Highlights:
Aspergillus terreus was isolated from soil contaminated with toxic metals.
A. terreus showed high tolerance index to lead (0.9) and mercury (0.8).
Minimum inhibitory concentration with lead and mercury was greater than 500 ppm.
Cadmium was the most toxic with indexes of 0.56 and 0.2 at 50 ppm and 100 ppm, respectively.
The index was high (0.9) at 64 ppm for the multimetal system (cadmium, chromium, mercury and lead).
Introduction
Releasing toxic metals into the environment, due to mismanagement of anthropogenic activities, is an issue of great global concern. Due to the toxic nature of metals, their ability to accumulate, and the fact that they cannot be converted into non-toxic forms, these substances represent a threat to the environment and public health (Desai, Patel, & Joshi, 2016). There are just over 20 toxic metals, but those of highest priority are arsenic, lead, mercury, and cadmium (Agency for Toxic Substances and Disease Registry [ATSDR], 2019). Such metals, even at very low concentrations, are cytotoxic, carcinogenic, and mutagenic; recently, they have been studied as potential initiators or promoters of cardiovascular diseases (atherosclerosis, hypertension, and myocardial infarction) (Meng, Wang, Li, Yin, & Zhang, 2017; Sevim, Dogana, & Comakli, 2020; Zolfaghari, 2018). These elements have also been reported to affect reproductive health in men (Tariba, 2020).
Some methods to remove toxic metals include ultrafiltration, reverse osmosis and chemical precipitation. Most of them are very expensive and not very efficient when the concentration of metals is very low; in addition, their process generates by-products that are also toxic (Fu & Wang, 2011; Gunatilake, 2015). Due to the above and combined to a change in awareness of environmental problems, there is a need to look for more environmentally friendly and economically viable alternatives (Dhankhar & Hooda, 2011). For this reason, attention has been focused on the search for microorganisms tolerant to toxic metals and with the capacity to remove these metals.
Among microorganisms, fungi are considered superior to single-celled organisms. In the fungi kingdom, filamentous or also known as microscopic fungi are multifaceted components, ubiquitous in the air and subsoil, and often become a dominant group in metal-contaminated habitats (Zafar, Aqil, & Ahmad, 2007). Studies have shown that fungal strains, isolated from contaminated areas, have potential to tolerate toxic conditions; due to their morphological diversity, they are better able to adapt to extreme values of pH, temperature, and nutrient availability (Iram et al., 2009; Kabata-Pendias, 2010); and develop constitutive and adaptive physiological and genetic mechanisms (Bellion, Courbot, Jacob, Blaudez, & Chalot, 2006; Gadd, 2007; Gube, 2016). Moreover, mycelial growth maximizes mechanical and enzymatic contact with the contaminant due to the increased cell-to-surface ratio. The main advantage of fungal strains isolated from contaminated sites is that they are adapted not only to the presence of contaminants, but also to the environmental conditions of the site. Therefore, the objective of the present study was to isolate a microscopic fungal strain from a site with toxic metals and to evaluate the tolerance to these substances as an indicator of the potential for use in removal processes of this type of contaminants.
Materials and Methods
Samples and reagents
A total of four surface samples (10 cm depth) of Technosols (International Union of Soil Sciences [IUSS], 2015) were collected from an abandoned lead extraction mine, located 2 km to the south of the municipality of San Felipe de Jesús, Sonora, Mexico (29° 50' 58.43" N, 110° 14' 48.85" W). Samples were placed in sterile containers and transported to the laboratory for further analysis. As a source of cadmium (Cd2+), chromium (Cr6+), mercury (Hg2+) and lead (Pb2+) metal salts: CdCl2, K2Cr2O7, HgCl2 and (CH3COO)2 Pb(3H2O were used, respectively. Potato dextrose agar (PDA, Difco™) was used as growth medium.
The composition of metals in the soil samples was determined by Energy Dispersive Spectroscopy (EDS) through Transmission Electron Microscopy (TEM).
Isolation of fungal strain
A 1 g soil sample was placed in 100 mL of sterilized distilled water. The mixture was stirred for 20 min at room temperature and serial dilutions (10-1 to 10-4) were prepared. Aliquots of 100 μL of each dilution were taken, placed in Petri dishes with PDA and incubated at 28 °C for 7 days. Colonies were randomly selected, and the seeding procedure was repeated until isolation and purification of fungal strain.
Preparation and inoculation of the spore suspension
The purified strain was inoculated into a 250 mL Erlenmeyer flask with 50 mL PDA and incubated at 28 °C for 7 days. A total of 100 mL of distilled water was added and placed on a magnetic stirrer for 5 min at room temperature. Concentration of spores in the suspension was estimated with a Neubauer chamber. A suspension of about 108 spores∙mL-1 was prepared for inoculation of the culture medium supplemented with toxic metals. Petri dishes with solidified PDA and metals had a hole drilled in the center, where the spore suspension was added to obtain circular-shaped colonies.
Identification of the isolated fungal strain
Fungi were identified based on the main macro and microscopic morphological characteristics in a seven-day culture. Macroscopic characteristics considered were color, shape, and colony type. Microscopic characteristics considered were spore morphology and size, conidiophore, and hyphae, which were documented by light microscopy and scanning electron microscopy (SEM). Structures were stained with lactophenol blue for light microscopy. Moisture-free mycelial samples were used for electron microscopy.
Tolerance index
The tolerance index of the isolated strain was evaluated with cadmium, mercury and lead individually at concentrations of 50, 100, 250, 350 and 500 ppm. As and those metals are considered the most toxic by ATSDR (2019); however, no source of As was available so it was not possible to include it in the study. A multimetal system (mixture of metals) of cadmium, chromium, mercury and lead was also evaluated at concentrations of 4, 8, 16, 16, 64, 80, 120, 200 and 400 ppm; the proportion of each metal was equal. In both systems (single and multimetal), Petri dishes with PDA supplemented with metals were inoculated with fungal spores and incubated at 28 °C for 7 days; every 24 h the radius of the developed colonies was measured. PDA inoculated with the fungal strain, but without metals, was considered as the control treatment. All treatments were carried out in triplicate. Tolerance index was calculated by dividing the radius of the metal colony by the radius of the control colony.
Minimum inhibitory concentration
The minimum inhibitory concentration (MIC) is the lowest concentration to which a substance inhibits the growth of a microorganism (Ezzouhri, Castro, Moya, Espinola, & Lairini, 2009). In this study, the MIC was obtained from the tolerance index experiments.
Statistical analysis
After assessing the normality of the data (Shapiro-Wilk), the differences in the mean values of tolerance indices between metal concentrations were estimated by one-way ANOVA with days as blocks. Significant differences were studied using the Tukey's test (P < 0.05). All tests were performed using GenStat v. 20 (VSN International, 2019).
Results and Discussion
Soil elemental composition
The elemental composition derived by TEM-EDS, indicates the presence of 15 elements (O, Si, Al, Mg, S, Ca, P, Fe, Cl, Ni, K, Zn, Pb, As and Mn) in the soil sample, from which the studied strain was isolated. The presence of Al, Pb and As stands out due to their potential toxicity to a great diversity of living organisms, from microorganisms to man; according to TEM-EDS analysis, these elements are found in proportions of 0.1, 0.11 and 0.14 %, respectively (Table 1). ATSDR (2019) rates As as the most toxic substance, followed by Pb.
Table 1 Elemental composition of soil from the San Felipe de Jesus mine, Sonora, Mexico, determined by energy dispersive spectrometry analysis by transmission electron microscopy (EDS-TEM).
| Element | Percentage (%) |
|---|---|
| O | 33.88 |
| Si | 29.78 |
| Al | 12.71 |
| Mg | 5.33 |
| S | 4.77 |
| Ca | 4.57 |
| P | 3.55 |
| Fe | 2.99 |
| Cl | 0.80 |
| Ni | 0.55 |
| K | 0.45 |
| Zn | 0.29 |
| Pb | 0.14 |
| As | 0.11 |
| Mn | 0.10 |
In addition, the diversity of microorganisms is generally low in stressful environments caused by the presence of toxic substances. Some microorganisms, like plants and animals, have developed strategies that allow them to successfully survive and maintain themselves in stressed communities where other organisms cannot proliferate; for example, Aspergillus and Penicillium produce abundant quantities of spores that resist dispersal and manage to survive long periods of inactivity (Atlas & Bartha, 2001). This coincides with that found in this study, because the isolated strain corresponds to the genus Aspergillus. Limits of tolerance to stressors are what largely determine the inhabitants of stressed environments (toxic compounds, high levels of solar radiation and water scarcity) (Atlas & Bartha, 2001).
Identification of the fungal strain
From its morphology, the isolated microscopic fungal in this study was identified as Aspergillus terreus Thom 1918. Fungi showed fast-growing colonies at 28 °C on PDA, white to light yellow during the first two days of culture, changing to brown on the third day; the colonies on the reverse side were also brown. The mycelium was observed branched with hyaline hyphae greater than 1 μm in diameter. Aspergillus terreus produced abundant spores with a diameter of 1.5 μm (Figure 1a), periform vesicles of 10 to 15 μm (Figure 1b) and 150 μm long conidiophores (Figure 1c). These morphological features are consistent with those reported for A. terreus by Klich (2002) and Atiqah and Zakaria (2017).
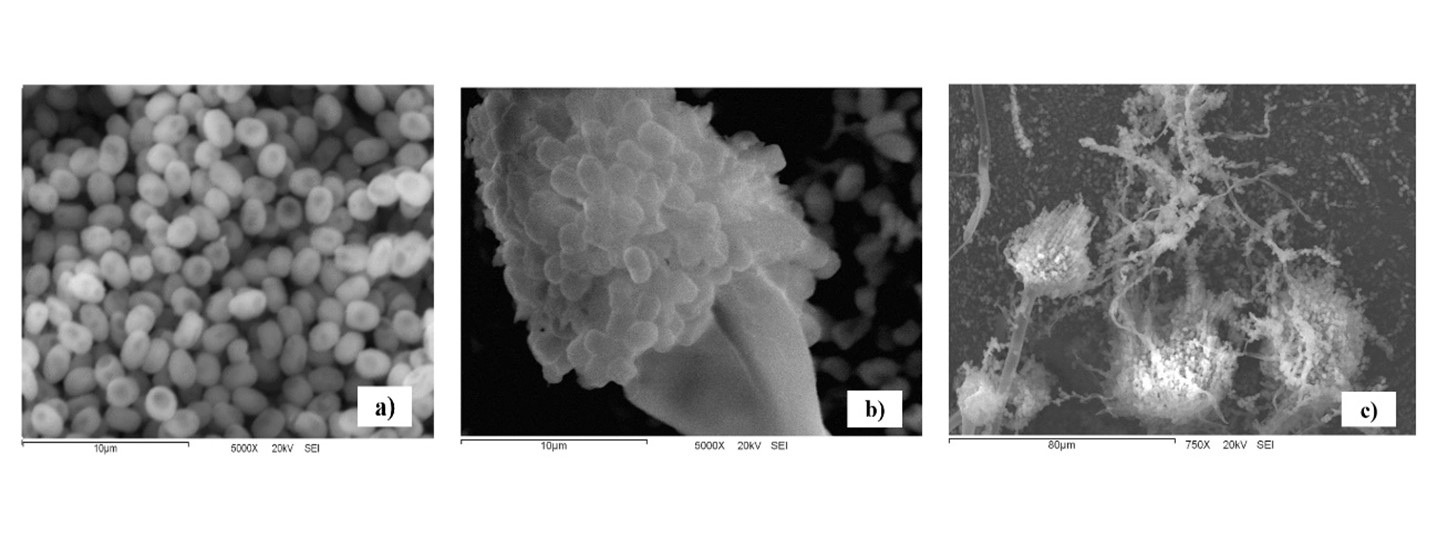
Figure 1 Microscopic features of Aspergillus terreus by scanning electron microscopy (JEOL JSM-5410 LV): a) spores, bar indicates 10 µm; b) conidiophore, bar indicates 10 µm; c) hyphae, conidiophore and spores, bar indicates 80 µm.
Soil microbial community is usually very diverse, especially the microscopic colony found between 10 and 30 cm depth. The microscopic fungi isolated were mainly from Fusarium, Penicillium, Trichoderma, Aspergillus and Geotrichum, which have cosmopolitan distribution (Jiang, Wang, Xue, Cao, & Zhang, 2016; Mohammadian, Ahari, Arzanlou, Oustan, & Khazaei, 2017; Oladipo, Awotoye, Olayinka, Bezuidenhout, & Maboeta, 2018). However, mycological diversity in stressful environments, due to the presence of toxic substances such as metals, is usually lower compared to a healthy environment (Dixit et al., 2015). The presence of Aspergillus in environments contaminated with toxic metals has been reported in a number of studies (Awasthi, Pandey, & Khan, 2017; Rose & Devi, 2018; Villalba-Villalba, Cruz-Campas, & Azuara-Gómez, 2018; Zafar et al., 2007).
Tolerance index
The tolerance index was used to study the growth performance of A. terreus to various concentrations of toxic metals. ANOVA results indicated that days are not significant in the four treatments (Pb, Hg, Cd and mixed metals), but significant differences were found for the tolerance index in response to metal concentrations, as shown in Table 2. According to Oladipo et al. (2018), the metal tolerance index by fungi can be classified as follows: >1 very high, 0.8-0.99 high, 0.6-0.79 moderate, 0.4-0.59 low, and 0.0-0.39 very low.
Table 2 Effect of metal concentrations on the tolerance index (one-way ANOVA) of Aspergillus terreus.
| Metal | gl | F | P |
|---|---|---|---|
| Cd | 4 | 223.55 | <0.001 |
| Pb | 4 | 4.2 | 0.009 |
| Hg | 4 | 4.51 | 0.007 |
| Multimetal | 7 | 33.41 | <0.001 |
Figure 2 indicates that A. terreus was very tolerant to lead at all concentrations tested, with the lowest average tolerance at 100 ppm, with statistical significance (P < 0.05). However, the tolerance index was always greater than 0.9, even at day 7 of growth. Similar results were reported by Sanyal, Rautaray, Bansal, Ahmad, and Sastry (2005), who found that lead was not toxic to Fusarium oxysporum Schltdl. 1824, which grew without problem after exposure to concentrations similar to those reported in this study. Maini et al. (2019) observed high tolerance to lead in Penicillium and Taloromyces; however, the concentrations they evaluated were lower (0.5, 1, and 3 ppm) than those reported in this study. Several Aspergillus species have been reported to exhibit high tolerance to toxic metals (Mungasavalli, Viraraghavan, & Jin, 2007; Thippeswamy, Shivakumar, & Krishnappa, 2014); such is the case of A. niger Tieghem 1867 with the ability to grow at 5 000 ppm lead (Iskandar, Zainudin, & Tan, 2011). High tolerance of A. terreus to lead may be due to constant exposure to this metal in its local environment, i.e., in the soil from which fungi were isolated. For certain fungal species, exposure to toxic substances in their habitat can trigger adaptive changes that allow them to remain viable under such stress conditions (Valix, Tang, & Malik 2001). Therefore, the high tolerance of these fungi to lead may be related to the mechanisms developed to manage the influx of lead; moreover, in this study, no lag phase (adaptation phase) delay effect of A. terreus was detected due to lead.
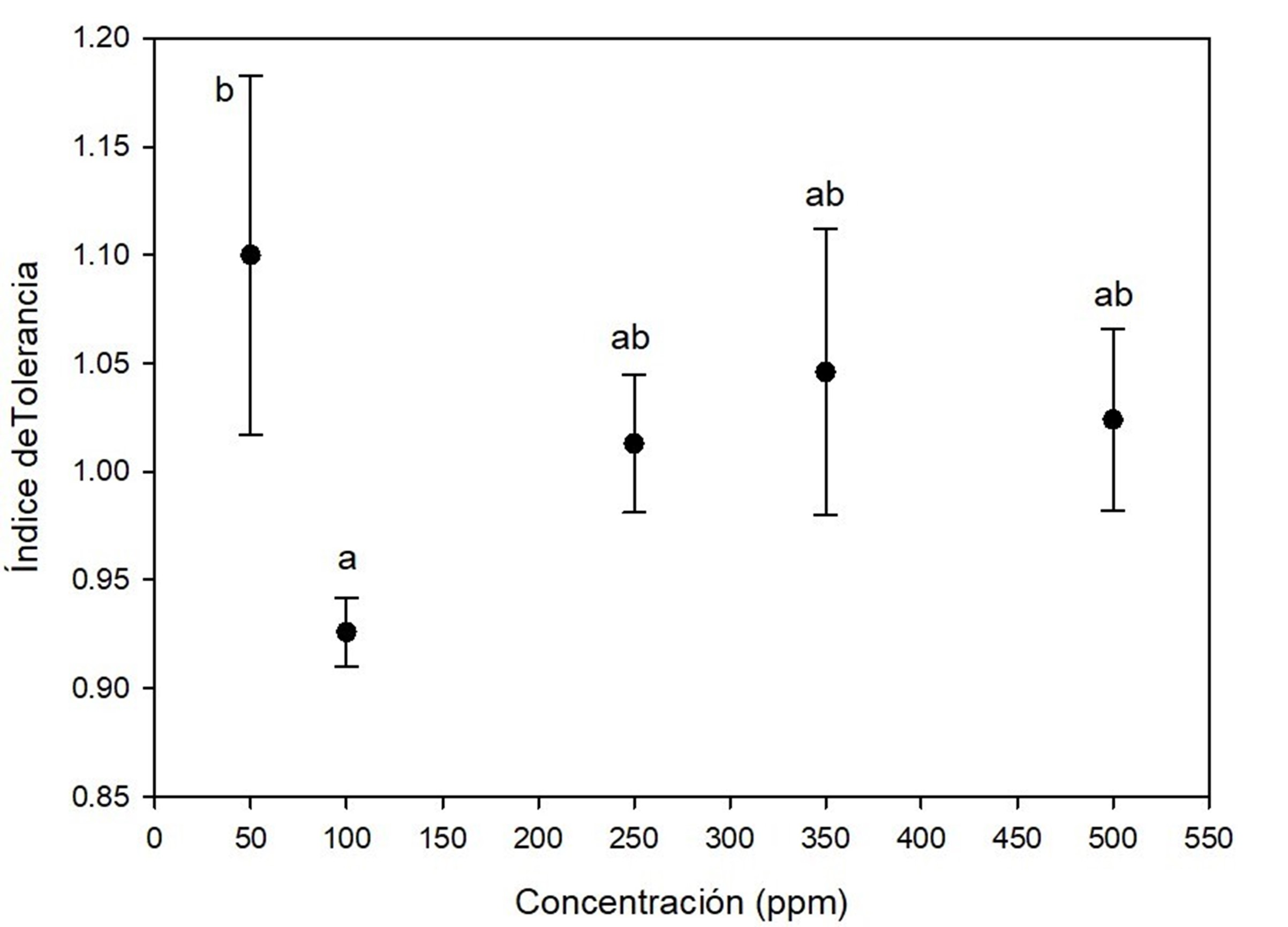
Figure 2 Tolerance index of Aspergillus terreus at five concentrations of (CH3COO)2 Pb(3H2O as a source of lead. Different letters indicate significant differences in tolerance index between concentrations according to the Tukey's test (P < 0.05). The bar shows the standard deviation of the mean.
On the other hand, Figure 3 shows that A. terreus has good growth performance by exposure to mercury, because tolerance indexes ranged from 0.7 and 1.0 in almost all the experiments. At concentrations of 350 ppm and 500 ppm there was effect of elongation on the lag phase of the fungi; despite this, the fungi were able to adapt to the presence of the toxic metal and reach the exponential phase. Kurniati, Arfarita, and Imai (2014) reported that A. flavus Link 1809 have a tolerance index of 0.8 at 25 ppm mercury, while Khan et al. (2019) observed that A. niger and A. terreus, isolated from effluents contaminated with toxic metals, were able to grow in 26.66 ppm mercury and remove more than 90 % of this metal.
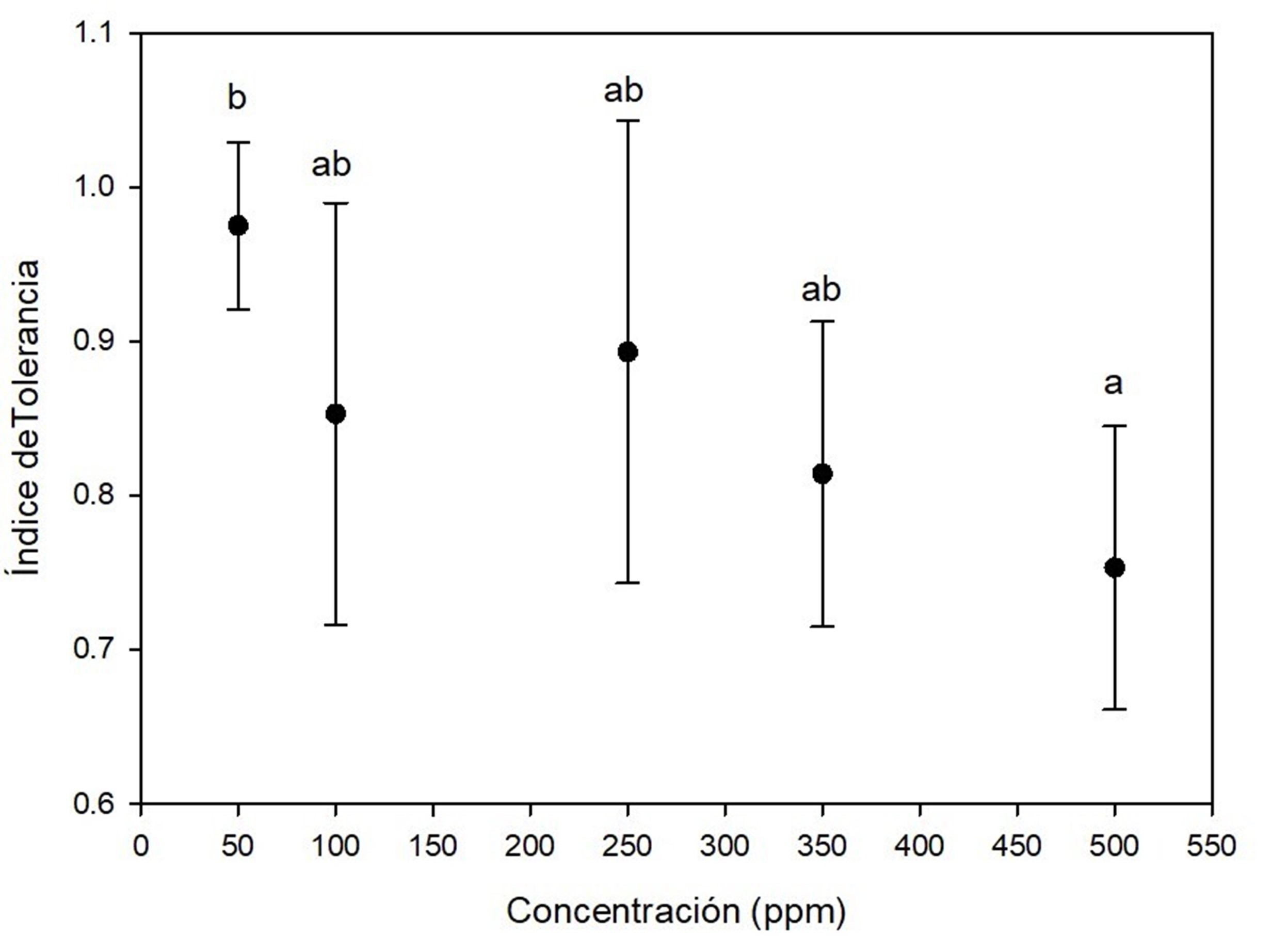
Figure 3 Tolerance index of Aspergillus terreus at five concentrations of HgCl2 as a source of mercury. Different letters indicate significant differences in tolerance index between concentrations according to the Tukey's test (P < 0.05). The bar shows the standard deviation of the mean.
Cadmium was the most toxic for A. terreus, because all concentrations had a significant effect on tolerance index, as shown in Figure 4. The fungi showed low tolerance at 50 ppm (0.56 ± 0.9) on the seventh day of growth and very low tolerance at 100 ppm on days 6 (0.17 ± 0.019) and 7 (0.20 ± 0.018). Growth was observed until day 6 for the las concentration, i.e., there was a marked effect of elongation of the lag phase of the fungus due to cadmium. At concentrations of 250 ppm, 350 ppm and 500 ppm, A. terreus showed no growth. This high toxicity of cadmium for fungi has also been documented in another research (Joo & Hussein, 2012; Oladipo et al., 2018). Similar to the present study, Dey et al. (2016) reported no growth of A. terreus at 500 ppm cadmium.
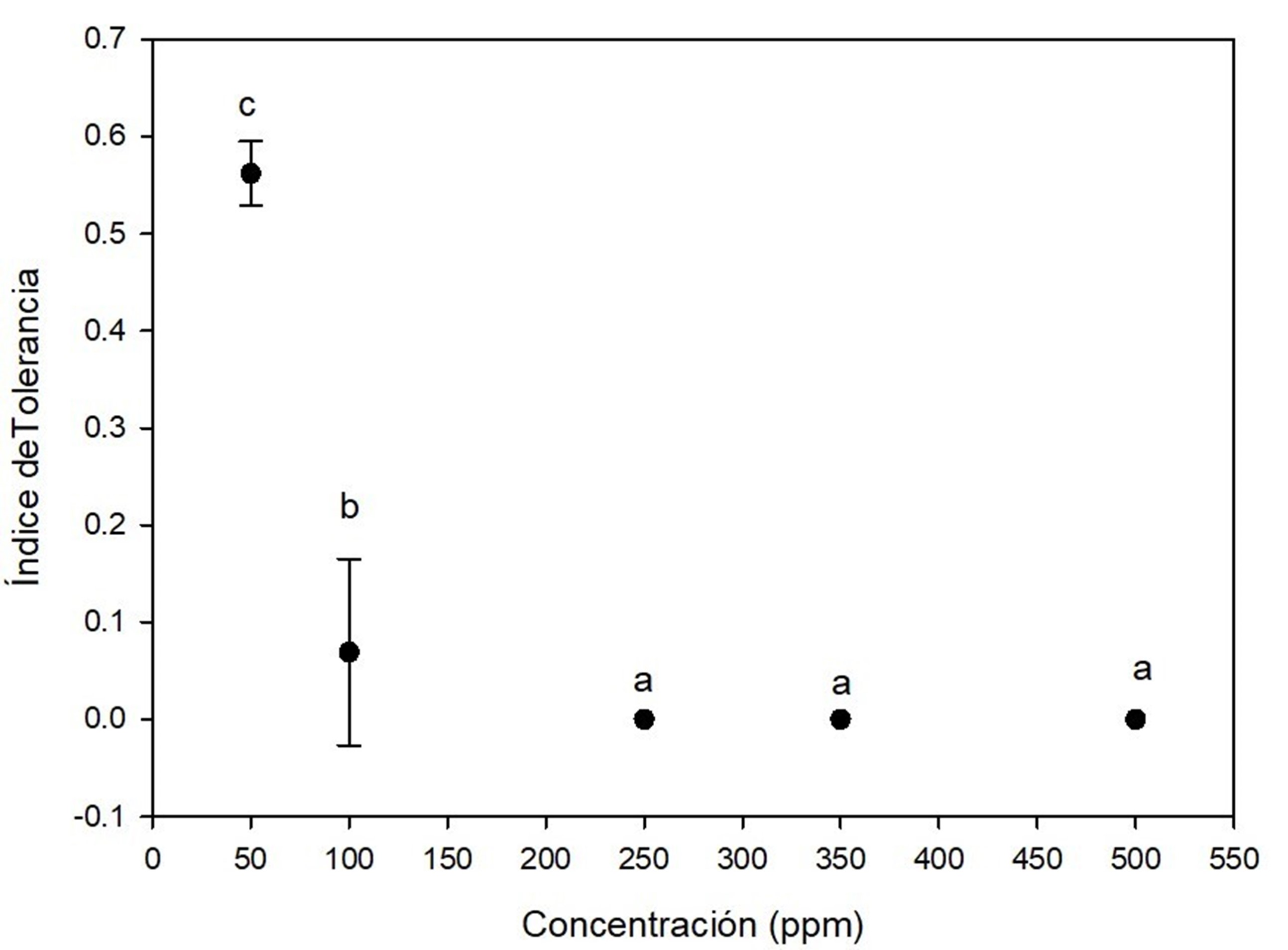
Figure 4 Tolerance index of Aspergillus terreus at five concentrations of CdCl2 as a source of cadmium. Different letters indicate significant differences in tolerance index between concentrations according to the Tukey's test (P < 0.05). The bar shows the standard deviation of the mean.
This study showed tolerance of A. terreus in the following order: lead > mercury > cadmium, indicating high cadmium toxicity. Several studies confirm that various fungi isolated from the same source of metal-contaminated sites have demonstrated different levels of resistance (Chakraborty, Mukherjee, & Das, 2012; Gururajan & Belur, 2018; Kumar et al., 2018; Zafar et al., 2007).
In the multimetal system (mixture of cadmium, chrome, mercury and lead) a lag phase elongation effect was observed compared to the control. This could be attributed to the toxicity induced by the mixture of metals on fungi growth. After the adaptation phase to the toxic environment, the fungi resumed its exponential growth. Based on Figure 5, the tolerance index to the metal mixture was very high at 4 ppm (0.9 ± 0.08), 8 ppm (1.0 ± 0.03), 16 ppm (1.2 ± 0.08) and 64 ppm (0. 9 ± 0.16) over the seven days of culture; moderate at 80 ppm (0.7 ± 0.06), 120 ppm (0.7 ± 0.06) and 200 ppm (0.6 ± 0.08); and low at 400 ppm (0.5 ± 0.01). Dey et al. (2016) studied a multimetal system similar to the one in this study, which consisted of a mixture of Cd, Cr, Cu, Ni, Pb, and Zn at concentrations of 6, 12, 18, and 30 mg∙L-1. These researchers observed that Aspergillus fumigatus Fresenius 1863 showed good adaptability to the growth medium, as well as an effect on the lag phase lasting 6 to 7 h in the metal-free control treatment and 17 to 18 h in the multimetal system.
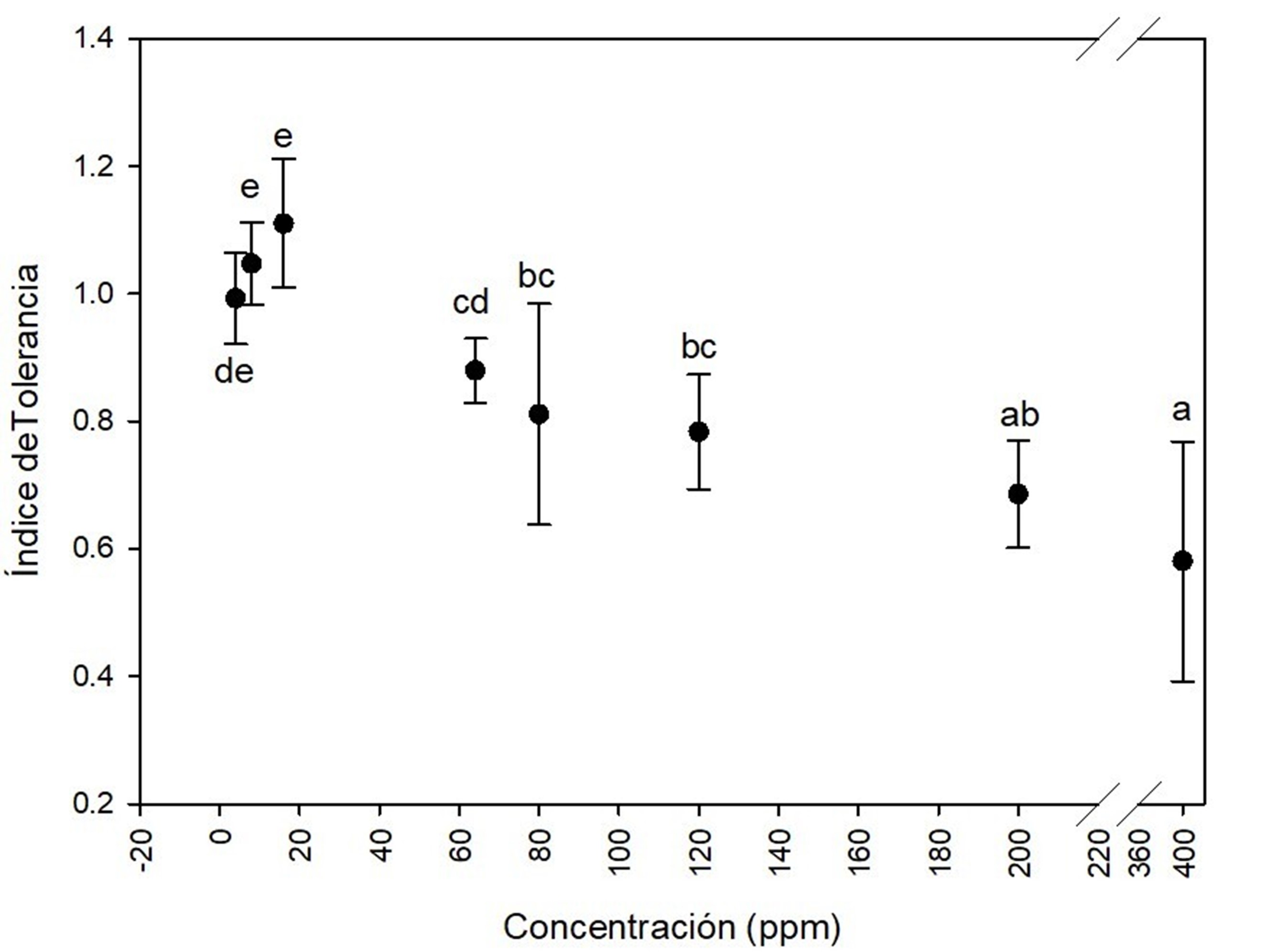
Figure 5 Tolerance index of Aspergillus terreus at several concentrations of (4, 8, 16, 64, 80, 120, 200 and 400 ppm) of the mixture of cadmium (CdCl2), chrome (K2Cr2O7), mercury (HgCl2) and lead [(CH3COO)2Pb(3H2O] in equal proportion of each metal. Different letters indicate significant differences in tolerance index between concentrations according to the Tukey's test (P < 0.05). The bar shows the standard deviation of the mean.
Minimum inhibitory concentration
In all individual lead and mercury treatments, and the multimetal system, fungi had growth even at the highest concentrations (500 ppm individual treatment and 400 ppm multimetal system). For this reason, the MIC could only be determined in the individual evaluation with cadmium, finding that A. terreus no longer grew after supplementation of the growth medium with 100 ppm (Table 3).
Table 3 Minimum inhibitory concentration (MIC) of Aspergillus terreus on growth medium supplemented with 50, 250, 350 and 500 ppm cadmium, mercury and lead individually, and with a mixture of cadmium, chrome, mercury and lead in equal proportions at concentrations of 4, 8, 16, 64, 64, 80, 120, 200 and 400 ppm.
| Treatment | MIC (ppm) |
|---|---|
| Cadmium | 100 < MIC < 250 |
| Mercury | MIC > 500 |
| Lead | MIC > 500 |
| Multimetal | MIC > 400 |
Gururajan and Belur (2018) isolated fungal strains from soil samples from a metal-contaminated scrap metal landfill and evaluated the MIC; the strains were reported as "isolates", no genus or species names were mentioned. They found MICs of 50 ppm mercury, 100 ppm cadmium and 400 ppm lead. Several MIC studies have been reported for microscopic fungi, but most are for individual metal exposure. For Bauveria bassiana Vuill. 1912, a MIC of 100 ppm was found for Cu II, Ni II and Cd VI individually, 200 ppm for Cd II and 250 ppm for Zn II (Gola et al., 2016). Mishra and Malik (2012) observed that Aspergillus lentulus Balajee & K. A. Marr 2005 has MIC >12 000 ppm for Cr III, 5 000 ppm for Pb II, 850 ppm for Cu II, 550 ppm for Cr VI and 300 ppm for Ni II. Variations in survival rates and patterns of tolerance index reinforce studies that propose that tolerance to toxic substances depends on the innate characteristics of each fungal species (Iskandar et al., 2011; Say, Yilmaz, & Denizli, 2003); moreover, constant exposure of fungi to toxic metals may also be implicated in the ability to develop tolerance mechanisms (Gorbushina & Krumbein, 2000).
Microscopic fungi have evolved several adaptive biological strategies or mechanisms to tolerate toxic metals and avoid cellular damage. Such strategies include chelation and cell wall binding (extracellular sequestration) and physical intracellular sequestration of the metal by binding to proteins and other ligands (Pumpel & Paknikar, 2001). Binding to the cell wall is called biosorption. Extracellular mechanisms are mainly involved in preventing the entry of metals into the cell, whereas intracellular mechanisms aim to reduce the action of toxic compounds in the cytosol. In the first case, the fungal cell excretes organic molecules that do not belong to the cell wall matrix for chelation of metal ions (Gadd, 1993). The cell surface of certain filamentous fungi is negatively charged, due to the presence of anionic structures, such as glucan and chitin (Gupta, Nayak, & Agarwall, 2015). This gives the microorganisms the ability to bind metal cations, whereas, in the intracellular mechanism, metal tolerance is apparently determined by transport proteins that remove toxic metal ions from the cytosol out of the cell or sequester metals in the vacuole (Bellion et al., 2006).
It has also been reported that toxic metal stress can be reduced by melanin synthesis. Melanin refers to dark pigments synthesized by organisms of all biological kingdoms, including fungi (Nosanchuk & Casadevall, 2003b). Melanins have been suggested to play several roles in fungal biology (Cordero & Casadevall, 2017) and, with their multiple properties, it seems likely that these help fungi survive various types of stresses, both biotic and abiotic. The latter include high ultraviolet radiation, reactive oxygen species, and toxic metals. Melanins form granules that can accumulate on the cell surface or be released into the extracellular space (Malofe, Solhaug, Minibayera, & Beckett, 2019) and, thanks to their functional groups, exhibit high binding affinity and binding capacity for many metal ions (Cordero & Casadevall, 2017).
The strategies that each species develops may vary depending on the isolation site, type, and metal concentration (Rose & Devi, 2018). Aspergillus has the ability to uptake copper through an active process, thanks to metallothionein synthesis (Kermasha, Pellerin, Rovel, Goetghebeur, & Metche, 1993). On the other hand, Chakraborty et al. (2013) found that Aspergillus foetidus Thom & Raper 1945 converts soluble Pb to insoluble Pb and could retain it on the mycelial surface. It has also been reported that some Aspergillus species have evolved an enzymatic mechanism to reduce chromium toxicity. This mechanism is mediated by an antioxidant enzyme system involving peroxidases, catalases and ascorbate peroxidase (Srivastava & Thakur, 2006). Bǔcková, Godočiková, Šimonovičová, and Polek (2005) found a positive relationship between the level of total catalases and increased metal tolerance in cultures of A. niger isolated from mining areas. Catalase production is one of the mechanisms that cells use to protect themselves against damage caused by reactive oxygen species. Therefore, these authors concluded that regulation of catalase production by A. niger may be an adaptive response to environmental stress generated not only by high metal concentrations, but also by changes in pH and nutrient content, which often follow soils impacted by mining waste. It is likely that A. terreus, isolated in the present study, uses adaptive mechanisms similar to those mentioned above.
Changes in morphology
Microscopic fungi with intense green, brown or black mycelium have been found in the Sonoran Desert (Ranzoni, 1968). Figure 6 shows changes in morphology and pigmentation of mycelium by exposure to metal mixture. From 16 ppm, changes in brown coloration are observed compared to the control. Other studies have also recorded this phenomenon when exposing mycelium to metals in growth medium (Yazdani, Yap, Abdullah, & Tan, 2010). Fazli et al. (2015) observed changes in coloration in several fungi due to cadmium exposure: discoloration in Aspergillus versicolor Tirab. 1908 and Cladosporium sp., pink color of Paecilomyces sp. changed to white, and Terichoderma sp. and A. fumigatus changed from green to white. Pigmentation of fungal cells ranging from red, yellow, black or other shades may occur in some species. This may be due to the precipitation of metals in the cell wall, in response to fungi producing various extracellular compounds such as complexing agents, precipitating agents, polysaccharides and pigments with the ability to bind metals (Gadd, 2007). In general, the possible explanation for these morphological changes may be due to the vast detoxification/tolerance mechanisms that each strain has evolved. The variation in tolerance to toxic metals may be due to the presence of one or more types of tolerance strategies or resistance mechanisms of fungal species (Zafar et al., 2007); furthermore, alteration of microscopic structure of fungal biomass (rough-looking hyphae and flattened spores) treated with 100 ppm cadmium was observed.
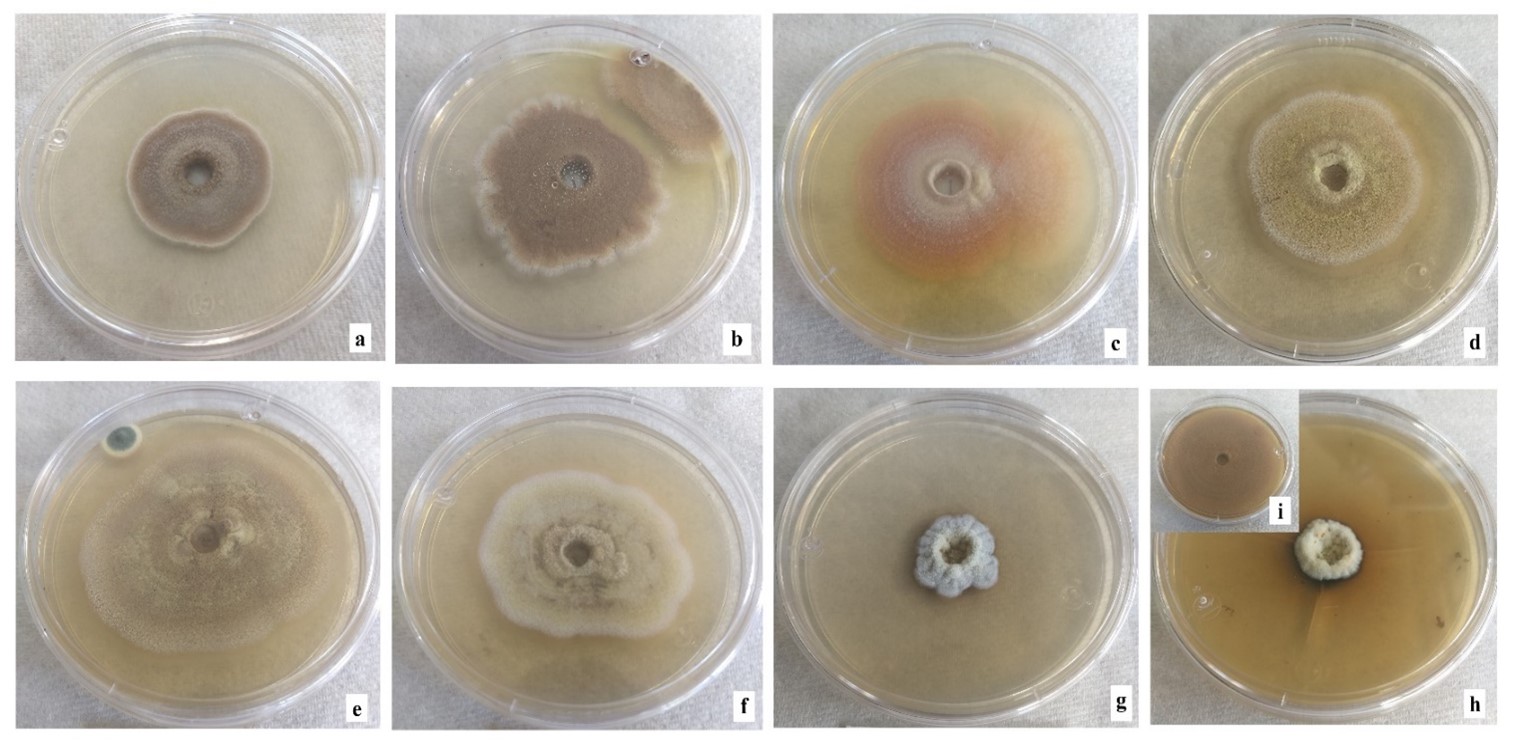
Figure 6 Morphology of Aspergillus terreus colonies in multimetal system. (a) 4 ppm, (b) 8 ppm, (c) 16 ppm, (d) 64 ppm, (e) 80 ppm, (f) 120 ppm, (g) 200 ppm and (h) 400 ppm of the mixture of cadmium (CdCl2), chrome (K2Cr2O7), mercury (HgCl2) and lead [(CH3COO)2Pb(3H2O] in equal proportion of each metal, i) control without metals.
Conclusions
Fungal strain of A. terreus, isolated in this study, showed high tolerance to the presence of lead and moderate tolerance to mercury in all the concentrations evaluated, and the multimetal system (cadmium, chrome, mercury and lead). This suggests that microorganisms isolated from soils contaminated with toxic metals develop tolerance mechanisms to these substances; however, the level of tolerance is influenced by the type of metal. According to these results, A. terreus may have the potential to remove the toxic metals studied in this research.











 texto en
texto en 


