
Escalante García J. I. (1996); PhD thesis: The effect of temperature on the hydration of Portland cement and composite cement pastes; University of Sheffield. [ Links ]
Damtoft JS, Lukasik J, Herfort D, Sorrentino D, Gartner EM (2008) "Sustainable development and climate change initiatives"; Cement and Concrete Research, 38; pp. 115-127. [ Links ]
Esteves L. P., Cachim P. B., Ferreira V. M. (2010) "Effect of fine aggregate on the rheology properties of high performance cement-silica systems"; Construction and Building Materials, 24, pp. 640-649. [ Links ]
Gesoglu M., Guneyisi E., Özbay E. (2009) "Properties of self-compacting concretes made with binary, ternary, and quaternary cementitious blends of fly ash, blast furnace slag, and silica fume; Construction and Building Materials, 23, pp. 1847-1854. [ Links ]
Golapan M. K. (1993) "Nucleation and pozzolanic factors in strength development of class F fly ahs concrete"; ACI Materials Journal, pp. 117-121. [ Links ]
Gutteridge W. A., Dalziel J. A. (1990) "The effect of a secondary component on the hydration of Portland cement, Part II: Fine hydraulic binders"; Cement and Concrete Research, 20, pp. 853-861. [ Links ]
Haha M. B, Saout G. Le, Winnefeld F., Lothenbach B. (2011) "Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast furnace slags"; Cement and Concrete Research, 41, pp. 301-310. [ Links ]
Hwang C. L., Shen D. H. (1991) "The effects of Blastfurnace slag and fly ash on the hydration of Portland cement"; Cement and Concrete Research, 21, pp. 410-425. [ Links ]
Isaia G. C., Gastaldini A.L.G:, Morales R., (2003) "Physical and pozzolanic action of mineral additions on the mechanical strength of high performance concrete"; Cement and Concrete Composites Vol. 25, pp. 69-76. [ Links ]
Janotka I., Puertas F., Palacios M., Kuliffayová M., Varga C. (2010) "Metakaolin sand-blended-cement pastes: Rheology, hydration process and mechanical properties"; Construction and Building Materials, 24, pp. 791-802. [ Links ]
Juenger M.C.G., Siddique R. (2015) "Recent advances in understanding the role of supplementary cementitious materials in concrete", Cement and Concrete Research 78, pp. 71-80 [ Links ]
Khatib J. M., Sabir B. B., Wild S. (1996) "Pore size distribution of metakaolin paste"; Cement and Concrete Research,. 26, pp. 1545-1553. [ Links ]
Langan B. W., Weng K., Ward M. A. (2002) "Effect of silica fume and fly ash on heat of hydration of Portland cement"; Cement and Concrete research, 32, pp. 1045-1051. [ Links ]
Lothenbach B., Winnefeld F., Figi R. (2007) "The influence of superplasticizers on the hydration of Portland cement"; Empa, Dübendorf, Switzerland. [ Links ]
Mansour S. M., Abadlia M. T., Bekkour K. (2010) "Improvement of Rheological Behaviour of Cement Pastes by Incorporating Metakaolin"; European Journal of Scientific Research, 42, pp. 428-438. [ Links ]
Martínez-Alvarado M. J; (2009); Tesis: Estudio de la hidratación de la escoria granulada de alto horno (EGAH) a diferentes temperaturas; Maestría en Ciencias en Ingeniería Metalúrgica; Escuela Superior de Ingenierías e Industrias Extractivas; Instituto Politécnico Nacional, México, D.F. [ Links ]
Meredith P., Donald A.M. Meller N., Hall C. (2004) "Tricalcium aluminate hydration: microestructural observations by in-situ electron microscopy"; Journal of Materials Science 39, pp. 997-1005. [ Links ]
Mollah M. Y. A., Adams W. J., Schennach R., Cocke D. L. (2000) "A review of cement - superplasticizers interactions and their models"; Advances in Cement Research 12, pp. 153-161. [ Links ]
Ping D., Zhonghe S., Wei C. , Chunhua S., 2013. Effects of metakaolin, silica fume and slag on pore structure, interfacial transition zone and compressive strength of concrete., Construction and Building Materials, 44: 1-6. [ Links ]
Plank J., Dai Z., Andres P.R. (2006), "Preparation and characterization of new Ca-Al-polycarboxilate layered double hydroxides"; Materials Letters 60; pp. 3614-3617. [ Links ]
Plank J., Zhimin D., Keller H., Hössle F. V., Seidl W. (2010) "Fundamental mechanisms for polycarbozylate intercalation into C3A hydrate phases and the role of sulfate present in cement"; Cement and Concrete Research, 40. pp. 45-57. [ Links ]
Roy D. M., Arjunan P., Silsbee M. R. (2001) "Effect of silica fume, metakaolin, and low-calcium fly ash on chemical resistance of concrete"; Cement and Concrete Research, 31, pp. 1809-1813. [ Links ]
Schneider M, Romer M, Tschudin M, Bolio H. (2011), "Sustainable cement production at present and future", Cement and Concrete Research; 41, pp. 642-650. [ Links ]
Schöler A. , Lothenbach B., Winnefeld F., Zajac M. (2015) "Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone poder" Cement and Concrete Composites, 55, pp.374-382. [ Links ]
Sharfuddin A., Obada K., Wendy A., 2008. Chloride penetration in binary and ternary blended cement concretes asmeasured by two different rapid methods ., Cement and Concrete Composite, 30:576-582 [ Links ]
Slanicka S. (1999) "The influence of fly ash fineness on the strength of concrete"; Cement and Concrete Research, 21, pp. 285-96. [ Links ]
Snelson D.G., Wild S., O'Farrel M. (2008), "Heat of hydration of Portland Cement-Metakaolin-Fly ash (PC-MK-PFA) blends"; Cement and Concrete Research, Vol. 38, pp. 832-840. [ Links ]
Talero R. (2005), "Performance of metakaolin and Portland cements in ettringite formation as determined by ASTM C 452-68: kinetic and morphological differences"; Cement and Concrete Research,. 35, pp. 1269-1284. [ Links ]
Talero R., Rahhal V. (2009); "Calorimetric comparison of portland cements containing silica fume and metakaolin"; Journal of Thermal Analysis and Calorimetry, 96, pp. 383-393. [ Links ]
Yun G., Geert De S., Guang Y., Zhuqing Y., Zhijun T., Kai W., 2013, A microscopic study on ternary blended cement based composites. Construction and Building Materials, 46: 28-38. [ Links ]
Wild S., Khatib J.M., Jones A. (1996) "Relative strength pozzolanic activity and cement hydration in superplasticised metakaolin concrete"; Cement and Concrete Research. 26; pp. 1537-1544. [ Links ]
Winnefeld F., Becker S., Pakusch J., Götz T. (2007) "Effects of the molecular architecture of comb- shaped superplasticizers on their performance in cementitious systems", Cement and Concrete Composites, 29, pp. 251-262. [ Links ]
Received: February 16, 2015; Accepted: July 18, 2015











 text in
text in 





