Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.37 no.1 Texcoco ene. 2019 Epub 21-Ago-2020
https://doi.org/10.18781/r.mex.fit.1810-1
Review articles
Biological, anaerobic and reductive soil disinfestation to the soil for control of harmful organisms to plants
1 Campo Experimental La Laguna INIFAP, Bulevard. José Santos Valdez 1200 Pte., Colonia Centro Matamoros, Coahuila, México. C.P. 27440;
2 Universidad Autónoma Chapingo, Unidad Regional de Zonas Áridas. Carretera Gómez Palacio - Ciudad Juárez Km 40, Bermejillo, Durango. C.P. 35230.
The need to reduce the use of synthetic pesticides, lower costs, increase efficiency for the control of phytopathogens and to carry out organic agriculture, are reasons to improve and develop new control alternatives. The biological disinfestation (BSD), anaerobic (ASD) or reductive soil (RSD) are synonymous; in this review, the term RSD is used and the reason explained. The RSD is a method applied to the soil to reduce or eliminate bacteria, fungi, weeds, and nematodes that damage agricultural crops. This technique consists in adding a source of easily oxidized organic carbon such as crop residues, seeds, green manure, etc., cover with plastic and saturate or flood the soil. In this way, the carbon source decomposes in an anaerobic condition, the soil acidifies, the oxidation-reduction potential reaches reductive values (-100 to -400 mV) and short-chain volatile fatty acids (VFAs) are generated. The VFAs are lethal for weeds and phytopathogens in the soil. In this essay the subject of the RSD, shows a historical approach, the principles that sustain it, proposals for improvement and evaluation of its variants are made, and practical applications are presented.
Key words: Volatile fatty acids; flood; oxidation-reduction potential; pH
La necesidad de disminuir el uso de pesticidas sintéticos, bajar costos, incrementar eficiencia para el control de fitopatógenos y de realizar una agricultura orgánica, son razones para mejorar y desarrollar nuevas alternativas de control. La desinfestación biológica (DBS), anaerobia (DAS) o reductiva del suelo (DRS) son sinónimos; en ésta revisión, se utiliza el término DRS y se explica la razón. La DRS constituye un método aplicado al suelo para disminuir o eliminar bacterias, hongos, malezas y nematodos que dañan a los cultivos agrícolas. Esta técnica consiste en agregar una fuente de carbono orgánico de fácil oxidación como residuos de cosecha, semillas, abonos, etc., cubrir con plástico y saturar o inundar el suelo. De esta manera, la fuente de carbono se descompone en condición anaerobia, el suelo se acidifica, el potencial oxido-reducción alcanza valores reductivos (-100 a -400 mV) y se generan ácidos grasos volátiles de cadena corta (AGVs). Los AGVs son letales para malezas y fitopatógenos en el suelo. En este ensayo el tema de la DRS, muestra un enfoque histórico, los principios que la sustentan, se hacen propuestas de mejora y evaluación de sus variantes, y se presentan aplicaciones prácticas.
Palabras clave: ácidos grasos volátiles; inundación; potencial oxido-reducción; pH
Before the use of chemical products for the control of pests and diseases, crop rotation and the incorporation of organic matter in the soil were the two most widely used methods in agriculture for this purpose (Bailey and Lazarovits, 2003). The latter is still being used, but the results for the control of plant diseases in the soil have a wide variation related to the amount and type of organic matter, the crop to be protected and the pathogen to be controlled (Bonanomi et al., 2007). Recently, the control of plant pests and diseases promoted the use of selective organic matter or its byproducts (Meghvansi and Varma, 2015). In particular, the decomposition of organic matter in the soil under anaerobic conditions led to the appearance of biofumigation and its variants, all of which have the intention of substituting methyl bromide and pesticides, and contributing towards organic agriculture (Meghvansi and Varma, 2015; Shennan et. al., 2014; Shrestha et al., 2016).
Soil biofumigation has focused on the incorporation of residues, complete plants, or seeds of the species of the Brassicaceae family into the soil, some of the most important of which are Brassica carinata, B. juncea and B. napus, followed by covering the soil with plastic, where residues decompose, producing toxic compounds for organisms that are harmful to the crops (Ríos-Cano, 2017). Variations of biofumigation are the biological soil disinfestation (BSD), later named anaerobic soil disinfestation (ASD), and finally reductive soil disinfestation (RSD). The purpose of disinfestations is to eliminate or reduce organisms in the soil that harm crops by incorporating species of Brassicaceae and of other plants, organic compounds and crop residues in an environment where the soil is saturated with water and covered with plastic (Momma et al., 2008). It was initially named BSD, since the degradation of the material added implies a microbiological process; next, the term ASD was coined due to the importance of the lack of oxygen; finally, the term RSD is replacing the previous ones because, along with anaerobiosis, it is also crucial for the oxidation-reduction potential (ORP) to reach a reductive level with values of -100 to -400 mv, the pH of the soil to acidify and for volatile fatty acids (VFAs) to be produced. However, the terms BSD and ASD are still being used and are gradually being replaced with RSD; this revision will consider RSD as a synonym of BSD and ASD.
RSD is a relatively new technique, not widely known in Mexico, and with causes that are not entirely known, but they are being investigated in different countries. Therefore, the aims of this revision are to show the advances, achievements and to propose how to improve the efficiency and efficacy of this technique, used for the control of organisms in the soil that are harmful for crops. The topic of RSD has been revised before, evaluating different inputs for different types of pathogens, and even with an approach of meta-analysis (Momma et al., 2013; Shennan et al., 2014; Shrestha et al., 2016), which supports the aim of this work.
In consequence, the approach of this revision places emphasis on: i) historical development; ii) principles that back them; iii) proposals to improve and evaluate their variants. All this, supported by the integration of part of the recently produced knowledge, as well as by the bases of additional modifications to improve RSD.
Retrospective analysis of RSD. Recently in Mexico, the status of pesticides was analyzed in a study including fumigants; the criteria of the analysis are based on the danger found by international agencies, the most important of which are the International Health Organization, the United Nations Food and Agriculture Organization, and the Pesticide Action Network. Table 1 highlights the most commonly used fumigants in Mexico, some of which - such as methyl bromide, Chloropicrin, 1,3-Dichloropropene and metham-sodium are considered highly dangerous. Hence the need throughout Mexico and the world to seek new alternatives for a sustainable agriculture (Bejarano-González, 2017).
An alternative for the management of plant pathogens in the soil is RSD, which includes flooding, adding and decomposing organic matter, anaerobiosis, changes in pH and ORP, and the production of VFAs. In the past, each topic was researched separately as methods for the control of plant pathogens in the soils and will be regarded as precedents of RSD.
Flooding soils planted with banana trees resulted in the eradication of Fusarium oxysporum f. sp. cubense (Stover, 1955). It was later found that the sclerotia of Verticillium dahliae Kleb died or were unable to reproduce in flooded soils, where organic matter decomposed in anaerobic conditions (Ioannou et al., 1977; Menzies, 1962). In this way, the constants found were flooding and the decomposition of organic matter under anaerobic conditions, which induced the death of plant pathogens. At the same time, the decomposition of organic matter in flooded soils were related to the production of VFAs, which has been recorded in microbiology classics since the 1960s (Alexander, 1961); the same is true for changes in pH and ORP (Ponnamperuma, 1972).
Table 1 Soil fumigantsx,y,z or pesticide used in Mexico and their danger.
| Ingrediente activo | Formula | Usos principales | Peligrosidad1 | Peligrosidad2 |
|---|---|---|---|---|
| Bromuro de metilo | CH3Br | Fungicida, herbicida, insecticida y nematicida | 0/0/0/1 | ¿??? |
| Cloropicrina | Cl2CNO2 | idem | 1/0/0/0 | |
| 1,3-Dicloropropeno | C3H4Cl2 | idem | 0/1/0/0 | |
| Dibromuro de etileno | C2H4Br2 | Insecticida, nematicida. | NI | Toxico a humanos efecto dañino al ambiente |
| Yoduro de metilo | CH3I | Fungicida, herbicida, insecticida y nematicida. | NI | ídem |
| metam-sodio | C2H4NNaS2 | ídem | 0/1/0/0 | |
| Dazomet | C5H10N2S2 | ídem | NI | Toxico a humanos efecto dañino al ambiente |
| Dimetil disulfuro3 | CH3SSCH3 | Fungicida, nematicida | NI | NI |
| Tetratiocarbonato de sodio3 | CNa2S2 | Nematicida | NI | NI |
x Información obtenida de Bejarano-González (2017), el valor numérico entre las diagonales ( / / / /), indican toxicidad aguda, efectos a largo plazo, toxicidad ambiental e infringir acuerdos internacionales de protección al ambiente, salud o derechos humanos por uso de pesticidas, respetivamente / Information obtained from Bejarano-González (2017); the numeric value between slashes ( / / / /), indicate acute toxicity, long term effects, and the infringement of international environmental protection, health of human rights agreements due to the use of pesticides, respectively.
y Información obtenida de De la Cruz y Ramírez, (2018) / Information obtained from De la Cruz and Ramírez, (2018).
NI = Peligrosidad no incluida en las fuentes de información 1 y 2. / Harmness not included in information sources 1 and 2.
z La peligrosidad del Dimetil disulfuro y Tetratiocarbonato de sodio, son expuestas en las ligas: http://www.kooragro.com.mx/media/products/F.T._PALADIN_JoLxNOq.pdf y http://www.afipa.cl/web/files/afipa/arysta2015/ENZONE.pdf, respectivamente / The harmness of Dimethyl disulfide and sodium Tetrathiocarbonate are shown in the links http://www.kooragro.com.mx/media/products/F.T._PALADIN_JoLxNOq.pdf and http://www.afipa.cl/web/files/afipa/arysta2015/ENZONE.pdf, respectively.
VFAs are produced by microorganisms when these degrade the organic matter in the soil using a fermentative metabolism (Figure 1). Before RSD, acetic, butyric, formic, propionic acids and other VFAs were known to have biocide effects on fungi (Cochrane, 1958) and on bacteria (Goepfert and Hicks, 1969), and most recently, for other organisms (Samaniego-Gaxiola and Pedroza-Sandoval, 2013). However, it was in Japan where Okazaki (1985) found that the addition of glucose and flooding of the soil produced volatile compounds that killed the Fusarium oxysporum f. sp. raphani chlamydospores, in a device he designed (Figure 2); similarly, Samaniego-Gaxiola (1994) added glucose and flooded the soil, and observed the death of the Phymatotrichopsis omnivora (Duggar) Hennebert sclerotia. Meanwhile, Okazaki and Nose (1986) determined that the acetic, propionic and butyric VFAs were produced under these conditions and killed F. oxysporum; although this work and Okazaki’s (1985) appeared translated from Japanese and published in English around the year 2000. This provided evidence of the lethal effect of the VFAs for organisms during RSD, or at least in vitro, which has been confirmed in later works (Momma 2008; Momma et al., 2006; Samaniego-Gaxiola and Balagurusamy, 2013; Tenuta et al., 2002).
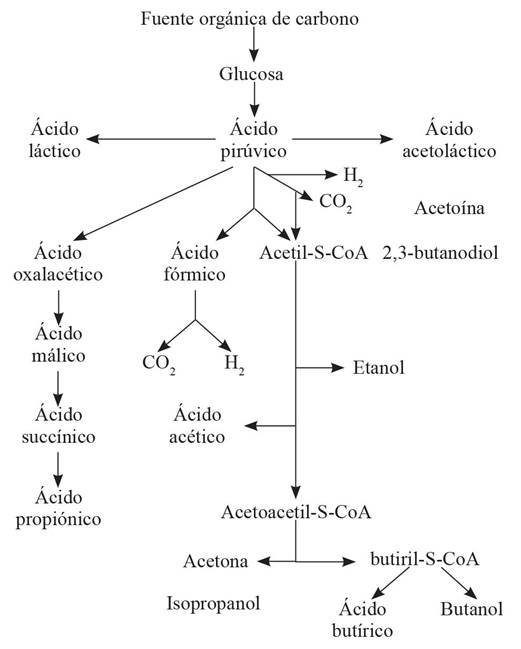
Figure 1 Volatile fatty acids produced the fermenting an organic source of carbon. Adapted from Steiner et al., 1977.
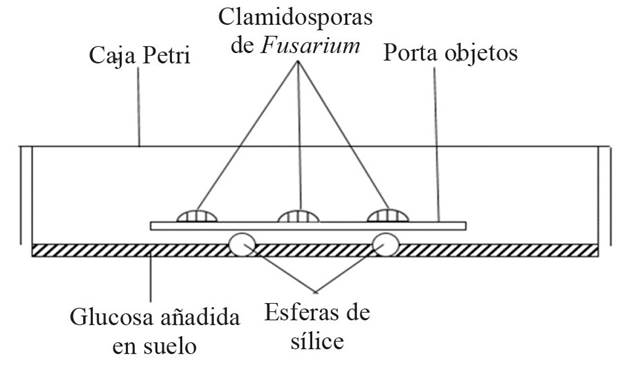
Figure 2 Okazaki device (1985) when chlamydospores Fusarium oxysporum died when adding glucose to the soil and flooding.
It is worth pointing out that the presence of VFAs in residues of plants such as cabbage (Brassica oleracea) has been known since the 1950s (Barnett and Ducan, 1953). The same is true for the phytotoxic effects of the VFAs in the soil in wheat bran residues and the amount of acids produced (Lynch, 1977; Lynch et al., 1980), which is important, since wheat straw and kale residues are commonly used to induce RSD and biofumigation, respectively.
Finally, the first two authors that included the principles of RSD were Shinmura et al. (1999) in Japan and Blok et al. (2000) in the Netherlands, both for the control of pathogenic fungi in the soil. The VFAs found in pig slurry were later found to kill Verticillium dahliae (Tenuta et al., 2002). Wheat bran was used to induce RSD and kill pathogenic bacteria and fungi in the soil (Momma et al., 2006). Ethanol has also been used to produce RSD (Momma et al., 2010); as well as grass, alfalfa, molasses, seeds, glucose, and others (Conn et al., 2005; Hewavitharana and Mazzola, 2016; Momma, et al., 2010; Shrestha et al., 2016).
The VFAs. These acids, produced in the RSD, are toxic to nematodes, fungi and bacteria, mostly when in a non-ionized form in the soil solution or another medium (Goepfert and Hicks, 1969; Katase et al., 2009; Tenuta et al., 2002). For example, acetic acid, in its non-ionized or dissociated form (VFAsni) can be represented as CH3CCOH, whereas in its ionized form, (VFAsi) as CH3CCO- + H+, and one or another will predominate based on the pH of the medium in which it is found; the principles and calculations to determine the ionized non-ionized forms are detailed by Samaniego-Gaxiola and Pedroza-Sandoval (2013). The concentration of VFAsni in the soil solution decreases dramatically as the pH becomes less acidic; Figure 3 indicates the concentration of four VFAsni based on the pH. The behavior of the VFAs is crucial to understand its toxicity for organisms. For example, 100 mmol L-1 of acetic acid, with a pH of 5.5 generates approximately 13.7 mmol L-1 in its non-ionized form (toxic), whereas 25 mmol L-1 of butyric acid at a pH of 4.5 generates 15.4 mmol L-1; in other words, the acidification of one unit (from 5.5 to 4.5) results in the use of four times less butyric than acetic acid with a similar production of VFAsni. It is worth pointing out that each VFAs has a different behavior based on the pH and particularly formic acid (Figure 3); likewise, the toxicity of V. dahliae and P. omnivora died based on the type, concentration and the time of exposure to the VFAs (Samaniego-Gaxiola and Balagurusamy, 2013; Tenuta et al., 2002).
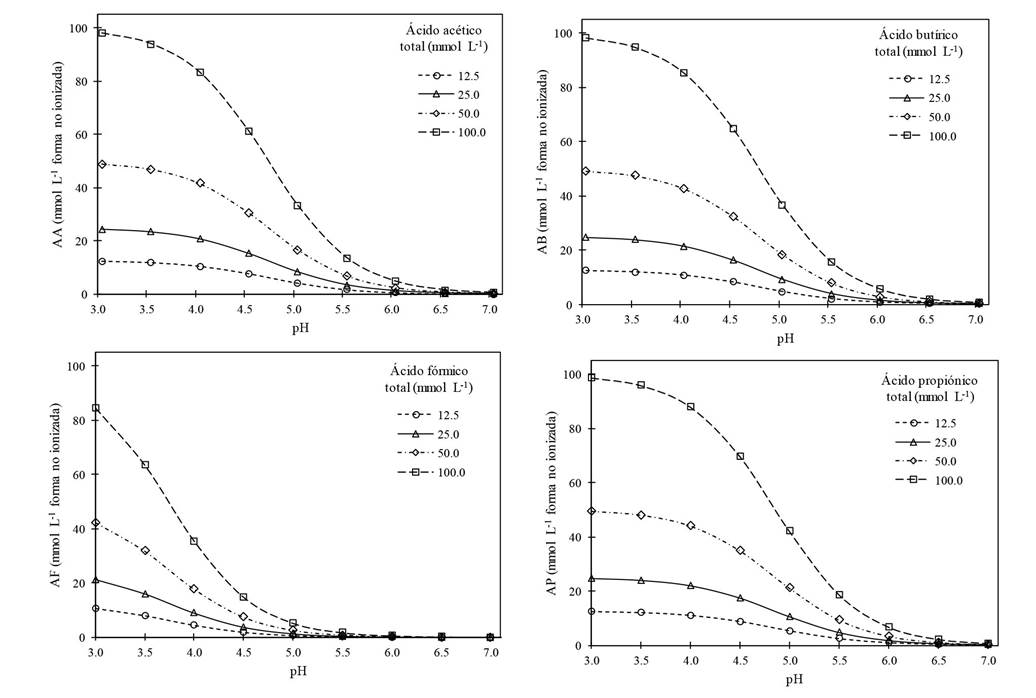
Figure 3 Non-ionized forms of acetic, butyric, formic and propionic acids at concentrations of 12.5, 25.0, 50.0 and 100.0 mmol L-1, all based on the pH of the solution. The graphs were created by the authors using the data of the mole cular weight and pH of each acid and the Henderson-Hasselbalch equation, all quoted by Samaniego-Gaxiola and Pedroza-Sandoval (2013).
Proposal, VFAs modified by the pH. In 1969, Goepfert and Hicks (1969) proved that only when acidifying with HCl the medium that contained VFAs, Salmonella typhimurium bacteria died; recently, Samaniego-Gaxiola et al. (2018) registered the death of the P. omnivora sclerotia only when acidifying with H2SO4 a solution obtained from the soil treated for RSD that contained VFAs. Therefore, under field conditions, addition of a strong acid after 2 to 7 days of starting the RSD process may very likely increase the toxicity of VFAs, and consequently kill susceptible organisms that one wants to eliminate or reduce. In the laboratory, we determined the amount of H2SO4 required to adjust the pH ~4 in soils with RSD (Samaniego-Gaxiola et al., 2018). Additional data are shown in this study, adapted from Estupiñán-Herrera et al. (2010), where changes in pH were induced in soil with added fructose and flooding, followed by additional reductions of the pH ~4 by adding H2SO4, Figure 4 A-D.
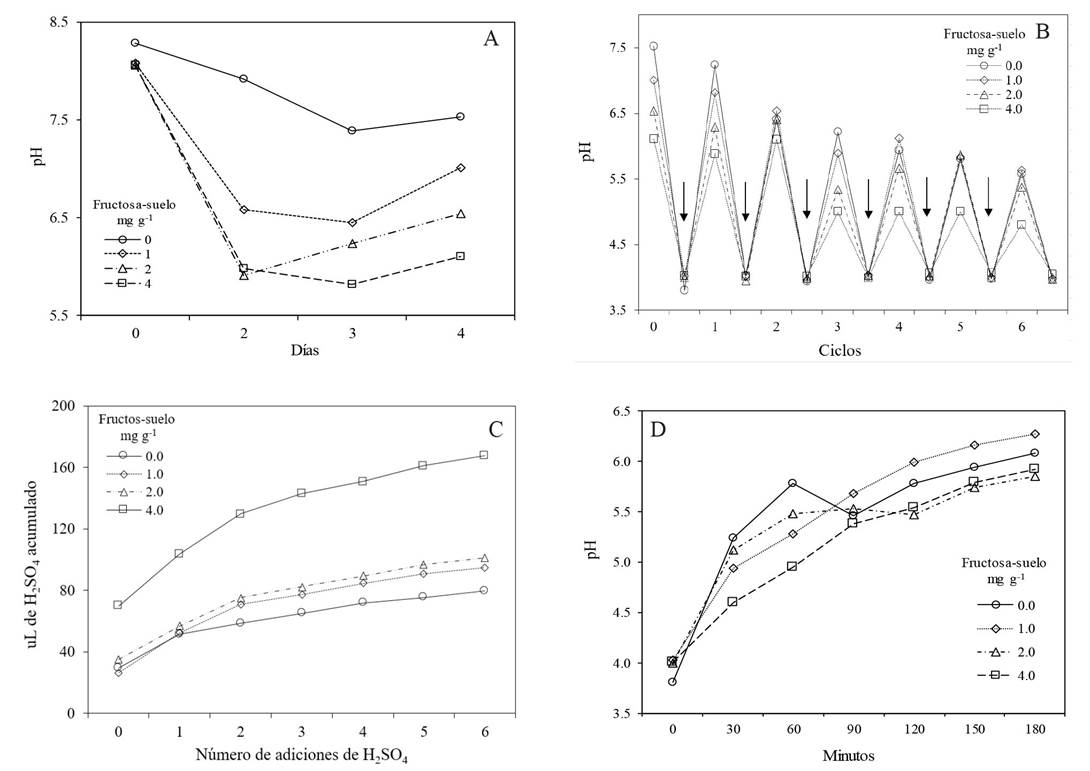
Figure 4 A-D. Changes in pH and amounts of H2SO4 needed to adjust the pH ~4 after adding fructose to the soil and flood ing it. A. Change in pH with different doses of fructose added to the flooded soil. B. Sevenfold reduction (six cycles) of the pH ~4 adding H2SO4 (arrows) to the soil solutions; solutions come from day 4 of Figure 4A. C. Accumulated amount (uL) of H2SO4 added sevenfold to adjust the pH ~4, which corresponds to the arrows in Figure 4B. D. Time of return of the pH after adjusting it with the acid to ~4, for the solutions of day 4 of Figure 4A. Adapted from Estupiñán-Herrera et al. (2010).
Figure 4 shows that the pH can fall to ~4. An agenda to investigate what we propose would be to evaluate the change in pH~4 after RSD when adding H2SO4; to do it in different types of soil in the field, using several inputs, and to determine the feasibility of the plant pathogens. Assuming that a soil type, after having reached its greatest reduction in pH after RSD, can be saturated with water in 25% of its volume, it would require around 3.3 tons of H2SO4 to adjust the pH ~4; all this, extrapolating to the data in Figure 4C to one hectare, complementary to Estupiñán-Herrera et al. (2010) and Samaniego et al. (2018).
Direct use of other compounds and/or VFAs. During the RSD, along with the VFAs, different compounds, lethal to the plant pathogens in the soil, have been found, along with other volatile compounds that have stood out for inhibiting pathogens (van Agtmaal et al., 2015). Therefore, the RSD may increase its efficiency and efficacy in combination with the addition of the known lethal compounds which are still to be determined. Some of these compounds may be added immediately after the pH and the ORP reach their maximum change.
In the RSD, along with VFAs, biocidal compounds may be formed, such as isothiocyanates and nitriles, which are products of hydrolysis (in the soil) of the glucosinolates, the latter of which are secondary metabolites contained in the plant species of the Brassicaceae family, produced in the soil, both in biofumigation and in the RSD (Blok et al., 2000; Ríos-Castaño, 2017). Fe2+ and Mn2+ ions in water in a reductive condition reached by the RSD were lethal to F. oxysporum, although Momma et al. (2011) suggest the confirmation and investigation of the mechanism that give these ions such an effect. Depending on the alkalinity or acidity of the soil, VFAs may continue decomposing in compounds equally lethal for plant pathogens in the soil; in an acid and alkaline pH, nitrous acid and ammonia will be formed, respectively (Lazarovits et al., 2005; Tenuta and Lazarovits, 2002).
VFAs may be present in large amounts in media such as pig slurry, the emulsions of industrially processed fish and other sources (Abbasi et al., 2009; Samaniego-Gaxiola and Pedroza-Sandoval, 2013; Tenuta et al., 2002); therefore, its potential use from previously mentioned sources. In addition, VFAs may be generated in the soil or aqueous solutions using molasses for later use. In earlier experiments carried out by us, using the method by Samaniego-Gaxiola et al. (2018) and where molasses, water and soil, or molasses and water were mixed, the pH and ORP were measured as shown in Figure 5; the pH and the ORP changed as expected, although the amounts of VFAs were not determined. Regardless of this, the formation of VFAs is expected when mixing molasses in soil and/or water.
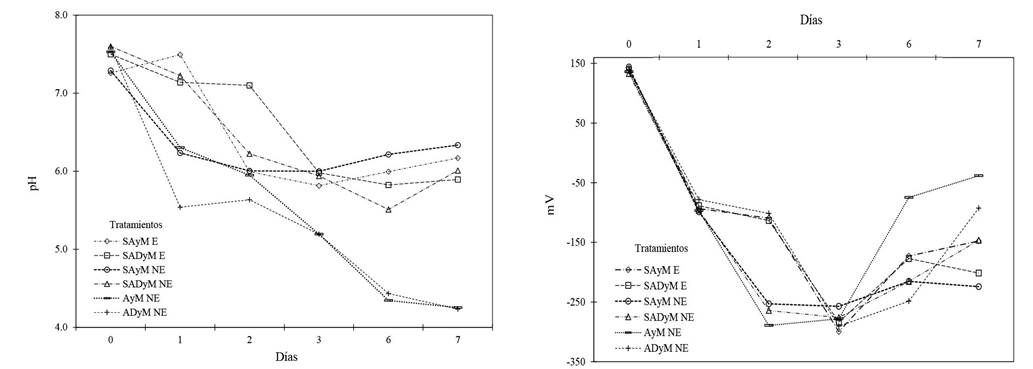
Figure 5 Treatments which registered changes in pH and ORP (mV). The soil, tap water and molasses were kept in an au toclave for 20 min before mixing ; soil, distilled water and molasses were kept in ; soil, tap water and molasses were mixed ; soil, distilled water and molasses were mixed ; mixture of tap water and molasses ; mixture of distilled water and molasses . When us ing soil, water and molasses, it was 150 g, 90 ml and 1% v/v (water-molasses), respectively; and when using water and molasses, we used 90 ml and 1% v/v, respectively. The autoclave was kept at ~121 °C and 280 kPa.
A way to induce RSD is the use of ethanol (Momma et al., 2010). Acetic, formic, and other acids could also be used directly in previously saturated soil to increase or complement the effects of the RSD, as in the case of the patent pending requested by He et al. (2012). The distribution of VFAs has also been carried out with the system of irrigation (Rosskopf et al., 2014).
The acidic pH, induced by the RSD, could be used to increase the toxicity of conventional fungicides or pesticides, i.e., combining both practices, and even an additional practice could be the addition of a strong acid after RSD and use of pesticides. In this regard, it was determined that in a buffer solution at a pH of 4 (base of acetate-acetic acid) and 1000 ppm of the fungicide Propiconazole (Tilt ®), the P. omnivora sclerotia died in 20 min by up to 60%; under the same condition, but at a pH of 7, 72 hours were required for the sclerotia to die in a similar percentage (Samaniego-Gaxiola, 2008). Likewise, the RSD has been used in combination with solarization as an alternative to substitute dusting with methyl bromide (Butler et al., 2014).
Inoculation of soil and plant with other organisms after RSD. Several biological changes have been reported after RSD, such as the appearance of antagonists of plant pathogens, an increase in nutrient cycles, an increase in crop growth, the reduction of the diversity of bacteria and fungi, and the appearance of dominant microorganism, all of this regarding soils without RSD (Huang et al., 2015, 2016; Hewavitharana and Mazzola, 2016; Liu et al., 2016). After RSD, we recorded the spontaneous or induced establishment of species of Trichoderma colonizing pathogens such as P. omnivora and Sclerotium rolfsii (Samaniego-Gaxiola et al., 2018; Shrestha et al., 2013); but also the reestablishment of previously eliminated pathogens such as Fusarium oxysporum f. sp. niveum and Monosporascus in a second cycle of the watermelon crop Citrullus lanatus (Liu et al., 2018). Moreover, after RSD, the inoculation of soil, seeds and seedlings with species of Trichoderma, or other fungi, and bacteria may have beneficial effects in the control of plant diseases; particularly when evaluating bacteria obtained from the RSD that show antagonism towards phytopathogens (Ueki et al., 2017, 2018), the endophytes of Trichoderma, bacteria and other fungi (Druzhinina et al., 2011; Eljounaidi et al., 2016: Santoyo et al., 2016).
A large amount of compounds, toxic to plant pathogens and beneficial to plants, are produced by bacteria and fungi in the soil, both in vitro and in the RSD (Hayat et al., 2010; Hewavitharana et al., 2014; Siddiquee et al. 2012); both the compounds and the microorganisms, after the RSD, may continue to be evaluated, introducing them or promoting their production for the control of pathogens. For example, 258 volatile compounds of Trichoderma harzianum were found, with an abundance of 50% of acetic acid when the extraction for their detection was carried out using methanol (Siddiquee et al. 2012), i.e., it is possible to investigate if the fungus is capable of producing this acid in a significant amount after an RSD.
Practical applications of the RSD. The next crops to be studied, such as alfalfa in its last year of planting, residues of crops such as cereals, grass trimmings, residues of crops such as pecan shells, as well as organic materials abundant in each region of Mexico, are some of the multiple sources of carbon that could be used and evaluated for an RSD.
For a particular evaluation, it may be worth considering the temperature at which RSD is most effective, and although it is scarcely studied, soil temperatures of 17 to > 30 °C have worked in a satisfactory manner (Shennan et al., 2014; Yossen et al., 2008). The types of organic matter and their amounts are also factors that have an effect on the RSD; in general terms, 4 mg of carbon are required for every gram of soil (4 mg g-1), which is equal to 2 Kg m-2 of rice hay/soil (bran) (Serrano-Pérez et al., 2017). Easily oxidized organic carbon (EOC) is a highly appropriate method that can be incorporated to the soil and could lead to a successful RSD. For example, glucose and wheat hay have 276 and 122 g Kg-1 of EOC, respectively; the former induces the greatest changes and effectiveness of RSD to control Fusarium oxysporum (Liu et al., 2016). Manure compost (11 Mg ha-1) was not effective to induce the typical changes of the RSD and did not control R. solani and Pratylenchus penetrans (Hewavitharana and Mazzola, 2016); meanwhile, manure and other types of organic matter decomposed in aerobic conditions have had inconsistent effects, and have occasionally favors phytopathogens such as R. solani (Bonanomi et al., 2007). Currently, manure (mature or composted) has not consistently showed induction of RSD, although additional approaches have to be explored.
It is important to mention that VFAs are lost by volatilization and microbial degradation, as it occurred from one to two days with concentrations of up to ~ 500 mmol L-1 contained in fresh pig and dairy cow manures (Kirchmann & Lundvall, 1993); however, when the soil has a constant supply of VFAs, its degradation could be delayed for months (Hrapovic and Rowe, 2002). Consequently, the constant use and application of fresh cattle slurry containing VFAs could be evaluated, taking into account whether or not pathogenic animal and human bacteria survive.
For the last 10 years, investigation on RSD has focused on improving its efficiency, although in Mexico, it is yet to be evaluated in different crops, soils and phytopathogens. However, the RSD could be particularly useful in southern Mexico, where rainfalls are abundant and soils are acidic, both of which are favorable factors for RSD.
Table 2 shows examples of inputs used to generate RSD and the control of organisms. This information could serve as a basis to be evaluated in soils, where organisms negatively affect crops. Figure 6 shows some aspects of RSD in alfalfa, chestnut and fig trees that are currently being evaluated.
Table 2 Inputs used to induce RSD to control harmful organisms to agricultural crops.
| Insumos | Cantidades | Organismos a controlar | Cultivo | Referencia |
|---|---|---|---|---|
| Brassica oleracea y Lolium perenne. | 5 y 8 Mg/ton peso seco para Brassica y Lolium, respectivamente. | F. oxysporum f. sp. asparagi, V. dahliae y Rhizoctonia solani | Ninguno (suelo) | Blok et al. 2000. |
| Paja de trigo | 1-1.6 % (paja-suelo, p/p) | F. oxysporum f. sp. lycopersici and Ralstonia solanacearum | Ninguno (suelo) | Momma et al., 2006. |
| Melaza | 13.9-27.7 m3 ha-1 | Nematodos | Tomate | Di Gioia et al., 2016. |
| Melaza | 8.2 Mg ha-1 | Macrophomina phaseolina F. oxysporum | Fresa | Rosskopf et al., 2014 |
| Pajas de trigo y arroz, etanol, orujo de uva, residuos de cebolla, pasta de mostaza. | ~ 9 Mg ha-1 como carbono contenido, excepto el etanol, aplicado (1%). | V. dahliae | Ninguno (suelo) | |
| Etanol | 2-0.5% (v/v), 50-200 L m-2 | Bacterias, hongos, malezas y nematodos | Hortalizas (8) | Shennan et al., 2014. |
| Paja de arroz | 4.9 Mg ha-1 | |||
| Etanol | 8.9 kL ha-1 | Frutas (4) | ||
| B. junacea | 4.9 Mg ha-1 | |||
| Césped | Hasta Mg ha-1 | Pratylenchus penetrans y R. solani | Manzano | Hewavitharana y Mazzola, 2016. |
| Paja de arroz | 4.4 Mg ha-1 | |||
| B. junacea | 4.4 Mg ha-1 | |||
| Paja de arroz | 2 kg m-2 | Phytophthora nicotianae | Pimiento | Serrano-Pérez et al., 2017. |
| Pasta de B. junacea | 2 kg m-2 | |||
| Orujo de uva | 4 kg m-2 | |||
| Residuos de cebada fermentada | 3.5 kg m-2 | |||
Conclusions
RSD has an enormous potential for use in Mexico, due to the large extensions of cereal planted, from which necessary sources of carbon can be obtained. In addition, reusing regular black plastic and the possible adaptation of sub-surface irrigation may complement this technology; in particular, RSD is highly profitable in greenhouse crops. Molasses are another very cheap source of carbon in some areas of Mexico, along with the availability of pig and cattle slurry. In this way, RSD has the advantage of being able to use a large diversity of carbon sources in comparison with biofumigation.

Figure 6 Left, plastic in experimental field covering the soil after applying molasses to induce RSD, where alfalfa was later planted. Center, molasses added on soil in micro plot where chestnut trees were to be planted. Right, plastic covering soil on which molasses were added, and where a fig tree died previously by a P. omnivora.
Acknowledgements
To the SAGARPA-CONACYT fund, through the project code 2011-13-175247, financing part of the information. Also, to Daniela Samaniego Castruita for revising the manuscript and its version in English.
REFERENCES
Abbasi, P. A., Lazarovits, G., & Jabaji-Hare, S. (2009). Detection of high concentrations of organic acids in fish emulsion and their role in pathogen or disease suppression. Phytopathology, 99(3), 274-281. https://doi.org/10.1094/PHYTO-99-3-0274 [ Links ]
Alexander, M. (1961). Introduction to soil microbiology. John Wiley and Sons, Inc. 472 p. [ Links ]
Bailey, K. L., & Lazarovits, G. (2003). Suppressing soil-borne diseases with residue management and organic amendments. Soil and tillage research, 72(2), 169-180. https://doi.org/10.1016/S0167-1987(03)00086-2 [ Links ]
Barnett, A. J. G., & Duncan, R. E. B. (1953). The volatile fatty acids present in fresh and in fermented marrow-stem kale. Plant and Soil, 4(4), 370-376. https://doi.org/10.1007/BF01435506 [ Links ]
Bejarano-González, F. (Editor). (2017). Los Plaguicidas Altamente Peligrosos en México. Red de Acción sobre Plaguicidas y Alternativas en México, A. C. 358 p. https://www.researchgate.net/profile/Omar_Arellano-Aguilar/publication/319515704_Los_Plaguicidas_Altamente_Peligrosos_en_Mexico/links/59b04922a6fdcc3f8889aca4/Los-Plaguicidas-Altamente-Peligrosos-en-Mexico.pdf (consulta, Octubre 2018). [ Links ]
Blok, W. J., Lamers, J. G., Termorshuizen, A. J., & Bollen, G. J. (2000). Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology, 90(3), 253-259. https://apsjournals.apsnet.org/doi/pdfplus/10.1094/PHYTO.2000.90.3.253 [ Links ]
Bonanomi, G., Antignani, V., Pane, C., & Scala, F. (2007). Suppression of soilborne fungal diseases with organic amendments. Journal of Plant Pathology, 89(3), 311-324. http://www.sipav.org/main/jpp/volumes/0307/030701.pdf [ Links ]
Butler, D. M., Kokalis-Burelle, N., Albano, J. P., McCollum, T. G., Muramoto, J., Shennan, C., & Rosskopf, E. N. (2014). Anaerobic soil disinfestation (ASD) combined with soil solarization as a methyl bromide alternative: vegetable crop performance and soil nutrient dynamics. Plant and soil, 378(1-2), 365-381. https://doi.org/10.1007/s11104-014-2030-z [ Links ]
Cochrane, V. W. (1958). Physiology of fungi. John Wiley & Sons Inc.; London. 524 p. [ Links ]
Conn, K. L., Tenuta, M., & Lazarovits, G. (2005). Liquid swine manure can kill Verticillium dahliae microsclerotia in soil by volatile fatty acid, nitrous acid, and ammonia toxicity. Phytopathology, 95(1), 28-35. https://doi.org/10.1094/PHYTO-95-0028 [ Links ]
De la Cruz, E., Bravo, V., & Ramírez, F. (2018). Manual plaguicida de Centroamérica. Instituto Regional de Estudios en Sustancias Tóxicas, Universidad Nacional. Costa Rica. http://www.plaguicidasdecentroamerica.una.ac.cr/ (consulta, Octubre 2018). [ Links ]
Druzhinina, I. S., Seidl-Seiboth, V., Herrera-Estrella, A., Horwitz, B. A., Kenerley, C. M., Monte, E., Mukherjee, P. K., Zeilinger, S., Grigoriev, I. V., & Kubicek, C. P. (2011). Trichoderma: the genomics of opportunistic success. NATURE REVIEWS| MICROBIOLOGY, 9, 749-759. https://doi.org/10.1038/nrmi cro2637 [ Links ]
Eljounaidi, K., Lee, S. K., & Bae, H. (2016). Bacterial endophytes as potential biocontrol agents of vascular wilt diseases-review and future prospects. Biological Control, 103, 62-68. https://doi.org/10.1016/j.biocontrol.2016.07.013 [ Links ]
Estupiñán-Herrera, C., Samaniego-Gaxiola, J. A., Cueto-Wong, C., y Balagurusamy, N. (2010). Inducción del cambio temporal del pH en la solución de suelos inundados y adicionados con fructosa y ácido sulfúrico. Pp. 710-714. Memoria de la XXII Semana Internacional de Agronomía FAZ-UJED. Noviembre 10-12. Gómez Palacio, Durango, México. 1277 p. http://faz.ujed.mx/files/Memoria_XXII_FAZ_UJED_2010.pdf [ Links ]
Goepfert, J. M., & Hicks, R. (1969). Effect of volatile fatty acids on Salmonella typhimurium. Journal of bacteriology, 97(2), 956-958. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC249784/pdf/jbacter00392-0512.pdf [ Links ]
Hayat, R., Ali, S., Amara, U., Khalid, R., & Ahmed, I. (2010). Soil beneficial bacteria and their role in plant growth promotion: a review. Annals of Microbiology, 60(4), 579-598. https://doi.org/10.1007/s13213-010-0117-1 [ Links ]
He, Z. L., Rosskopf, E. N., Lin, Y., Powell, C. A., Hu, C., Iriarte, F., & Kokalis-Burelle, N. (2012). U.S. Patent No. US 20120015809 A1. https://patentimages.storage.googleapis.com/2c/6b/12/21ba1c21271ff4/US20120015809A1.pdf [ Links ]
Hewavitharana, S. S., & Mazzola, M. (2016). Carbon source-dependent effects of anaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology, 106(9), 1015-1028. https://doi.org/10.1094/PHYTO-12-15-0329-R [ Links ]
Hewavitharana, S. S., Ruddell, D., & Mazzola, M. (2014). Carbon source-dependent antifungal and nematicidal volatiles derived during anaerobic soil disinfestation. European journal of plant pathology, 140(1), 39-52. https://doi.org/10.1007/s10658-014-0442-5 [ Links ]
Hrapovic, L., & Rowe, R. K. (2002). Intrinsic degradation of volatile fatty acids in laboratory-compacted clayey soil.Journal of contaminant hydrology,58(3-4), 221-242. https://doi.org/10.1016/S0169-7722(02)00038-4 [ Links ]
Huang, X., Liu, L., Wen, T., Zhu, R., Zhang, J., & Cai, Z. (2015). Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f. sp. cubense infected soil during and after reductive soil disinfestation. Microbiological research, 181, 33-42. https://doi.org/10.1016/j.micres.2015.08.004 [ Links ]
Huang, X., Liu, L., Wen, T., Zhang, J., Wang, F., & Cai, Z. (2016). Changes in the soil microbial community after reductive soil disinfestation and cucumber seedling cultivation. Applied microbiology and biotechnology, 100(12), 5581-5593. https://doi.org/10.1007/s00253-016-7362-6 [ Links ]
Ioannou, N. S. R. W., Schneider, R. W., & Grogan, R. G. (1977). Effect of flooding on the soil gas composition and the production of microsclerotia by Verticillium dahliae in the field. Phytopathology, 67, 651-656. https://www.apsnet.org/publications/phytopathology/backissues/Documents/1977Articles/Phyto67n05_651.pdf [ Links ]
Katase, M., Kubo, C., Ushio, S., Ootsuka, E., Takeuchi, T., & Mizukubo, T. (2009). Nematicidal activity of volatile fatty acids generated from wheat bran in reductive soil disinfestation. Nematological Research, 39(2), 53-62. https://doi.org/10.3725/jjn.39.53 [ Links ]
Kirchmann, H., & Lundvall, A. (1993). Relationship between N immobilization and volatile fatty acids in soil after application of pig and cattle slurry. Biology and fertility of soils, 15(3), 161-164. https://doi.org/10.1007/BF00361605 [ Links ]
Lazarovits, G., Conn, K. L., Abbasi, P. A., & Tenuta, M. (2005). Understanding the mode of action of organic soil amendments provides the way for improved management of soilborne plant pathogens. Acta Horticulturae, 698, 215-224. https://doi.org/10.17660/ActaHortic.2005.698.29 [ Links ]
Liu, L., Kong, J., Cui, H., Zhang, J., Wang, F., Cai, Z., & Huang, X. (2016). Relationships of decomposability and C/N ratio in different types of organic matter with suppression of Fusarium oxysporum and microbial communities during reductive soil disinfestation. Biological Control, 101, 103-113. http://dx.doi.org/10.1016/j.biocontrol.2016.06.011 [ Links ]
Liu, L., Chen, S., Zhao, J., Zhou, X., Wang, B., Li, Y., Zheng, G., Zhang, J., Cai, Z., & Huang, X. (2018). Watermelon planting is capable to restructure the soil microbiome that regulated by reductive soil disinfestation. Applied Soil Ecology, 129, 52-60. https://doi.org/10.1016/j.apsoil.2018.05.004 [ Links ]
Lynch, J. M. (1977). Phytotoxicity of acetic acid produced in the anaerobic decomposition of wheat straw.Journal of Applied Bacteriology,42(1), 81-87. https://doi.org/10.1111/j.1365-2672.1977.tb00672.x [ Links ]
Lynch, J. M., Gunn, K. B., & Panting, L. M. (1980). On the concentration of acetic acid in straw and soil.Plant and soil,56(1), 93-98. https://link.springer.com/article/10.1007/BF02197956 [ Links ]
Meghvansi, M. K., & Varma, A. (Eds.). (2015). Organic amendments and soil suppressiveness in plant disease management (Vol. 46). Dordrecht: Springer, Switzerland. 531p. [ Links ]
Menzies, J. D. (1962). Effect of anaerobic fermentation in soil on survival of sclerotia of Verticillium dahliae (Abst). Phytopathology, 52(8), 743. http://www.apsnet.org/meetings/meetingarchives/Pages/default.aspx [ Links ]
Momma, N., Yamamoto, K., Simandi, P., & Shishido, M. (2006). Role of organic acids in the mechanisms of biological soil disinfestation (BSD). Journal of General Plant Pathology, 72(4), 247-252. https://doi.org/10.1007/s10327-006-0274-z [ Links ]
Momma, N. (2008). Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. Japan Agricultural Research Quarterly: JARQ, 42(1), 7-12. https://doi.org/10.6090/jarq.42.7 [ Links ]
Momma, N., Momma, M., & Kobara, Y. (2010). Biological soil disinfestation using ethanol: effect on Fusarium oxysporum f. sp. lycopersici and soil microorganisms.Journal of general plant pathology,76(5), 336-344. https://doi.org/10.1007/s10327-010-0252-3 [ Links ]
Momma, N., Kobara, Y., & Momma, M. (2011). Fe2+ and Mn2+, potential agents to induce suppression of Fusarium oxysporum for biological soil disinfestation. Journal of General Plant Pathology, 77(6), 331-335. https://doi.org/10.1007/s10327-011-0336-8 [ Links ]
Momma, N., Kobara, Y., Uematsu, S., Kita, N., & Shinmura, A. (2013). Development of biological soil disinfestations in Japan. Applied microbiology and biotechnology, 97(9), 3801-3809. https://doi.org/10.1007/s00253-013-4826-9 [ Links ]
Okazaki, H. (1985). Volatile (s) from glucose-amended flooded soil influencing survival of Fusarium oxysporum f. sp. raphani. Japanese Journal of Phytopathology, 51(3), 264-271. https://www.jstage.jst.go.jp/article/jjphytopath1918/51/3/51_3_264/_pdf/-char/ja [ Links ]
Okazaki, H., & Nose, K. (1986). Acetic acid and n-butyric acid as causal agents of fungicidal activity of glucose-amended flooded soil. Japanese Journal of Phytopathology, 52(3), 384-393. https://www.jstage.jst.go.jp/article/jjphytopath1918/52/3/52_3_384/_pdf [ Links ]
Ponnamperuma, F. N. (1972). The chemistry of submerged soils. InAdvances in agronomy(Vol. 24, pp. 29-96). Academic Press. https://pdfs.semanticscholar.org/ed7f/45fc78cfd694ed285e17590058c6c7aa2e62.pdf [ Links ]
Ríos-Castaño, P. (2017). Control de la podredumbre radical causada por Phytophthora cinnamomi en dehesas mediante biofumigación con Brassica spp. Tesis Doctoral. Córdoba España. Pp. 176. http://helvia.uco.es/bitstream/handle/10396/15073/2017000001669.pdf?sequence=1 [ Links ]
Rosskopf, E. N., Burelle, N., Hong, J., Butler, D. M., Noling, J. W., He, Z., Booker, B., & Sances, F. (2014). Comparison of Anaerobic Soil Disinfestation and Drip-Applied Organic Acids for Raised-Bed Specialty Crop Production in Florida. Proc. VIIIth IS on Chemical and Non-Chemical Soil and Substrate Disinfestation. Acta Horticola, 1044:221-228. Doi: 10.17660/ActaHortic.2014.1044.26 [ Links ]
Samaniego-Gaxiola, J. A. (1994). Viabilidad de los esclerocios de Phymatotrichum omnivorum (Shear) Dugg. en suelos inundados y complementados con glucosa. Revista Mexicana de Fitopatología, 12(1), 125-133. http://rmf.smf.org.mx/# [ Links ]
Samaniego-Gaxiola, J. A. (2008). Efecto del pH en la sobrevivencia de esclerocios de Phymatotrichopsis omnivora Dugg Hennebert II expuestos a Tilt y Trichoderma sp. Revista Mexicana de Fitopatología, 26, (1) 32-39. http://www.redalyc.org/html/612/61226106/ [ Links ]
Samaniego Gaxiola, J. A., Ordóñez-Meléndez, H. J., Pedroza Sandoval, A., & Cueto-Wong, C. (2010). Relationship between the drying of the sclerotia of Phymatotrichopsis omnivora and its survival. Revista Mexicana de Micología, 32(1), 49-58. http://www.redalyc.org/pdf/883/88319899006.pdf [ Links ]
Samaniego-Gaxiola, J. A. (2013). Supervivencia de los esclerocios de Phymatotrichopsis omnivora en función del pH in vitro. Revista Mexicana de Ciencias Agrícolas, 4(3), 337-351. http://www.redalyc.org/service/redalyc/downloadPdf/2631/263127575001/1 [ Links ]
Samaniego-Gaxiola, J. A., & Balagurusamy, N. (2013). Survival of soil-borne fungus Phymatotrichopsis omnivora after exposure to volatile fatty acids. Journal of general plant pathology, 79(2), 105-109. https://link.springer.com/article/10.1007/s10327-013-0436-8 [ Links ]
Samaniego-Gaxiola, J. A., & Pedroza-Sandoval, A. (2013). Usos potenciales de los ácidos grasos volátiles en suelo, agua y aire. Terra Latinoamericana, 31(2), 155-163. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0187-57792013000300155 [ Links ]
Samaniego-Gaxiola, J. A., Pedroza-Sandoval, A., Chew-Madinaveitia, Y., & Gaytán-Mascorro A. (2018). Reductive disinfestation, desiccation and Trichoderma harzianum to control Phymatotrichopsis omnivora in pecan tree nursery. Revista Mexicana de Fitopatología, (sometido para publicación). [ Links ]
Santoyo, G., Moreno-Hagelsieb, G., del Carmen Orozco-Mosqueda, M., & Glick, B. R. (2016). Plant growth-promoting bacterial endophytes. Microbiological research, 183, 92-99. https://doi.org/10.1016/j.micres.2015.11.008 [ Links ]
Serrano-Pérez, P., Rosskopf, E., De Santiago, A., & del Carmen Rodríguez-Molina, M. (2017). Anaerobic soil disinfestation reduces survival and infectivity of Phytophthora nicotianae chlamydospores in pepper. Scientia horticulturae, 215, 38-48. http://dx.doi.org/10.1016/j.scienta.2016.12.003 [ Links ]
Shennan C, Muramoto J, Lamers J, Mazzola M, Rosskopf EN, Kokalis-Burelle N, Momma N, Butler DM, and Kobara Y. (2014). Anaerobic soil disinfestation for soil borne disease control in strawberry and vegetable systems: Current knowledge and future directions. Acta Hortic. 1044:165-175. https://doi.org/10.17660/ActaHortic.2014.1044.20 [ Links ]
Shinmura, A., Sakamoto, N., & Abe, H. (1999). Control of Fusarium root rot of Welsh onion by soil reduction. (Abstract in Japanese). Annals of the Phytopathological Society of Japan, 65(3), 352-353. https://ci.nii.ac.jp/els/contents110002733276.pdf?id=ART0003023797 [ Links ]
Shrestha, U., Ownley, B. H., Rosskopf, E. N., Dee, M. E., & Butler, D. M. (2013). Optimization of amendment C: N ratio in anaerobic soil disinfestation for control of Sclerotium rolfsii. In Proceedings of Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, San Diego, CA (pp. 14-1). [ Links ]
Shrestha, U., Augé, R. M., & Butler, D. M. (2016). A Meta-Analysis of the Impact of Anaerobic Soil Disinfestation on Pest Suppression and Yield of Horticultural Crops. Frontiers in plant science, 7, article 1254, 1-20. https://doi.org/10.3389/fpls.2016.01254 [ Links ]
Siddiquee, S., Cheong, B. E., Taslima, K., Kausar, H., & Hasan, M. M. (2012). Separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC-MS using three different capillary columns.Journal of chromatographic science,50(4), 358-367. https://doi.org/10.1093/chromsci/bms012 [ Links ]
Stanier, R. Y., Doudoroff, M., y Adelberg, E. A. (1977). Microgiología. Aguilar. España. 932 p. [ Links ]
Stover, R. H. (1955). Flood-fallowing for eradication of Fusarium oxysporum f. cubense: III. Effect of oxygen on fungus survival.Soil Science,80(5), 397-412. https://journals.lww.com/soilsci/Citation/1955/11000/FLOOD_FALLOWING_FOR_ERADICATION_OF_Fusarium.7.aspx [ Links ]
Tenuta, M., & Lazarovits, G. (2002). Ammonia and nitrous acid from nitrogenous amendments kill the microsclerotia of Verticillium dahliae. Phytopathology, 92(3), 255-264. https://apsjournals.apsnet.org/doi/pdfplus/10.1094/PHYTO.2002.92.3.255 [ Links ]
Tenuta, M., Conn, K. L., & Lazarovits, G. (2002). Volatile fatty acids in liquid swine manure can kill microsclerotia of Verticillium dahliae. Phytopathology, 92(5), 548-552. https://apsjournals.apsnet.org/doi/pdf/10.1094/PHYTO.2002.92.5.548 [ Links ]
Ueki, A., Takehara, T., Ishioka, G., Kaku, N., & Ueki, K. (2017). Degradation of the fungal cell wall by clostridial strains isolated from soil subjected to biological soil disinfestation and biocontrol of Fusarium wilt disease of spinach. Applied microbiology and biotechnology, 101(22), 8267-8277. https://doi.org/10.1007/s00253-017-8543-7 [ Links ]
Ueki, A., Kaku, N., & Ueki, K. (2018). Role of anaerobic bacteria in biological soil disinfestation for elimination of soil-borne plant pathogens in agriculture. Applied microbiology and biotechnology, 102 (15), 6309-6318. https://doi.org/10.1007/s00253-018-9119-x [ Links ]
van Agtmaal, M., van Os, G. J., Hol, W. G., Hundscheid, M. P., Runia, W. T., Hordijk, C. A., & de Boer, W. (2015). Legacy effects of anaerobic soil disinfestation on soil bacterial community composition and production of pathogen-suppressing volatiles. Frontiers in microbiology, 6, article 701, 1-12. https://doi.org/10.3389/fmicb.2015.00701 [ Links ]
Yossen, V., Zumelzu, G., Gasoni, L., & Kobayashi, K. (2008). Effect of soil reductive sterilization on Fusarium wilt in greenhouse carnation in Córdoba, Argentina. Australasian Plant Pathology, 37(5), 520-522. https://doi.org/10.1071/AP08039 [ Links ]
Received: October 02, 2018; Accepted: November 21, 2018











 texto en
texto en 


