Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista ALCONPAT
On-line version ISSN 2007-6835
Rev. ALCONPAT vol.6 n.2 Mérida May./Aug. 2016
https://doi.org/10.21041/ra.v6i2.132
Applied research
Effect of the addition of nanosilica particles on the properties of two cementitious matrices
1Universidad Autónoma de Nuevo León, Facultad de Ingeniería Mecánica y Eléctrica, Programa Doctoral en Ingeniería de Materiales, Ave. Universidad s/n, Ciudad Universitaria, San Nicolás de los Garza, Nuevo León, México, CP 66450.
This research focused on evaluating the effect of adding silica nanoparticles (NS) to two cementitious matrices, as ordinary portland and sulfoaluminate cement, in order to establish their influence on the mechanical and chemical properties. To conduct this evaluation, the NS were added in dosages of 0.30-to-5.0% by mass relative to cement. The results indicated that the compressive strength and resistance to chemical attack by sulfates were improved due to the addition of silica nanoparticles, in both matrices. Finally, the resistance to chemical attack by sulfates showed an improvement with the addition of silica nanoparticles when comparing with pure cement, suggesting an increase in the densification.
Keywords: reactivity; supplementary cementitious materials
Este trabajo de investigación evaluó el efecto de la adición de nanopartículas de sílice (NS) a dos matrices cementantes, base cemento portland ordinario y cemento sulfoaluminoso, con el fin de establecer su influencia en las propiedades mecánicas y de resistencia química de dichos materiales. Para esto, se adicionaron las NS en dosificaciones de 0.30% a 5.0% en peso. Los resultados indicaron que la resistencia a la compresión y al ataque químico por sulfatos, se ven mejoradas debido a la adición de NS. La resistencia al ataque químico por sulfatos se mejoró de forma importante con la presencia de NS en comparación al cemento sin adiciones. Este resultado sugiere que ambas matrices presentaron una mayor densificación.
Palabras claves: reactividad; materiales cementosos suplementarios
Este estudo avaliou o efeito da adição de nanopartículas de sílica (NS) em duas matrizes cimentícias, base cimento Portland comum e cimento sulfoaluminoso, a fim de estabelecer a sua influência sobre as propriedades de resistência mecânica e química dos referidos materiais. Para isso, foram adicionadas as NS em dosagens de 0,30% a 5,0% em massa. Os resultados indicaram que a resistência à compressão e ao ataque químico por sulfatos são aumentadas devido à adição do NS. A resistência ao ataque químico por sulfatos melhorou significativamente com a presença de NS em comparação com o cimento sem adições. Este resultado sugere que ambas as matrizes apresentaram uma maior densificação.
Palavras-clave: reatividade; materiais cimentícios suplementares
1. Introduction
Among the recent research in the construction industry, it is possible to identify the use of nanomaterials, added to the cementitious matrix, which can modify the rheological properties of the concrete. Some of the most commonly used compounds are: nanosilica, photocatalytic nanocomposites as TiO2, nano-CaCO3 and nanoclays; the use of nanomaterials in the development of hybrid materials was also investigated, where the nanoparticles are not added, but formed in the cementitious matrix.
Other types of materials that have been tested, are carbon nanofibers, cellulose nanofibers, including the utilization of nanomaterials to modify the properties of the aggregates used in the manufacture of concrete. One of the most widely used nanomaterial is silica (silica nanoparticles, NS) due to its pozzolanic behavior. The results generally agree that when using this material, the mechanical properties are improved, as reported by (Sobolev et. al., 2009), who added 5-70 nm NS; they reported that, both compressive strength (RC) and the flexural strength, of mortars with an addition of 0.25% of nano-SiO2 were improved in percentages of 16% at 24 hours and 18% at 28 h respectively. Other studies (Belkowitz et. al., 2010) have focused on the comparison of the properties obtained in the concrete when using microsilica particles and compared with NS (Mondal et. al. 2010, Shah et al, 2009), where the addition of up to 15% NS substantially improves the durability of concrete by increasing the rigidity of the CSH gel. These observations are also consistent with those reported when by (Hosseni et. al., 2010), where ferrocements mortars with 1-3% NS presented an improvement in the compressive strength and better densification in the interfacial transition zone, with a w/c ratios of 0.35 and 0.40. On the other hand, nanomaterials were studied to improve the performance of recycled aggregates (Hosseni et. al., 2009), where while the use of those nanoparticles improved the hydration reactions, the use of recycled aggregates generated lower strength and workabilities when using 1.5-3% of silica nanoparticles. Furthermore, the formation of a thin film of nanoparticles directly on the surface of the aggregates was studied (San Filippo et. al., 2009), the adhesion of aggregate and cement paste was improved, obtaining better mechanical properties. Additionally, they reported improvements up to 35% RC with an addition of 0.032% SiO2.
As mentioned above, the use of nanotechnology in the construction industry can modify the properties of cement and/or concrete produced with these materials. However, a key point of several studies includes the process of the addition of nanoparticles and its proper dispersion in the matrix. As mentioned by (Sobolev et. al., 2009), the distribution of nano-SiO2 in the cement paste plays an essential role on the performance of the cement and the products obtained. In other nanomaterials, such as carbon nanotubes, the problem of dispersion was also reported, (Shah et. al., 2009) and the biggest drawback of the incorporation of carbon nanotubes was the poor dispersion obtained.
It is worth notice that through this article a relevant contribution concerning the study of a sulfoaluminate cement with addition of NS is presented, since there is very little information related to this (Raki et. al., 2010, Jewell, 2015 Chung et. al., 2012). Additionally, the sulfates attack in both matrices with the NS additions is an interesting topic that has not been extensively studied.
2. Experimental procedure
Two cementitious matrices were used: (a) Ordinary Portland Cement CPO 40, according to Mexican standard NMX-C-414-ONNCCE-2010 and (b) Sulfoaluminate Cement (CSA) which main characteristic is that contains, in addition calcium sulfoaluminate (C4A3S), besides the traditional cement phases (C3S, C2S, C3A and C4AF). The results of chemical composition of both cements are presented in Table 1.
Table 1 Chemical composition of both types of cements.
| Oxides (mass%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 | P2O5 | Mn2O3 | CaO | P.P.I | R.I. | A.T. |
| CPO 40 | 19.07 | 4.97 | 1.98 | 62.54 | 1.55 | 4.14 | 0.36 | 0.68 | 0.21 | 0.13 | 0.067 | 1.2 | 4 | 1.06 | 0.813 |
| CSA | 18.67 | 4.44 | 1.66 | 63.14 | 0.83 | 3.91 | 0.2 | 0.15 | 0.18 | 0.07 | 0.03 | 0.6 | 6.42 | 0.5 | 0.295 |
* P.P.I.: lost on ignition; R.I: insoluble residues; A.T.: Total alkalis.
Table 1 shows that the content of SO3 of both cements is in the range of 4.0%, i.e. 4.14% for CPO and 3.91% for the CSA. It is also noted that the CaO content is slightly higher in the CPO compared to the CSA cement (63.14% vs. 62.54%), indicating a higher content of limestone addition. Quantification of both cements phases was carried out using Rietveld refinement with HighScore Plus Software, version 3.05. Rietveld refinement results are presented in Figure 1 and Table 2. The results of the mineralogical quantification confirmed that both cements, have different contents of the main clinker phases, as C3S, C2S, C3A. The analysis also showed the presence of CaCO3 in both cements, although in the CSA cement was around 15% compared with 2.5% for the CPO. The main mineralogical differences were the presence of 15% Yelimite or C4A3S, identified only in the CSA cement. The nanosilica used was a commercial product and its characterization is presented in Table 3.
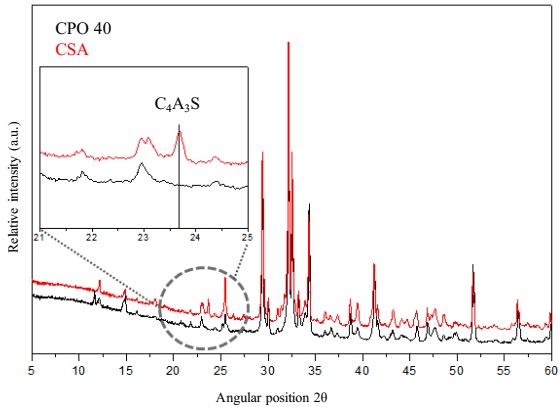
Figure 1 Comparison of diffraction patterns for both cements; In detail C4A3S phase observed in CSA.
Table 2 Results of Rietveld quantification for the studied cements.
| Component | Chemical formulae | CPO 40 | CSA |
|---|---|---|---|
| C3S- Alite, de la Torre et al | Ca3SiO5 | 61.9 | 36.4 |
| C2S - Belite | Ca2SiO4 | 18.9 | 27.3 |
| C3A- Cubic Aluminate | Ca9(Al6O18) | 5.0 | 1.2 |
| C3A- Aluminate | Ca9(Al6O18) | ---- | 1.8 |
| Calcium Ferroaluminate | Ca2Fe1.52Al0.48O5 | 3.7 | 3.7 |
| Yelimite | Ca3Al6O2CaSO4 | ---- | 1.5 |
| Anhidrite | CaSO4 | 0.8 | 3.2 |
| Gypsum | CaSO4·2H2O | 7.2 | 5.5 |
| Calcium Carbonate | CaCO3 | 2.5 | 15.1 |
| Calcium oxide | CaO | ---- | 4.3 |
| GOF | 8.6 | 11.2 |
Table 3 Characterization of the nanosilica.
| Characteristic | Specification | Result |
|---|---|---|
| Presentation | Liquid | Liquid |
| Color | Semi-Transparent white | Semi-Transparent white |
| Specific gravity, 20°C | 1.134±0.03 | 1.22 |
| Viscosity, 20°C, Brookfield, Sp 00/100 rpm | < 30 cps | ND |
| pH | 10±1 | 10.3 |
| Solid % | -------------- | 32.5 |
*Where ND = Not determined.
The NS were added in replacement levels of 0, 0.3, 1 and 5% by weight. It is noteworthy that even though for this type of materials the use of higher dosages than 1.0% could be unviable due to the costs, in the present investigation these levels were utilized to determine their influence on mortars properties. The mixing process used was similar to that indicated in the NMX-C-085-ONNCCE-2010 Mexican Standard, with a slight modification; mixing water was added first, then SP, and mix for 60s prior to adding NS and mix again for 1 minute; afterwards the process was carried out as indicated by the standard. It was necessary to use 0.5% SP, to ensure dispersion of the NS. After 24 h, the specimens were demolded and placed in a saturated calcium hydroxide (to avoid leaching) for the different curing periods.
The samples were characterized by compressive strength (RC); subsequently solid fractions of the samples were immersed in acetone and vacuum dried at 50°C for 24 h, to stop the hydration reactions and be analyzed by scanning electron microscopy (SEM). Also, the samples were characterized by isothermal conduction calorimetry.
3. Results
4.1 Isothermal calorimetry
Isothermal calorimetry tests were carried out in pastes for both type of cements; the results are shown in Table 4. To evaluate the effect on the heat generated during the hydration reactions, samples were prepared with 0.50% SP and 1.0 and 5.0% NS. The pastes were had a water/cement (w/c) ratio of 0.40 and 0.50 (see Table 4).
Table 4 Samples prepared for characterization with isothermal calorimetry, cements were prepared with NS and SP.
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Type of cement | CPO 40 | CPO 40 | CSA | CSA | CPO 40 | CPO 40 | CSA | CSA |
| NS (mass%) | --- | --- | --- | --- | 1 | 5 | 1 | 5 |
| SP (mass%) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| w/c ratio | 0.5 | 0.4 | 0.5 | 0.4 | 0.5 | 0.4 | 0.5 | 0.4 |
Figure 2 shows that the induction period ended after 2.0 hours for the CPO 40, while for CSA, finished before. It is at this time, when the setting period starts and the C-S-H layer breaks, with the continuation of the hydration process. Results shows that lower w/c ratios slightly accelerate the hydration reaction; this effect is more noticeable for CPO 40.
As shown in Figure 2, when adding 5% SP for both type of cements, a shift of the exothermic curve was observed, indicating an extended sleeping period, causing the curve became more pronounced with a lower crest, having a maximum heat release at 15 h for CPO 40 with 0.5% SP, compared to 7 h without SP, i.e. the addition of 0.5% SP increases the starting time for the development of RC two times. A similar behavior is shown for CSA; where the effect of SP appears to be slightly higher, since cement without SP registers its exothermic maximum at 4.7 h, whereas with the addition of 0.5% SP, the exothermic maximum is observed at 13h, i.e., almost three times slower than the plain cement.
On the other hand, at the same w/c ratio, it is observed that the CSA presents a different heat evolution curve compared with CPO 40, where the main peak due to the exothermic reaction of C3S was at 6.5 h for CPO 40, and 4.5 h for CSA. Additionally, the exothermic peak reached a maximum heat flux for CSA of 0.07 W/g, while for the CPO 40 of 0.06 W/g. This indicates that the hydration reaction of cement CSA starts faster, and the initial RC should be greater in CSA; however, the RC values were higher at 24 hours for CPO 40 (18 MPa) compared to CSA (13.1MPa). This could be due to the dilution effect of CSA, which according to the results of the chemical composition, contains a higher content of limestone, compared with CPO 40. The results also show a shorter setting period of CSA with respect to CPO 40. After the pure cements analysis, the effect of NS addition was studied.
These results confirm those reported by Puertas (Puertas et. al., 2001 and Puertas et. al., 2005), indicating that regardless of the nature of the SP added, the hydration reactions of calcium silicates were delayed, extending the setting period. This effect can be explained due to an adsorption of the polymer in the cement grains, forming a layer barrier around grains, and preventing the transport of water molecules to the cement particles, in addition to the formation of complexes between Ca2+ ions formed in the initial hydration and the anions of the polymers that affect nucleation and precipitation of Ca(OH)2. However, the RC of mortars with SP indicated that at 24 h, the RC of pure CPO 40 was lower than those containing 0.5% SP, i.e., 18 MPa and 23.5 MPa respectively.
The isothermal calorimetry results indicated a significant extension of the initial setting time, however, this result does not necessarily imply any relation with the RC expected by these cements, since the setting process and the development of strength process are independent. It is noteworthy that that for CS, the effect was different, and the addition of SP decreased the RC at 24 hours, from 13.1 to 8.2 MPa.
Figure 3 also showed that by adding the nanoparticles to the same w/c ratio the evolution heat curves shifted to shorter times, suggesting that the processes of setting and hydration reactions were accelerated by the addition of the silica nanoparticles. This trend was observed for both types of cements.
This behavior confirms that reported by (Qing et. al., 2007), they observed that with the increase in the percentage of NS, the workability decreased, and the hydration process was accelerated compared to other pozzolanic materials such as silica fume. Other authors (Björnström et. al., 2004, Morteza et. al., 2014 and Li et. al., 2004) found that nanosilica also accelerates the process of hydration and C-S-H gel formation due to its particle size; moreover, with the increase of the addition percentage of NS, the heat of hydration of the mixture incremented.
Hence, it is possible also to observe that at higher NS load, the exothermic maximum is reached at lower time. According to curves for CPO 40, form 0 to 1.0% NS, the time at which the maximum was reached moved from 14.5 h to 11 h, and by adding 5% NS time fell from 11 to 7.5 h. For the CSA, the behavior was similar, i.e., with no additions the maximum was observed at 13.3 h, with 1% NS at 8.8 h and for 5% NS at 6 h.
This experimentally observed behavior had a parabolic shape and can be explained through the following equation:
where: f (x) = maximum time of heat release, x = % of nanoparticles; a, b and c are coefficients of each of the systems.
Equation for the system with CPO 40:
Equation for the system with CSA:
From Figure 4 it can be concluded that, while adding nanoparticles to the cementitious matrix accelerates hydration reactions and therefore increase RC, there is a point where the increase in the nanoparticles content will not have an improvement effect in the cement. In Figure 4 it can also be noted that higher that 3.5% contents of NS, the time where the exothermic maximum is reached, increase again. This would confirm that the nanoparticles presence, accelerated the development of the initial RC, but only up to this content.
4.2. Compressive strength results.
Samples with 0.5%SP and 0.3%, 1% and 5% NS were used to estimate the compressive strength. The results of 24 h, 3, 7, 28, 90 and 150 days are presented in Figure 5. It is worth to notice that, for this first set of results, the water added to the mix was corrected considering the water supplied by NS (which was provided in suspension), in order to keep the ratio w/c of 0.485 constant.
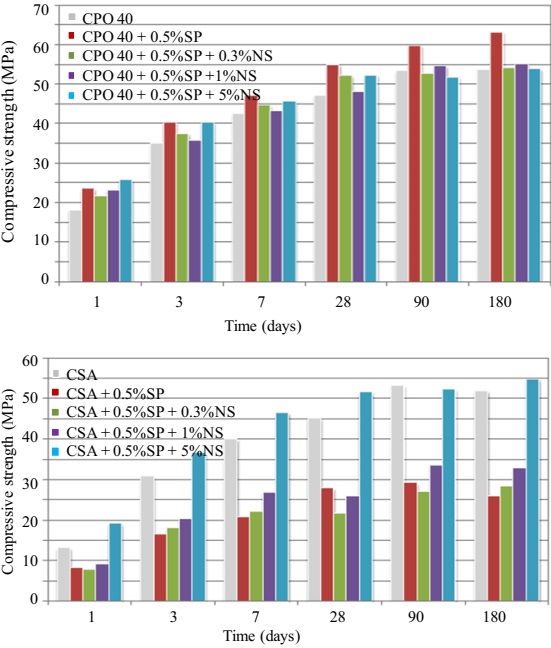
Figure 5 Compressive strengths results of the CPO 40 and CSA, without adjusting the amount of water.
From the results, it can be observed that for the CPO 40, the best RC was obtained for the mixture with SP. The RC results also showed that all the systems with NS tested at 24h exceed the results obtained by reference (neat CPO 40), with an increase up to 43% (25.8 MPa vs 18 MPa), for sample with 5% NS. After 3 days of hydration, these percentages decreased, observing an increase of 15% of for mixtures with 5% NS (40 MPa vs 34.9 MPa). At 7 days, the improvement was of 11% for CPO + SP while, with the incorporation of 5%NS the RC was 8% higher than that obtained by the reference. At 28 days, the samples with SP developed a RC 17% RC higher that the reference (54.9 vs. 47.1 MPa), while the increase for samples with NS was around 11% compared to the reference (52.1 and 52.3 MPa for samples with 0.3% and 5% NS vs 47.1 MPa for the reference). After 150 days of curing, all samples with NS reaching almost the same value as obtained by reference and only the sample with SP developed a 18% greater 34.9 MPa than the neat CPO.
The effect shown for CSA, was different as that observed for CPO 40, since the addition of SP, did not favor the development of RC, but rather inhibited the hydration processes. Therefore, all the RC results for the mixtures containing 0.5% SP, showed lower RC with respect to the reference, as 63%, 54%, 52%, 62% and 55% at 24 h, 3, 7, 28, 90 and 150 days respectively due to the decrease in the aforementioned reactivity, since SP are designed for ordinary Portland cement and the sulfate phases contained in the CSA may negatively affect the functionality of the SP.
This behavior was observed for CSA cement in all samples, except for the mixture with 5% NS, where it possible to observe an increase in the RC of about 47% at 24h, 19% at 3 days, 16% at 7 days, and 14% after 28 days. According to (Ma et. Al., 2014) when adding SP, the RC for belite-sulfoaluminate cementsat 24 h decreased, however, at 28 days RC were equivalent to the reference and even higher when dosed in percentage of 0.075%. This was to the delay in the ettringite formation after 1 day of hydration, especially at high concentrations, resulting in a poor RC. This could explain that the 0.5% CSA load used in this research could be relatively high for the nature of this cement.
As can be seen in Figure 6, at similar values of fluidity, by adjusting the mixing water for the same fluidity; RC results for CPO 40 with NS change with respect to those previously described, although the best results are observed in presence of SP, the results with the NS are very similar, and even superior at 24 h for samples with 0.3% and 1% NS.
These results are relevant because it raises a question relative with the Mexican standard specifications of consumption of water to use when it comes to assessing ordinary cements. As is known, the standard indicates that for an ordinary Portland cement, the w/c ratio recommended to evaluate RC is 0.485. The results obtained in this research suggest that for systems with SP systems, or nanoparticles, it is best to seek the fluidity and water required to adjust to this fluidity and do not use a fixed w/c ratio.
4.3. Chemical resistance to sulfate attack
The sulfate attack tests were carried out according to the procedure specified in ASTM-C-1012. Figures 7 and 8 show the results for each type of cement measured during 550 days, include the standard sets a minimum duration of 12 months. From the results, it is possible to observe that neat CPO 40 and CSA do not have good resistance to chemical attack by sulfates, since the change in length exceeds the allowable expansion value of NMX-C-414-ONNCE-2010 standard of 0.1% at 125 days for CPO 40 and at 50 days for CSA.
The addition of nanoparticles in percentages of 5%, promotes the chemical resistance to sulfates, for both types of cement. In the case of CPO 40, the benefit of adding nanoparticles is so favorable that the cement changes from a cement without resistance to sulfate attack ("RS" in the nomenclature used in the Mexican standard NMX-C-414-2010-ONNCCE) to be a resistant to sulfates at least up to 550 days, with contents of 0.5% of SP and 5% NS. The addition of less than 5%NS did not improve the resistance.
For the CSA, the addition of nanoparticles showed a lower resistance to chemical attack by sulfates compared to CPO 40, since CSA without additions exceeded the limit established by NMX-C-414-2010-ONNCCE standard after only two months of exposure. However, by adding SP and NS, cement remained with characteristics of resistance to chemical attack up to 275 days (9 months). This change was 94.8% for 180 days, which was the maximum age at which the reference specimen cement volume remained stable with respect to neat CSA.
According with the results observed for CPO 40 the addition of both SP and NS, in low dosages for the latter was not beneficial in terms of resistance to chemical attack, however, with all the additions, the CSA resistance was improved compared with pure CSA.
As mentioned above, this behavior can be explained by a densification of the matrix, which increased with the content of NS, and thus a reduction in porosity and sulfate attack tends to be slower than in cement samples without NS. The NS would be acting as nucleation sites for the generation of gel C-S-H and to promote reduction in porosity. This behavior was confirmed by other investigators (Quercia et. al., 2012 and Li et. al., 2004), they reported that the addition of NA increased RC and decreases permeability through the pozzolanic reaction, resulting in an increased denser structure with a further formation of C-S-H. According to (Khater et. Al., 2006), with the use of modified montmorillonites, the permeability was 100 times reduced compared to neat cement.
Thus, durability of the cements could be modified and improved with the presence of NS, due to the filler effect of these nanoparticles, densifying the matrix and decreasing the porosity and permeability, this in turn increases the RC, as for microsilica.
4. Conclusions
Lower w/c ratios accelerated the hydration reactions and this effect was more pronounced for the CPO 40 for the CSA.
The CSA reacted faster than CPO 40, and consequently higher initial compressive strength.
Adding SP retarded hydration reactions of cement and its effect was reversed when using NS.
Following from that, NS accelerated the hydration reactions, up to a limit, otherwise this effect was reverted. In this study the optimal acceleration was observed at 3.5% NS.
SP addition substantially improved the compressive strength of CPO 40, for the case of the CSA, the behavior was the opposite, with the addition of SP, cement developed only 50% of the compressive strength achieved by the reference, except when using 5% NS, where the results were superior compared with those obtained for pure CSA. These results were obtained at a w/c constant ratio.
With constant fluidity, the results indicated hat with 1% NS the compressive strength was improved up to about 60% compared to cements without additions; due to an acceleration effect acting as nucleation sites and also its pozzolanic behavior, which was more pronounced at early ages, as observed in the calorimetry results.
In general, it was found that adding NS in both cements, the sulfate attack was not as pronounced as compared to samples without any additions.
For the case of CPO 40 this addition gives the concrete the characteristic of resistance to chemical attack by sulfates, when originally it is an ordinary Portland cement; increasing its added value. However, lower than 5%NS contents do not offer advantages in in this chemical resistance.
For the CSA, the presence of NS reduced the sulfate attack up to 95% compared to neat CSA. This is relevant because the CSA without adding any additional material exceeded the limit established by the Mexican Standard for sulfate resistant cement only after two months of exposure.
5. Acknowledgements
Financial support from CONACYT and CEMEX for the realization of this project is widely appreciated.
REFERENCES
ASTM C1012/C1012M - 15, Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution. [ Links ]
Belkowit J. S., Armentrout D. (2010) “An investigation of Nano Silica in the Cement Hydration Process”, Proceeding 2010 Concrete Sustainability Conference, National Ready Mixed Concrete Association, U.S.A., pp. 1-15 [ Links ]
Björnström J., Martinelli J., Matic A., Borjesson L., Panas I. (2004), “Accelerating effects of colloidal nano-Silica for beneficial calcium-silicate-hydrate formation in cement”, Chemistry Physic Letters; 392, pp. 242-248 [ Links ]
Chung D. L. (2012) “Carbon materials for structural self-sensing, electromagnetic shielding and thermal interfacing”, Elsevier, CARBON 50, pp. 3342-3353 [ Links ]
Hosseni P., Booshehrian A., Delkash M., Ghavami S., Zanjani M. K. (2009), “Use of Nano-SiO2 to Improve Microstructure and Compressive Strength of Recycled Aggregate Concretes”, Nanotechnology in Construction 3, pp 215-221 [ Links ]
Hosseni P., Booshehrian A., Farshchi S. (2010), “Influence of Nano-SiO2 addition on Microstructure and mechanical Properties of Cement Mortars for Ferrocement”, Transportation Research Record; Journal of the transportation Research Board No. 2141, pp. 15-20 [ Links ]
Jewell R. B. (2015) “Influence of Calcium Sulfoaluminate Cement on the Pullout Performance of Reinforcing Fibers: An Evaluation of the Micro-Mechanical Behavior”, PhD Thesis, Civil Engineering, University of Kentuky. [ Links ]
Li H., Xiao H., Yuan J., Ou J. (2004) “Microstructure of cement mortar with nano-particles”, Composites Part B: Engineering, 35, pp. 185-189 [ Links ]
Ma B., Ma M., Shen X., Li X., Wu X. (2014), “Compatibility between a polycarboxylate superplasticizer and the belite-rich sulfoaluminate cement: Setting time and the hydration properties”, Construction and Building Materials, 51, pp. 47-54 [ Links ]
Mondal P., Shah S. P., Marks L. D., Gaitero J. J. (2010), “Comparative Study of the effect of Microsilica and Nanosilica in concrete”, Transportation Research Record; Journal of the transportation Research Board No. 2141, pp. 6-9 [ Links ]
Morteza B., Baghbadrani M., Aslani F. (2014), “Performance of nano-Silica modified high strength concrete at elevated temperatures”, Construction and Building Materials, 68, pp. 402-408 [ Links ]
Norma NMX-C-085-ONNCCE-2010, Industria de la construcción - Cementos hidráulicos - Determinación estándar para el mezclado de pastas y morteros de cementantes hidráulicos. [ Links ]
Norma NMX-C-414-ONNCCE-2010, Industria de la construcción-Cementantes- Especificaciones y método de ensayo. [ Links ]
Puertas F., Vázquez T. (2001), “Hidratación inicial del cemento. Efecto de aditivos superplastificantes”, Materiales de Construcción 51(262), pp 53-61. [ Links ]
Puertas F., Santos H., Palacios M., Martínez S. (2005), “Polycarboxylate superplaticizer admixtures: effect on hydration, microestructure and rheological behavior in cement pastes”, Advances in Cement Research, 17, pp. 77-89 [ Links ]
Qing Y., Zenan Z., Deyu K., Rongshen C. (2007), “Influence of nano-SiO 2 addition on properties of hardened cement paste as compared with silica fume”, Construction and Building Materials 21(3), pp. 539-545 [ Links ]
Quercia G., Zpuesz P., Hüsken G., Brouwers J. (2012), “Effects of Amorphous Nano-Silica additions on Mechanical and Durability Performance of SCC Mixtures”, Proceedings of the International Congress on Durability of Concrete (ICDC 2012), 18-21 June, Trondheim, Norway, pp. A2-A4 [ Links ]
Raki L., Beaudoin J., Alizadeh R., Makar J. Sato T. (2010), “Cement and Concrete Nanoscience and Nanotechnology”, Materials, 3(2), 918-942 [ Links ]
San Filippo J. M., Muñoz J. F., Isabel Tejedor M., Anderson M. A., Cramer S. M. (2009), “Nanotechnology to Manipulate the aggregate-Cement Paste Bond Effects on Mortar Performance”, Nanotechnology in Construction 3, pp. 29-33 [ Links ]
Shah S. P., Konsta-Gdoutos M. S., Metaxa Z. S., Mondal P. (2009), “Nanoscale Modification of Cementitious Materials”, Nanotechnology in Construction 3, Proceedings of the NICOM3, pp. 125-130 [ Links ]
Sobolev K., Flores I., Torres-Martinez L. M., Valdez P. L., Zarazua E., Cuellar E. L. (2009) “Engineering of SiO 2 Nanoparticles for Optimal Performance in Nano Cement-Based Materials”; Proceedings of the Nanotechnology in Construction 3 (NICOM3) 01/2009; pp. 139-148. [ Links ]
Received: February 08, 2016; Accepted: April 10, 2016











 text in
text in 








