1. Introduction
Wool is one of the most luxurious fibers used in textiles and clothing by virtue of its appearance, performance, and comfort attributes. In the contrary, wool is the dirtiest natural fiber as it contains around 150 kg/ton of wool wax, 40 kg/ton of suint, 150 kg/ton of dirt, 20 kg/ton of vegetable matter and only 640 kg/ton of wool fiber (Simpson & Crawshaw, 2002). All non-keratinous component in wool fibers are usually removed before wool can be used in textile manufacture, in a number of subsequent wet processes such as scouring and carbonization.
Several wet processes are usually carried out during manufacturing of wool garments; Viz. scouring (Mowafi et al., 2020), carbonization (El-Sayed et al., 2010), bleaching (Abou El-Kheir et al., 2015), and dyeing (El-Hawary et al., 2016). Other processes are used to impart certain functions to wool; viz. enhancing its resistance to felting shrinkage (Kantouch et al., 2007; Kantouch et al, 2011), pilling (Kantouch et al., 2008), moth attack (Jose et al, 2017), and microorganisms (Kantouch et al, 2013; Mowafi et al. 2014). Furthermore, many investigations have been reported to improve the dimensional stability (El-Sayed, 2006), wettability (Kantouch et al, 2012), dyeability (El-Shemy et al., 2020), and printability (Kantouch et al., 2006; El-Shemy et al., 2020) of wool. Most of these wet processes result in discharge of by-products; some of which are categorized as polluting substances. Wool wax is produced during wool scouring. It is a natural substance consists of fats and oils (Lisovac & Shooter, 2003). Wool wax in its crude form is dark, highly viscous and greasy paste. So, the crude wool wax must be refined before it can be used for different fields (El-Sayed et al., 2018). The refined wool wax is generally known as lanolin.
Lanolin is a pale-yellow substance with pronounced emollient properties. It is insoluble in water, but it can mix without separation with twice its weight of water, sparingly soluble in cold alcohol, more soluble in hot alcohol, and freely soluble in ether and chloroform. Lanoline has versatile properties that make it an appropriate candidate for various possible industrial applications (Patel et al., 2001).
Lanolin exhibits anti-corrosive, non-toxicity, biodegradability and lubrication properties. Consequently, it meets the requirements of some industrial applications in different fields. However, only few applications of lanolin in textile processing were reported in the literature. Lanolin was applied to nylon hosiery to obtain variation in the appearance and handle or feel of the hosiery (Doyle & Copenhaver, 1958). In 1968, du Pont patented a process for coating polyester fibers with a mixture of lanolin and oily silicon for imparting a cashmere-like feel to polyester fabrics (Abashian, 1968). Lanolin was successfully utilized to impart superhydrophobic character to viscose fiber (Khattab et al., 2019). It was applied in pigment printing of textiles as a binder (El-Shemy et al., 2020). Lanoline was also reported as an emollient that gives a soft finish to some textiles (Sengupta & Behera, 2014). The utilization of all by-products produced during the preparatory wet processes of natural fibers; Viz. keratin from wool wastes and sericin from degumming of natural silk, is an ecologically and economically-demanded work (Abou Taleb et al., 2019; Abou Taleb et al., 2020; El-Newashy et al., 2019; El-Sayed et al., 2019; Mowafi et al., 2018).
Softeners are of great importance in textile processing to achieve a soft handle to improve wearability. There are four main types of softeners; namely anionic, cationic, nonionic, and amphoteric (Bajaj, 2001). The nonionic softeners are the most widely used types which comprise polyethylenes, glycerides, ethoxylates, and ethoxylated fatty acids. Nonionic softeners are characterized by excellent compatibility with other ingredients in the finishing bath, effective fiber lubrication and high resistance to discoloration or yellowing (Doyle & Copenhaver, 1958). Fatty acids and their derivatives have been reported as cores for softener compositions (Bahmaei et al., 2011; Duru & Şahin, 2019; Friedli et al., 2001; Tyagi et al., 2006). However, none of these formulations comprised lanolin or fatty acids extracted from it.
This work is devoted to extraction, purification, and characterization of wool wax from the scouring effluent of different wool breeds. Fatty acids will be extracted from the purified wool wax (lanolin) and then reacted with polyethylene glycol to form a condensate with nonionic softening effect. The prepared condensate will be used in softening of wool fabric surface without deterioration of the fabric’s inherent customer-demanded properties.
2. Materials and methods
2.1. Materials
Raw wool fleece from different localities; namely Australia, South Africa, Argentine and Peru were kindly supplied by Mediterranean Wool Industries (MWI) Company, El-Sadat City, Egypt. Egyptian wool fleece (Barki) of main fiber diameter 36 µm was obtained from the local market. Pure scoured crossbred twill weave wool fabrics (176 g/m2 were purchased from Misr Company for Spinning and Weaving, Egypt. The fabric density in warp and weft directions is 35 and 33 thread/cm, respectively. The yarn counts (Nm) in warp and weft directions is 39 and 63, respectively.
Polyethylene glycol (PEG) of molecular weights 2000 was purchased from Sigma - Aldrich. The crosslinking agent epichlorohydrin (ECH) was provided by Clariant. Egyptol PLM is a non-ionic wetting agent based on nonyl phenol ethoxylate, was kindly supplied by Starch and Yeast Company, Alexandria, Egypt. Absolute ethanol (90 %), magnesium chloride hexaydrate, calcium chloride dihydrate, sodium carbonate, and sodium chloride were purchased from ADWIC, Cairo, Egypt.
A commercial non-ionic silicon softener was used to compare its softening action with that of the prepared condensate (its name is not given here to evade confrontation with the manufacturer).
2.2.1. Separation of impurities
Dry vegetable contaminants were thoroughly removed from all wool fleeces in our labs using forceps and other related tools. Water-soluble impurities (salts and sweat) were removed by rinsing with tap water. The samples were left to dry at ambient temperature.
2.2.2. Scouring of wool and extraction of wool wax
Scouring of wool fleeces was carried out using aqueous solution of sodium carbonate (2g/L) for 1 h at the boil. Wool fleece was then drained from the bath and the boiled water containing wax was then centrifuged for 15 min at 8500 rpm to get rid of excess dusty materials. Wool wax was separated from the residual water using rotatory evaporator
2.2.3. Recovery and purification of wool wax
About 5 g of obtained wax was thoroughly mixed with Na2SO4, to eliminate water, and placed into the cartridge with 125 mL of solvent (dichloromethane). Extraction was conducted for 4 h and finally the solvent was evaporated to calculate the percentage of extracted wax (López-Mesas et al., 2003).
Purification of wool wax was undergone by bleaching in a bath containing 4 volume hydrogen peroxide at 40ºC for 1h in presence of sodium silicate as a stabilizer for H2O2 (López-Mesas et al., 2007).
2.2.4. Separation of fatty acids
Wool wax (100 g) from Egyptian wool fleece was added to 400 mL of 1 M ethanolic sodium hydroxide solution and the mixture was refluxed for 6 h at pH 8.5 (adjusted by hydrochloric acid). Simultaneously, 400 mL of ethyl ethanoate and 200 mL of distilled water were added to the mixture with continuous stirring for 30 min at 60 °C. A 100 mL of 0.8 molar solution CaCl2.2H2O was added to the mixture at the same temperature to form calcium soap. The resulting mixture was cooled to 20 °C, stirred for 30 min and allowed to stand overnight, whereby the mixture separated into three layers. The supernatant and the middle layers are retained by decanting and filtering the mixture. Solvent was removed from the supernatant layer. The middle layer, which was obtained by filtering the remaining layers, was added to 100 mL of water and the mixture was acidified to pH 4.0 with HCl, washed with 100 mL water 3 times and dried (Senda et al., 1976). Finally, we obtained 76 g of wool fatty acid (WFA) of an acid value 114.5.
2.2.5. Preparation of fatty acids/PEG condensate
In a 500 mL round flask equipped with the Dean and Stark apparatus, equimolar amounts of PEG and WFA are mixed together (assuming that the main fatty acid in WFA is C16). The mixture was melted at 60 ºC in a thermostatic oil bath with porcelain balls (2 mL in diameter) followed by thorough mixing with 5 g of H2SO4 (98 %, w/v) as a catalyst/kg reaction mixture. The temperature was raised until 150°C and the reaction was further continued for 8 h. Finally, the contents of the flask were cooled down to room temperature.
The produced esters were separated from unreacted fatty acid and PEG by dissolving the products in ethyl acetate at a liquid/product ratio of 10:1 at 35 °C, and transferred to a separating funnel (about 3 times the volume of the ethyl acetate solution). Five successive washing cycles were conducted for the ester solution, each with an equal volumes of an aqueous solution containing NaCl (30 wt. %) and NaOH (2 wt. %) to remove the fatty acid and catalyst), followed by 5 successive washings, each equivolume amount, to remove salinity and remnant alkalinity. The ethyl acetate layer was then evaporated in a rotatory evaporator at 80°C in a vacuum until dryness to get the mixture of esters (Slade, 1998).
2.2.6. Treatment of wool fabric with the prepared condensate
Adopting pad-dry-cure method, wool fabrics were padded with a solution containing 30 and 60 g/L WFA/PEG condensate, Egyptol PLM (2 g/L), in the presence of 5 g/L ECH and 5 g/L MgCl2. 6H2O, to a wet pick up of 80%. The samples were dried at room temperature overnight, cured at 120 °C for 6 min, and cooled at room temperature for at least 24 hours before testing.
In another experiment, wool fabrics were treated with commercial silicon softener using pad-dry-cure technique. The samples were padded with 30 and 60 g/L silicon softener, to a wet pick up of 80%. The samples were dried at ambient temperature and cured at 135 °C for 4 min.
2.3.1. Determination of the amount of extracted wool wax
The loss in weight of wool fleece after scouring or Soxhlet extraction was calculated from the following relation:
Weight loss (%) = [(W1 - W2)/W1] × 100
Where W1: is the weight of wool sample before extraction/scouring.
W2: is the weight of wool sample after extraction/scouring.
Consequently, the amount of extracted wool wax after evaporation of water was also determined by subtraction.
2.3.2. Physical properties of lanolin.
The physical properties of the extracted lanolin from different breeds were monitored. These include color, odor, melting point, moisture content and ash content. The melting point was measured according to the Japanese Standards of Cosmetic Ingredients (Central Pharmaceutical Affairs Council, 1985).
2.3.3. Chemical Properties of Lanolin
Saponification value
Saponification value is a measure of the free acid and saponifiable ester groups. It is expressed as the number of milligrams of potassium hydroxide required to neutralize the free acids and to saponify the esters contained in one gram of the material.
The standard method ASTM D5558 - 95 (2017) was adopted for this test. An accurately weighed wool wax sample was introduced into 250 ml Erlenmeyer flask. 25 mL of ethanolic potassium hydroxide and 25 mL of neutralized ethanol, and 3 glass beads were added in the flask. The flask was shaken frequently until all solid material was dissolved or well dispersed and the solution was refluxed for one hour. Each flask was removed from the hot plate and 1 mL phenolphthalein was added. The solution was titrated against 1N HCl with vigorous swirling until the pink color just disappears. The saponification value of lanolin was calculated from the following relation:
Iodine value
Iodine Value is a measure of the degree of unsaturation of the material. It is expressed as grams of iodine absorbed, under the prescribed conditions, by 100 grams of the material. The iodine value of the extracted lanolin was determined according to the ASTM-D-1959 standard method.
Acid value
Acid value is expressed as milligrams of potassium hydroxide required to neutralize the free acids in one gram of the material. It is measured in accordance with the standard test method ASTM Method D, 1980-87 & 1991.
Hydroxyl Value
This method determines the number of hydroxyl group present which can be acetylated under the conditions of this test. Hydroxyl value (or acetyl value) is defined as the number of milligrams of potassium hydroxide necessary to neutralize the acetic acid which combines on acetylation of 1 g of sample. The hydroxyl value is measured according to the standard method ASTM 111A-1.
2.3.4. Characterization of the prepared WFA/PEG condensate
Total conversion
The extent of conversion of reactants to total esters (mono and di) was calculated by determining the amount of fatty acid before and after the reaction. This was done by measuring the acid values before (A1) and after (A2) the reaction (ASTM Method D, 1980-87 & 1991). Corrections were made to calculate the acid value of catalyst (Ac). The total conversion was calculated from the following relation:
% Total conversion = [(A1 - A2)/(A1 - Ac)] × 100
FTIR spectroscopy was carried out for the ester products of the condensation reaction. FTIR spectra were done by the Perkin Elmer spectrum one FTIR with an optical system that conducts data collection over a total range of 7800-370 cm-1 with the best resolution of 0.5 cm-1. The samples were prepared for testing by mixing a 1mg sample into 200 mg KBr, forming a disc and scanning in a spectrophotometer.
2.3.5. Fabric testing
The add-on was determined by measuring the difference in the weight of the untreated and WFA/PEG condensate-treated samples. Weight add-on measurements were performed three times for each sample and were calculated using Equation:
Where W1 is the initial weight of the sample and W2 is the weight of WFA/PEG-treated sample.
Surface roughness of wool fabric samples were measured by KAWABATA Evaluation system for fabrics-Surface tester KES-FB4-A. (KATO-TECH CO. LTD, Japan). Air permeability of the fabrics was examined according to ASTM D737 standard method on an FX 3300 air permeability tester (TEXTEST AG, Switzerland) at a pressure of 100 Pa. Yellowing index was evaluated by the Color-Eye 3100 spectrophotometer supplied by SDL inter, England (Welch & Peter, 1997). The fabric area shrinkage was assessed using the IWS TM-31 standard method (Wascator).
Water contact was measured on OCA-15EC (Dataphysics GmbH, Germany) with software.
Contact angle properties were carried out with 10 µL drops of triple distilled water. Wool substrates were connected to glass cover slips using double sided adhesive tape to create a planar surface.
The fiber morphology was detected by scanning electron microscopy using scanning electron microscope (ZEISS LEO 1530 Gemini Optics Lens scanning electron microscopy with scanning voltages of 30 kV).
3. Results and discussion
In an attempt to introduce the Egyptian coarse wool as a suitable candidate, like finer wool grades, for extraction and utilization of wool wax, we conducted a comparative study for the amounts and properties of lanolin derived from different wool breeds.
3.1. Extraction of wool wax
Removal of wool wax from different wool breeds was carried out using aqueous solution of sodium carbonate; the alkalinity of Na2CO3 solution helps in hydrolysis of the thioester bond that binds wool wax with wool keratin (Koerner et al., 1995). To assign the proper conditions for removal of wool wax from wool fleece using sodium carbonate solution, this process was carried out using various concentrations of Na2CO3, at different reaction temperatures and times. Results of these investigations are shown in Tables 1-3.
Table 1 Effect of temperature on efficiency of sodium carbonate in removal of wool wax from different wool breeds (2 g/L Na2CO3 for 1 h).
| Wool breed | Amount of extracted wool wax
(%)at |
|||
| 100
°C |
90
°C |
75
°C |
60
°C |
|
| Egyptian | 13.1 | 12.9 | 11.2 | 9.4 |
| Australian | 26.3 | 26.1 | 22.8 | 18.8 |
| South African | 24.5 | 22.6 | 21.9 | 18.0 |
| Peruvian | 25.4 | 22.0 | 21.7 | 17.7 |
| Argentinean | 25.0 | 21.9 | 21.6 | 17.4 |
Table 2 Effect of time on efficiency of sodium carbonate in removal of wool wax from different wool breeds (2 g/L Na2CO3 at 90 °C).
| Wool breed | Amount of extracted wool wax (%)
after |
|||
| 15
min |
30
min |
45
min |
60
min |
|
| Egyptian | 4.0 | 9.2 | 11.6 | 12.9 |
| Australian | 9.4 | 17.1 | 23.4 | 26.1 |
| South African | 8.3 | 16.6 | 22.8 | 22.6 |
| Peruvian | 7.8 | 16.2 | 21.7 | 22.0 |
| Argentinean | 7.0 | 16.1 | 20.9 | 21.9 |
Table 3 Effect of concentration of sodium carbonate on its efficiency to remove wool wax from different wool breeds (at 90 °C for 60 min).
| Wool breed | Amount of extracted wool wax
(%)using |
|||
| 0.5 g/L
Na2CO3 |
1.0 g/L
Na2CO3 |
1.5 g/L
Na2CO3 |
2.0 g/L
Na2CO3 |
|
| Egyptian | 5.7 | 10.1 | 12.1 | 12.9 |
| Australian | 11.5 | 20.0 | 21.9 | 26.1 |
| South African | 10.7 | 19.9 | 21.8 | 22.6 |
| Peruvian | 9.4 | 17.7 | 19.9 | 22.0 |
| Argentinean | 9.5 | 17.5 | 19.9 | 21.9 |
Data of Table 1 clarifies that as the temperature increases from 60 °C until 90 °C, the amount of wool wax extracted from raw wool fleece increases whatever the breed of wool used. Further increase in the extraction temperature to 100°C resulted in limited enhancement in the amount of extracted waxy material. Similar trend was encountered upon increasing the extraction time from 15 min up to 60 min (Table 2). Further increase in the extraction time (results are not shown here) has no appreciable effect on the amount of extracted wool wax.
As shown in Table 3, there is a direct relationship between the amount of extracted wool wax and the concentration of Na2CO3 used for this purpose. Higher concentrations of sodium carbonate were not used in this study to avoid conversion of cysteine residues into of lanthionine moieties along keratin macromolecules in an irreversible reaction which results in fiber deterioration (Zahn et al., 2003).
Based on the results declared in Tables 1-3, the appropriate conditions of removal of wool wax from raw wool fleece are: 2 g/L Na2CO3 at 90 °C for 1h.
3.2. Physical Properties of Extracted Lanolin
The physical properties of lanolin extracted from different wool breeds are shown in Table 4. Data of this table reveals that there is no appreciable difference between the physical properties of lanolin extracted from Egyptian wool with that extracted from Marino, Argentinean, Peruvian, and South African wool fleece. Only the ash content in Egyptian lanolin is almost twice that of the other wool fleece breeds.
Table 4 Physical properties of purified unbleached lanolin extracted from Egyptian wool fleece.
| Wool breeds | Color | Odor | Ash content (%) | Moisture content (%) | Melting point (°C) |
| Egyptian | Yellow-brown | Nearly
Putrid |
0.9 | 0.8 | 43 - 45 |
| Merino | Yellow-brown | nearly
putrid |
0.4 | 0.8 | 41 - 42 |
| South African | Yellow-brown | nearly
putrid |
0.4 | 0.9 | 42 - 43 |
| Argentinian | Yellow-brown | nearly
putrid |
0.4 | 0.7 | 42 - 43 |
| Peruvian | Yellow-brown | nearly
putrid |
0.5 | 0.7 | 41- 42 |
3.3. Chemical properties of extracted lanoline
The chemical composition of lanolin extracted from different breeds of wool fleece is summarized in Table 5. The iodine value of lanoline extracted from Egyptian wool fleece is slightly higher than the other extracted lanolin indicating the relatively higher degree of unsaturation in the former than the latter.
Table 5 Chemical composition of lanolin extracted from different wool fleece breeds. Conclusions.
| Wool breeds | Iodine Value |
Saponification value |
Acid value |
Hydroxyl value |
| Egyptian | 17.2 | 88 | 5.8 | 19.5 |
| Merino | 16.4 | 72 | 5.5 | 19.6 |
| South African | 16.6 | 79 | 5.7 | 19.4 |
| Argentinian | 16.7 | 77 | 5.5 | 19.9 |
| Peruvian | 16.7 | 78 | 5.4 | 19.8 |
The saponification value of Egyptian lanoline is remarkably higher than Merino lanolin. The other lanolin breeds extracted from other wool fleece breeds have intermediate saponification values between Egyptian and Merino wool fleece. This implies that the number of ester links and free carboxylic groups are the highest in Egyptian wool fleece and the lowest in merino wool.
The difference in acid as well as hydroxyl values of lanolin extracted from different wool fleece breeds is insignificant. These findings indicate that the hydroxy acid and alcohol contents are similar in all examined lanolin samples from different wool breeds.
3.4. WFA/PEG condensate
The ester formation reaction between monobasic fatty acid (WFA) and dihydric alcohol (PEG) usually leads to formation of a mixture of monoesters and diesters as shown in the following equation:
The total conversion from the reactants to the produced mono- or di-esters was found to be 18.7% in absence of the catalyst (H2SO4) and 79.2% in presence of the catalyst. As shown in the above equation, the reaction is reversible and therefore concentrated sulphuric acid was added to absorb the produced water and prevent the backward reaction. Consequently, the equilibrium was shifted to the forward direction and the total conversion percent increased.
The FTIR spectra of the WFA, PEG, and WFA/PEG condensate are shown in Figure 1. The FTIR spectra of WFA show a sharp intense band at 2915 cm-1 and a sharp medium band at 2849 cm-1 which correspond to the asymmetric and symmetric stretching vibration of aliphatic methylene groups, respectively (Carrer et al., 2018). The absorption band appears at 1705 cm-1 of the FTIR spectra of the extracted fatty acids is a characteristic band for the C=O stretching vibration of carboxylic acid. The FTIR spectra of PEG show a broad band at 3416 cm-1 which is characteristic for the O-H stretching vibration, another band at 2879 cm-1 which corresponds to the aliphatic -CH2- group, the band at 1464 cm-1 belongs to the -CH2- bending vibration, and the quite significant band observed at 1097 cm-1 stands for the C-O stretching incorporated in intermolecular H-bond formation together with the OH group (Dinça & Güner, 2017).
The FTIR spectra of the WFA/PEG condensate has a new band of medium intensity at 1727 cm-1 which corresponds to the C=O stretching vibration of the ester bond formed as a result of the reaction of carboxylic group of the fatty acids with hydroxyl end groups of the PEG. Usually, the normal range of ester bond appears at 1735-1750 cm-1 and the shift of the ester band to 1727 cm-1 may be a clue that there is some sort of α, β-unsaturated form.
3.5. Performance attributes of the finished fabric
Wool fabrics were treated with WFA/PEG with two different concentrations 3% and 6% and some of the inherent properties of wool were evaluated and the results were presented in Table 6. As might be expected, the weight-add on increases from 7.13% and 11.6% upon increasing the amount of condensate used for treatment of wool from 3% to 6 % (w/v). Data in Table 6 clarify the following:
Table 6 Physic-mechanical properties of wool fabric treated with WFA/PEG condensate.
| Treatment (w/v) |
Add on (%) |
Surface roughness (μm) |
Air permeability (cm3/cm2.S) |
Area shrinkage (%)c | Yellowness index |
||
|---|---|---|---|---|---|---|---|
| Before rinsing a |
After rinsing b |
3 x 5Ad | 3 x 5Ae | ||||
| Untreated | -- | 8.5 | 8.4 | 20.5 | 32.6 | 46.3 | 13.8 |
| 3% WFA/PEG | 7.13 | 1.5 | 1.9 | 12.1 | 12.6 | 17.1 | 14.4 |
| 6% WFA/PEG | 11.6 | 1.1 | 1.5 | 10.3 | 10.4 | 15.9 | 14.8 |
| 3% Silicone softener | 9.11 | 1.3 | 1.6 | 11.9 | 26.8 | 29.4 | 14.2 |
| 6% silicone softener | 11.7 | 1.1 | 1.3 | 10.0 | 23.5 | 25.7 | 14.7 |
a: sample was tested directly after curing followed by drying at ambient temperature
b: rinsing was carried out by running tap-water
c: Average of warp and weft measurements
d: 3 washes using the program cycle 5A
e: 5 washes using the program cycle 5A
Treatment of wool fabric with the prepared WFA/PEG condensate led to improvement of the fabric surface smoothness as indicated by the lower roughness values of the untreated as well as the treated samples. The results show also that rinsing of the treated sample resulted in loss of about 26 % or 36 % of its smoothness for the two used condensate concentration. However, the smoothness of the rinsed treated fabric is much better than that of the undertreated sample. The fabric softness effect imparted by WFA/PEG condensate is due to the lubrication of wool surface by coating thin film layer (Kang & Kim, 2001). The smoothness effect imparted to wool fabric surface by WFA/PEG is akin to those samples treated with the used silicone softener.
The air permeability of wool fabric treated with either the prepared or the commercial surface decreases remarkably relative to the untreated wool samples.
The resistance of wool to felting shrinkage was highly improved upon treatment with WFA/PEG condensate, presumably due to masking of the scales of wool fiber by a layer of WFA/PEG. This is in harmony with the findings of the scanning electron microscopic investigation. On the other hand, limited improvement in the resistance of wool to felting shrinkage was observed upon treatment with the used silicone softener. This is a clue that the prepared condensate has a dual function; namely the ability to impart smoothing action as well as shrink resistance. This implies that the prepared condensate is unique among other softeners. Results of the fabric roughness and felting shrinkage tests indicate that the durability of silicone softener-treated fabric is better than that treated WFA/condensate.
The degree of yellowness of wool fabrics treated with WFA/PEG condensate is found to be nearly similar to those samples treated with the used commercial silicone and the untreated fabric. Nevertheless, the increase in the yellowness index of the treated wool samples relative to the untreated one is within the acceptable limits.
3.6. Fiber morphology
The changes in the surface of WFA/PEG-treated wool fabric were traced using scanning electron microscopy. As shown in (Figure 2a), wool fabric surface is covered with scales which are the main reason of shrinkage of wool fabric during washing in domestic washing machine. The scale structure of wool is a secondary reason for its roughness. Figure 2b showed that the scales were covered by a layer of the WFA/PEG condensate. Consequently, the treated fabric exhibited better degree of smoothness and resistance to felting shrinkage (Bahi et al., 2007). This finding was found to be compatible with the results of contact angle and roughness.
3.7. Contact angle measurement
Figure 3 and Table 7 show the results of the static contact angle measurements of untreated as well as WFA/PEG treated wool fabrics. The hydrophobic nature of wool results in a high contact angle (130.3°). Surprisingly, the wettability of wool fabric was highly improved upon treatment with 3% or 6% of the prepared WFA/PEG condensate and the contact angle of the treated wool samples is zeros. And what is more surprising is that upon rinsing of the WFA/PEG treated wool fabric with running water, the contact angle of the rinsed sample is 113.5°, in case of treatment with 3% WFA/PEG condensate and 124.2° , in case of treatment with 6% WFA/PEG condensate. This analysis was repeated 7 times and the results were nearly similar.
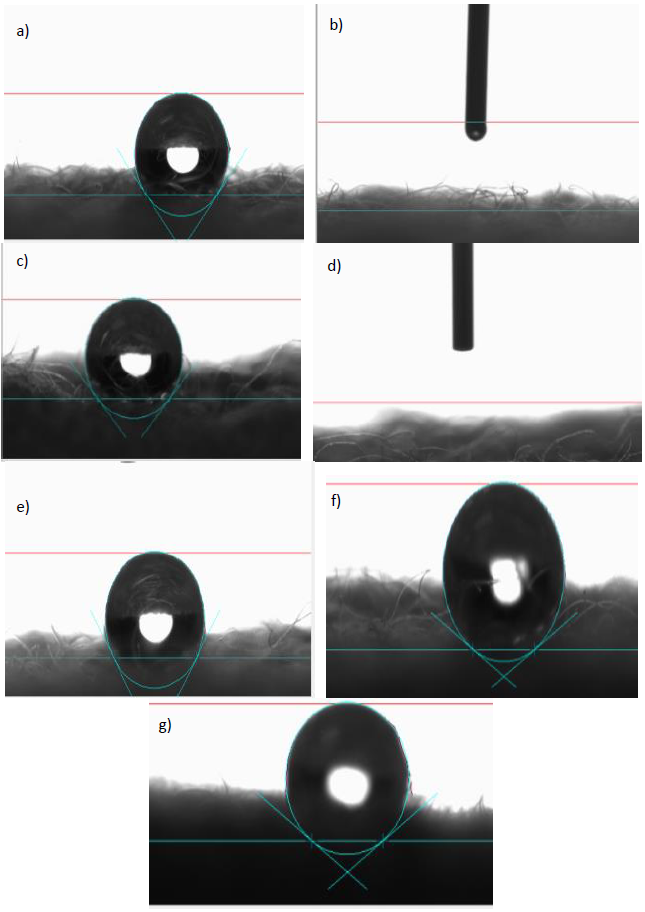
Figure 3 Static water contact angles of a) the untreated wool fabric, b) 3% WFA/PEG treated wool fabric before rinsing, c) 3% WFA/PEG treated wool fabric after rinsing, d) 6% WFA/ PEG treated wool fabric before rinsing, e) 6% WFA/PEG treated wool fabric after rinsing, f) 6% silicon softener ( pick up 80%) before rinsing, and g) 6% silicon softener ( pick up 80%) after rinsing.
Table 7 Static contact angles of untreated as well as WAF/PEG treated wool fabrics.
| Sample | Static water contact angle (°) |
|---|---|
| Untreated wool fabric | 130.3° |
| 3% WFA/PEG treated wool fabric before washing | 0° |
| 3% WFA/PEG treated wool fabric after washing | 113.5° |
| 6% WFA/PEG treated wool fabric before washing | 0° |
| 6% WFA/PEG treated wool fabric after washing. | 124.2° |
| 6% silicon softener ( pick up 80%) before rinsing | 149.5° |
| 6% silicon softener ( pick up 80%) after rinsing | 144.8° |
These results were confirmed by measuring the wettability of the treated and untreated samples according to the AATCC Test Method 39-1993. The drop of water spreads instantaneously on wool fabric treated with WFA/PEG (not rinsed) while it takes more than 300 second in case of both untreated wool fabric or that treated with WFA/PEG condensate followed by rinsing with running tap-water. These findings indicate that the treatment of wool with the prepared condensate didn’t withstand the effect of washing. Further work should be conducted to impart durability to tread fabrics against washing. On the other hand, the contact angles of wool fabric treated with commercial silicone softener before and after rinsing are 149.5° and 144.8°; results which assure the durability of this treatment.
4. Conclusions
The physical properties of lanolin extracted from Egyptian wool are similar to those of that extracted from Marino, Argentinean, Peruvian, and South African wool fleece, with the exception of the ash content, which is remarkably higher in lanolin extracted from Egyptian wool.
The similarity in chemical composition and physical properties of lanolin extracted from Egyptian wool fleece to those extracted from other wool breeds means supports the idea that the former can be utilized in the same applications commonly encountered in the market.
The fatty acids extracted from lanolin were successfully hybridized with PEG to form a condensate which is a suitable candidate to act as a surface coating for smoothness of wool fabric surface. The prepared nonionic softener imparts smooth hand to wool fabric without any adverse effect on the inherent properties of wool. The performance attributes of wool fabrics treated with the prepared condensate are similar to those of wool fabric treated with a commercial softener. Wool fabrics treated with WFA/PEG condensate exhibited adequate resistance to felting shrinkage during agitation in washing machine; this is an important advantage of the prepared softener over the other commercially available softeners.
Further work should be directed towards improvement of the treated fabric durability. Furthermore, we are currently undergoing sincere work to rationalize the enhanced wettability of treated sample.











 nueva página del texto (beta)
nueva página del texto (beta)




