INTRODUCTION
Heavy metal pollution has developed in many countries due to unregulated or illegal discharges to the soil or water systems (He et al., 2015). The sources of these include byproducts from chemical manufacturing (alloys, ornamental plating, green color glass, aluminum anodization, and oxidant agent in diverse chemical reactions), metallurgy (metal fabrication and industrial mining), and production of refractory materials (Jacobs and Testa, 2004). In the environment, chromium occurs in two stable redox states, chromium (III) (Cr III) and chromium (VI) (Cr VI). However, they differ in their bioavailability and mobility (Aharchaou et al., 2017). While Cr III is present in the soil where it is available as a micronutrient for plants, Cr VI is more toxic and considered a human carcinogen (ATSDR, 2012).
Cr III has a low solubility and low mobility, forms complexes with organic matter in soils and waters, and is considered relatively innocuous (Fendorf, 1995) this paper describes surface reactions that influence Cr chemistry in soils. Specifically, retention reactions of Cr (III. Nonetheless, its presence in water results from the release of Cr VI in wastewater and its subsequent biotransformation by both bacteria and eukaryotic cells (Norseth, 1986). Thereafter, in Cr VI-contaminated waters the presence of organic-Cr III complexes must be assessed because such complexes represent the main source for Cr III mobility and thus, the intoxication of exposed biota. Currently, information about the fate and toxicity of Cr III and its organic complexes is limited (Chatterjee & Luo 2010). Nevertheless, while Cr III plays an important role as an essential trace metal in plant metabolism (Bielicka et al., 2005), its role in animals remains controversial (Di Bona et al., 2011; Vincent, 2017).
These two forms of Cr differ in their solubility and mobility. In comparison to Cr III, Cr VI exhibits higher solubility and greater mobility and it because of the active sulfate transporter it through pass through biological membranes (Pereira et al., 2008). Besides Cr VI uptake via sulfate transporter, recent references highlight Cr VI-induced over-expression of several ABC transporters (Feng et al., 2018). Cr VI is considered the most toxic form of chromium due to its strong oxidant power; once it has passed through the cell’s membrane Cr VI initiates complex mechanisms involving different biochemical pathways and multiple targets (Rudolf & Červinka, 2006). Althought Cr VI is reduced by enzymatic and non-enzymatic reactions to produce less reactive forms like Cr III and CrV, such reduction reactions produce reactive oxygen species (ROS) that alter the redox environment within the organism, thereby inducing oxidative stress (Arzate-Cárdenas & Martínez-Jerónimo, 2011).
With at least 2000 species rotifers are a very diverse group of micrometazoans that play significant roles in aquatic ecosystems in both the microbial loop and classic food webs (Segers, 2007; Wallace et al., 2015). Rotifers have been used for ecotoxicological studies because of their ease to culture and maintenance, their parthenogenetic mode of reproduction, their short life cycle and generation time, their relatively high growth rates, and their wide geographical distribution (Sarma et al., 2006; Segers, 1996; Pérez-Legaspi & Rico-Martínez, 2001).
The genus Lecane (Family Lecanidae) is found in shallow waters, littoral areas, and eutrophic environments (Keppeler et al., 2010). While many species of Lecane are cosmopolitan, depending on the zoogeographical region between 6.5 to 22% are endemic (De Manuel, 1994; Segers, 1996). Besides their geographic distribution some Lecane spp. are used in activated sludge systems, to improve the quality of the process and removal of organic contaminants (Fiałkowska & Pajdak-Stós, 2008). Pérez-Legaspi & Rico-Martínez, (2001) used three Lecane species: L. hamata (Stokes, 1896), L. luna (Müller, 1776), and L. quadridentata (Ehrenberg, 1832), to assess the effect of several chemical compounds and suggested these rotifers as alternatives test species. Klimek et al., (2013) have suggested that Lecane inermis (Bryce, 1892) could represent a better option in toxicological evaluations because is more sensitive than species used in the more commonly used Brachionus test.
The process of bioconcentration begins when a chemical is absorbed by an organism from the environment through its respiratory or dermal surfaces or through ingestion; concentration of the substance increases with subsequent uptake. The degree to which bioconcentration occurs over the concentration in the environment is expressed as the bioconcentration factor (BCF) and can only be measured under controlled conditions in which dietary intake of the chemical is not included (Arnot & Gobas, 2011). Acute and chronic toxicity tests are helpful to assess the impact of environmental pollutants. In the last few years, bioconcentration has been used to allow better assessment of toxicity together with toxicological tests (Gagneten et al., 2009). Bioconcentration tests using rotifers as model organisms is advantageous because these invertebrates are easily cultured, their life cycles of are short, and their life history parameters are easily determined. Moreover, the stationary stage of analytes (like pesticides and semiochemicals) are reached in a few days (Rivera-Dávila et al., 2021). Lethal Body Burden (LBB) is defined as the body burden in micrograms (μg) per body weight or mmol/kg at which a specific toxic effect is detected: e.g., mortality, reduction in reproduction, enzymatic inhibition. Some authors have applied LBB to evaluate toxicity of compounds and metals in rotifers (Hernández-Flores et al., 2020; van Wezel et al., 1995).
The maximum limits of chromium in water for the protection of aquatic biota and drinking water quality are set in specific guidelines. However, the Mexican regulations are set higher than those of several other countries (DOF, 2022). For instance, the U.S. Environmental protection agency has a standard of 0.1 mg/L (USEPA, 2008); in Canada and Europe drinking water guideline for chromium is a maximum acceptable concentration of 0.05 mg/L (Government of Canada, 2018; HBM4EU, 2020). The Australian Government (2011) has established 0.003 mg/L (for Cr III) and 0.001 mg/L (for Cr VI).
Given the many variations in international governmental norms and legislation dealing with Cr III and Cr VI in water, and taking into consideration the role of both Cr species affecting different trophic levels in freshwater ecosystems and the quality of drinking water in several regions of Mexico; this contribution set a goal of analyzing how Cr III and Cr VI affect the demographic response of Lecane papuana (a zooplanktonic native species of Mexico), using acute and chronic tests, and determined the Bioconcentration Factor (BCF) for both Cr chemical speciations.
MATERIALS AND METHODS
Culture of test organisms. Lecane papuana was originally collected at El Ocote, Aguascalientes (21.464’N, 102.313’W), Mexico (Saucedo-Ríos et al., 2017), and cultured in the laboratory for more than five years prior to the beginning of the experiments. To start experiments we placed 60 to 80 parthenogenetic females in Petri dishes (~ 70 mm diameter) filled with ~ 50 mLs of moderately hard, reconstituted water (MHRW) (USEPA, 2002). Stock cultures were maintained in a bioclimatic chamber (Revco Scientific, Inc.) at 25 ± 2 ºC. Rotifers to be used in the toxicity tests were obtained by removing amictic embryos from the stock culture and placing them into Petri dishes with MHRW, without food supplementation, and kept in a bioclimatic chamber at 25 ± 2 ºC.
Acute toxicity tests. ACS grade ≥98% potassium dichromate (K2Cr2O7) and chromium (III) potassium sulfate dodecahydrate (KCr(SO4)2·12H2O) (Fisher Chemical, Hampton NH) were used as Cr VI and Cr III species, respectively. A standard solution (1000 mg/L) of each was made with deionized water; later these standard solutions were used in our experiments. pH was monitored throughout the experiments to ensure that Cr III and Cr VI species were maintained during the experiment.
To do these experiments we placed, 10 neonates (<24 h old) into the wells of a 24-well polystyrene plate (Corning Inc. New York) prefilled with the appropriate solution of MHRW. Each well had a total volume of 1 mL. All treatments were replicated four times. These plates were incubated in a bioclimatic chamber (Revco Scientific, Inc.), without food, for 48 h with a photoperiod of 16:8 h (light: dark), at 25 ± 2ºC. Following exposure, the number of dead or immobilized animals was recorded. We only accepted experimental runs where mortality in the control plates was less than 10%. The LC50 values were estimated with the dcr package in R.
Chronic toxicity tests. Briefly, eight replicates at six concentrations (0.0, 3.125, 6.25, 12.5, 25, and 50% of the corresponding acute LC50) were used to assess chronic toxicity. For each replicate, five neonates of < 24-h old were placed in 2 mL of EPA medium. All experiments were conducted in 24-well polystyrene plates (Costar Co., USA). After five days, the number of individuals per well was counted and used to estimate the intrinsic rate of population increase, r, as follows:
where Nt is the final number of individuals, N0 is the initial number of rotifers, Ln is the natural logarithm, and t is the exposure period (5 d). Statistical analyses were performed using GraphPad Prism version 6.0.0
Biomass determination. Ten thousand non-ovigerous, adult females of L. papuana were separated (3X), rinsed with deionized water, and dried at 60°C (Fisher Scientific, Isotemp® 500 Series; Waltham, MA) in microtubes until we recorded a constant mass using an analytical balance (Chyo, model JK-200, Japan, ± 0.001 g). The difference in the dry weight of the empty microtube with the microtube with the rotifer biomass is the dry weight expressed as nanograms per individual.
Bioconcentration determinations. We placed 600 rotifers in small Petri dishes (1 mm diameter) with 2 mL of either Cr III or Cr VI solution without food. The concentrations tested correspond to the LC50, LC10 and LC1 for every metal species (n = 4). Rotifers cultured in MHRW without metals served as a negative control. These Petri dishes were placed into a bioclimatic chamber (Revco Scientific, Inc.) at 25 ± 2ºC for 24 h with a photoperiod of 16:8 h (light: dark). After this exposure, rotifers were collected in Petri dishes and carefully rinsed with deionized water to eliminate excess chromium. Then the rotifers were placed in an microtube with 1 mL of deionized water and 500 µL of nitric acid (65%), at 4°C, until analysis.
We quantified chromium in rotifers exposed to all metal concentrations tested in our experiments. Exposure concentrations of the acute toxicity tests were also analyzed with three replicates to obtain actual concentrations instead of nominal concentrations. We performed atomic absorption spectrophotometry with an Analyst 800 Spectrometer (Perkin Elmer, Norwalk, CT) at three settings: (1) Transversely heated graphite furnace, (2) longitudinal Zeeman-effect background correction, and (3) AS-60 autosampler. Chromium was quantified according to the Mexican normative NOM-117-SSA1-1994 (DOF, 1995). To do this we performed Cr analysis methodology with 5 points of a calibration curve, with a minimum r2 of 0.995. The percentage variability was less than 5% (% RSD) and the maximum standard deviation was 5%, analyzing reagent blanks and fortified and replicated samples, with a variation less than 20 % of the analyte. The detection limit of this method was 0.372 µg/L. In all cases blanks were below detection limits.
The accumulated amount (q) of chromium (µg/g dry weight) was calculated according to Hernández-Flores et al., (2020):
Where: C0 , initial chromium concentration in the medium (µg/L); Ct , metal concentration at time t (µg/L); V, total volume of sample in liters (L) and W dry weight of rotifers in grams (g).
The Bioconcentration Factor (BCF) (dimensionless) was determined according to the following formulas:
RESULTS
Acute and chronic toxicity tests. Results of the acute toxicity tests showed that L. papuana is more tolerant to Cr III than it is to Cr VI; the LC50 for Cr III was 15x higher than the respective value for Cr VI (Table 1). Actual exposure concentrations are similar to nominal concentrations (98.75% of similarity, n = 4 for three concentrations tested at beginning of acute exposure). Table 2 shows LC50 of several invertebrate species.
Table 1 Toxicity values obtained from the acute toxicity test with Lecane papuana.
| Chromium species | LC50, mg/L | LC10, mg/L | LC1, mg/L | r2 |
| (CI 95%) | (CI 95%) | (CI 95%) | ||
| Cr III | 2.613 | 0.1028 | 0.0117 | 0.92 |
| (2.13-3.10) | (0.061-0.143) | (0.0005- 0.0004) | ||
| Cr VI | 0.177 | 0.0788 | 0.0132 | 0.96 |
| (0.13-0.23) | (0.041-0.116) | (0.0044- 0.0264) |
Table 2 Comparisons of values for chromium in other invertebrates
| Species | values mg/ L | Source |
| Brachionus calyciflorus (Monogononta: Brachionidae) | 0.64 - 1.051 (24 h) Cr III | Hernández-Ruiz et al. (2016) |
| Lecane quadridentata (Monogononta: Lecanidae) | 4x10-6 (24 h) Cr VI | |
| 1.279 (24 h) Cr III | ||
| 4.7 x 10-5 (24 h) Cr VI | ||
| Lecane papuana (Monogononta: Lecanidae) | 2.613 (48 h) Cr III | This study |
| 0.177 (48 h) Cr VI | ||
| Brachionus calyciflorus (Monogononta: Brachionidae) | 8.3 (48 h) Cr VI | Snell and Moffat (1992) |
| Daphnia exilis (Anomopoda: Daphniidae) | 0.1170 (48 h) Cr VI | Martínez-Jerónimo et al. (2008) |
| Daphnia magna (Anomopoda: Daphniidae) | 0.2076 (48 h) Cr VI | Martínez-Jerónimo et al. (2006) |
| Daphnia pulex (Anomopoda: Daphniidae) | 0.13 (48 h) Cr VI | Velandia and Montañez (2010) |
| Lecane hamata (Monogononta: Lecanidae) | 4.41 (48 h) total Cr | Pérez-Legaspi and Rico-Martínez (2001) |
| Lecane luna (Monogononta: Lecanidae) | 3.26 (48 h) total Cr | |
| Lecane quadridentata (Monogononta: Lecanidae) | 4.50 (48 h) total Cr | |
| Daphnia magna (Anomopoda: Daphniidae) | 0.015 (24 h) Cr VI | CCME (1999) |
| Procambarus clarkia (Decapoda: Cambaridae) | 500 (96 h) Cr VI | |
| Simocephalus vetulus (Anomopoda: Daphniidae) | 0.015 (24 h) Cr VI |
Chronic toxicity exposure concentrations were based on the corresponding LC50 values for both chromium species (3.125, 6.25, 12.5, 25, and 50% of each LC50) and we obtained their respective intrinsic growth rates. Cr III affects L. papuana significantly at 0.163 mg/L (6.25% of its respective LC50); Cr VI affects significantly at 0.0885 mg/L (50% of its respective LC50) (Figure 1).
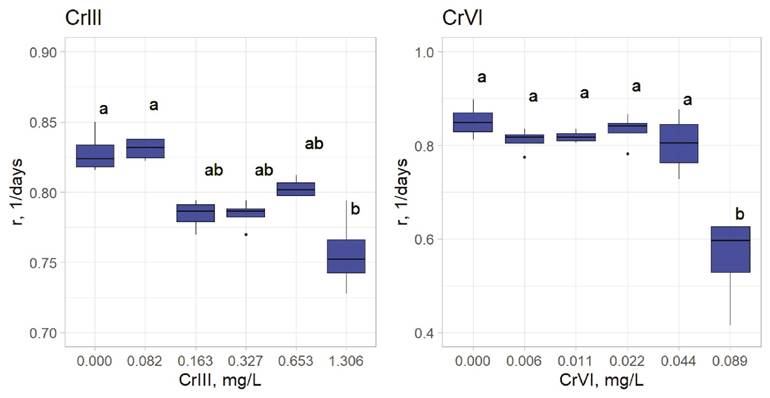
Figure 1 Intrinsic growth rates (r) of Lecane papuana exposed to five different concentrations of Cr III and Cr VI. Significant differences were established through one-way ANOVA and Bonferroni’s multiple comparison tests. Different letters above the boxes indicate significant differences (P<0.05). (N = 4). Statistical analyses were performed with the packages agricolae and ggplot2 in R.
Bioconcentation determinations. In metal-exposed rotifers the content of Cr III was significantly higher (LC10, 0.1028 mg/L) when compared to controls (Figure 2). Exposure to 0.163 mg/L (6.25% of the LC50) of Cr III negatively affected the intrinsic growth rate of L. papuana (Figure 1). Rotifers exposed to Cr VI bioconcentrated this metal at 0.177 mg/L (the respective LC50) (Figure 2). Fecundity was significantly affected when Cr VI reached 0.0885 mg/L (Figure 1), which corresponds to the 50% of the respective LC50.
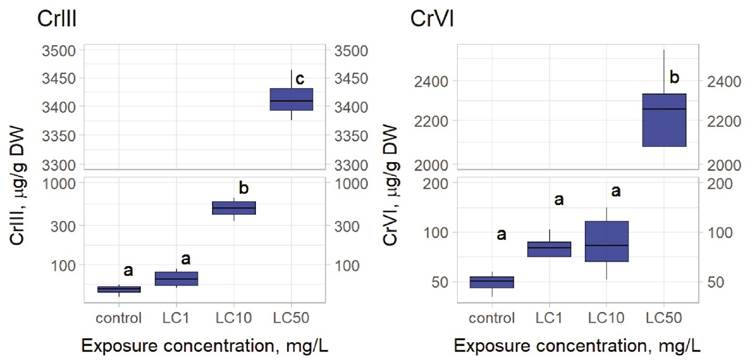
Figure 2 Results of bioconcentration on Lecane papuana exposed to three different concentrations of chromium. For Cr III: LC1, LC10 and LC50 correspond to 0.012, 0.103, and 2.613 mg/L, respectively. For Cr VI: LC1, LC10 and LC50 correspond to 0.013, 0.0.079, and 0.177 mg/L, respectively. Significant differences were established through one-way ANOVA and Bonferroni’s multiple comparison tests. Different letters above the boxes indicate significant differences (P<0.05). (N = 4). Statistical analyses were performed with the packages agricolae and ggplot2 in R.
Table 3 presents results of Cr content in rotifers exposed to Cr, determining the respective BCF and LBB for both redox forms. These data show that L. papuana accumulated higher amounts of Cr III and that the LBB for Cr III was higher (1.6X) than that of Cr VI. The BCF for Cr III was 9.4-fold lower than that of Cr VI.
Table 3 Bioconcentration, Body burdens, and chronic toxicity (“r” inhibition) of chromium in Lecane papuana after 24-h exposure to Cr III or Cr VI. n = 4.
| Chromium species | (µg of Cr/rotifer) | Accumulated Cr concentration in L. papuana (µg/g) | BCF | LBB mmol/kg DW | EC50 “r” | LOECchronic mg/L | NOECchronic mg/L | MATC | ACRr |
|---|---|---|---|---|---|---|---|---|---|
| III | 1.71x10-4 ± 3.15x10-6 | 3420 ± 62.93 | 1308.84 | 65.76 | 0.1181 | 0.163 | 0.0816 | 0.115 | 22.66 |
| VI | 1.089x10-4 ± 8.97x10-6 | 2177 ± 179.38 | 12299.44 | 41.86 | 0.1127 | 0.0885 | 0.04425 | 0.062 | 2.83 |
DISCUSSION
This contribution assessed the acute and chronic effects of Cr III and Cr VI in the rotifer L. papuana including acute and chronic body burdens (LBB and CBB) and bioconcentration factors (BFC) to make progress in the understanding on the fate and potential environmental effects of chromium in non-target freshwater organisms.
We found L. papuana to be a good model to assess the toxicity of Cr III and Cr VI and as a monitor of chromium bioaccumulation. Our results in the acute and chronic toxicity tests indicate that L. papuana presents similar sensitivity to toxicants as Cladoceras but offers the advantage to assess the effect of contaminants in the sediment-water interphase (Garza-León et al., 2017).
Acute toxicity tests. As expected from published research, Cr VI caused toxicity at a lower concentration than Cr III (Table 1). This is because Cr VI passes through cell membranes and can be actively transported into the cell by the sulfate transporter. Once inside of a cell, Cr VI generates reduced intermediates of chromium that with hydrogen peroxide (H2O2) generate reactive oxygen species (ROS) via Fenton and Haber-Weiss reactions. The result of that metabolism is substantial oxidative damage (Ercal et al., 2001). Moreover, Cr VI can be reduced by enzymatic and non-enzymatic reactions and then interact with some endogenous reductants, such as glutathione (GSH), cysteine, or nucleotides (Arzate-Cárdenas & Martínez-Jerónimo, 2011). However, cells are not very permeable to Cr III and these mechanisms do not occur in the presence of Cr III. Cr III is accumulated at cation-binding sites of the cell membrane. While Cr III is toxic due to its capacity to form complexes with proteins and organic compounds, it is not as toxic as Cr VI (Albert, 1997; Dayan & Paine, 2001; Gagneten & Imhof, 2009).
As observed in Table 2, Cr VI is more toxic for L. papuana than for Brachionus calyciflorus (Pallas, 1766) (a 48h). However, Cr VI is more toxic for L. quadridentata than for L. papuana. Cr III is more toxic to B. calyciflorus and L. quadridentata, in comparison to the effects elicited on L. papuana (Table 2). As observed, Cr III promoted lower toxicity than Cr VI.
Several authors have found differences in chromium susceptibility within the family Lecanidae (Saucedo-Ríos et al., 2017) and in comparison to Brachionidae (Sarma et al., 2006).
In addition, cladocerans as Daphnia exilis Herrick 1895, Daphnia magna Straus 1820, and Daphnia pulex (Leydig, 1860), exhibited LC50 values for Cr VI very close to those ones found for L. papuana (Tabla 2). All these tests were carried out in similar conditions to the ones employed in this work (except for the variation in temperature); which it might suggest that L. papuana is as sensitive as the freshwater model organisms used worldwide such as Daphnia magna (Martínez-Jerónimo et al., 2008). Some authors have suggested that Neoartic cladocerans like D. magna are not good model species to predict the effects of contaminants in neotropical ecosystems (Gutierrez et al., 2010) a representative calanoid copepod, we carried out two (acute and chronic. Moreover, it has been described that this rotifer species is also susceptible to the effects of pesticides at environmental concerning concentrations (Garza-León et al., 2017).
For protection of aquatic biota, the maximum allowable limit of chromium is 0.5 mg/L. However, that level is at least 3 times higher than the LC50 values recorded for L. papuana. Moreover, 0.5 mg/L is even higher for some other native species that are more sensitive to this metal. International guidelines report lower limits for chromium concentration in comparison to the Mexican normative. Nevertheless, these limits are higher than concentrations that cause deleterious effects on rotifers. Thus, while substantial variation exists for acceptable chromium limits among countries, Mexican law is less protective. Our results add information to the need to establish lower protective values for Cr to better protect native freshwater species from acute toxicity.
Chronic toxicity test. The intrinsic population growth rate of L. papuana was significantly affected by exposure to Cr (Figure 1). Lecane papuana was affected significantly at 0.163 and 0.0885 mg/L, which correspond to LOEC values for Cr III and Cr VI, respectively. Snell & Moffat (1992) carried out a study with similar conditions as ours, in which Brachionus calyciflorus was exposed to Cr VI and obtained a LOEC of 3.2 mg/L; representing lower toxicity to Brachionus than for L. papuana.
Hermens et al., (1984), found a chronic Cr VI EC50 value of 0.27 mg/L for D. magna. Wong & Pak, (2004) reported a EC50 of at least 0.268 mg/L for larval development inhibition in the copepod Mesocyclops pehpeiensis Hu 1943. Our value for Cr VI lethal effects was lower that both chronic values (Table 1). Gutierrez et al., (2010) found EC50 values from 0.170 to 0.599 mg/L in the copepod Notodiaptomus conifer (Kiefer, 1936), for 48 and 24 h respectively. Planktonic organisms are more tolerant to Cr VI than L. papuana.
The effects of metals in lifetable parameters have been analyzed in many zooplanktonic species of rotifers and cladocerans (Sarma et al., 2006). A decrease in peak population of a species due to stress may mainly result in (a) poor filtration, consumption or assimilation of the food and (b) reduced neonate production or higher rate of mortality (Sarma et al., 2006).
Bioconcentration determinations. The criterion to classify a chemical substance as “bioaccumulative” requires the BCF to be comprised between 1000 and 5000 (Arnot & Gobas, 2011). Thus, Cr VI in L. papuana can be classified as “very bioaccumulative” because its BCF was higher than 5000. In contrast, Cr III has a BCF of 1,308.84, which is about 10X lower than one obtained for Cr VI (BCF = 12,299.44). Thus, there is a significant difference in the accumulation rate between Cr III and Cr VI. These results suggest that the patterns of accumulation of the two chromium chemical species and the mechanisms of toxicity are different (Rainbow, 2007) all of which have the potential to cause toxic effects. Subsequent tissue and body concentrations of accumulated trace metals show enormous variability across metals and invertebrate taxa. Accumulated metal concentrations are interpreted in terms of different trace metal accumulation patterns, dividing accumulated metals into two components - metabolically available metal and stored detoxified metal. Examples of different accumulation patterns are described from crustaceans but have a general applicability to all aquatic invertebrates. Toxicity does not depend on total accumulated metal concentration but is related to a threshold concentration of internal metabolically available metal. Toxicity ensues when the rate of metal uptake from all sources exceeds the combined rates of detoxification and excretion (if present). These differences are clear when we compare the concentrations used for each chemical species; while for Cr III we used 2.613 mg/L, for Cr VI we used a 15-fold lesser concentration (0.177 mg/L). Even so, the Cr VI BCF was an order of magnitude higher (10-fold). This behavior of chromium chemical species was already reported for other freshwater rotifer species like L. quadridentata and B. calyciflorus, where Cr VI was more efficiently bioconcentrated than Cr III (Hernández-Ruiz et al., 2016). A comparison of bioconcentration studies shows that L. papuana bioconcentrated more Cr VI than freshwater fishes (Table 4).
Table 4 Bioconcentration Factor for Chromium (VI) in different taxa
| Species | Accumulated Cr concentration (µg/g) | BCF | Exposure concentration (mg/L) | LC50 | Source |
| Argyrodiaptomus falcifer (Calanoida: Diaptomidae) | 50 (2 d) Cr VI | 231 | 0.350 | NA | Gagneten et al. (2009) |
| Daphnia magna (Anomopoda: Daphniidae) | 80 (2 d) Cr VI | 281 | |||
| Catla catla (Cypriniformes: Cyprinidae) | 800 (7 d) Cr VI | NA | 2.19 | 105.87(24h); 51.41 (48h) | Sanyal et al. (2017) |
| Labeo bata (Cypriniformes: Cyprinidae) | 400 (7d) Cr VI | 24.53(24h); 17.13(48h) | |||
| Puntius sarana (Cypriniformes: Cyprinidae) | 100 (21 d) Cr VI | 59.31(24h); 29.44(48h) | |||
| Hyalella Azteca (Amphipoda: Hyalellidae) | 134.59 (4 weeks) Cr VI | 200 | NA | NA | Norwood et al. (2006) |
| Lecane papuana (Monogononta: Lecanidae) | 2,177 (24 h) Cr VI | 12,299.44 | 0.177 | 0.177 | This study |
| Mugil Cephalus (Mugiliformes: Mugilidae) | 700 (96 h) Cr VI | NA | 160 | 65.01 (96h) | Rajkumar and Tennyson (2013) |
| Zilchiopsis collastinensis (Decapoda: Trichodactylidae) | 200 (14 d) Cr VI | 766.9 | 2 and 5 | NA | Gagneten and Imhof (2009) |
The results of Bioconcentration of L. papuana, show that even at low concentrations Cr III is bioconcentrated in the organism (Figure 2). However, adverse effects are detected at a slightly higher concentration (see LOEC value in Figure 1). Regarding Cr VI, bioconcentration starts at LC50 value, but chronic effects are observed at very low concentrations at ½ of the LC50 value (see figures 1 and 2). However, we have to consider that acute tests only last 24h, while chronic tests last 5 days.
Due to the scarcity of data on Cr VI accumulation in invertebrates, we decided to compare our data with freshwater invertebrates and freshwater/coastal fishes (Table 4). In the fish Puntius sarana (Hamilton, 1822) exposed to 2.19 mg/L (12-fold higher than the exposure concentration for L. papuana) with supplemental food; the concentration of Cr VI found in the fish indicates that L. papuana bioconcentrated 21-fold more Cr VI than the fish. Bioacummulation of Cr VI in the fish Catla catla (Hamilton, 1822) (exposed to 2.19 mg/L) was 2.7-fold lower than L. papuana (Table 4). The fish Mugil cephalus (Linnaeus, 1758), bioaccumulated at least 3-fold less Cr VI with an exposure concentration at least 56-fold higher (160 mg/L) than L. papuana. Therefore, we can infer that the rotifer L. papuana bioconcentrates more Cr VI than these freshwater fishes. These differences can be explained by the presence of more sophisticated mechanisms to excrete Cr VI in fishes and the fact that the metabolism of small organisms is more accelerated with respect to larger organisms (Gutierrez et al., 2010).
Hyalella azteca (Smith, 1874), bioaccumulated 16-fold lower concentration of Cr VI than what we found in L. papuana (Table 4), but this study was carried out in a period of 4 weeks of exposure (ours was 24 h) and the exposure concentration was 0.176 mg/L, almost the same concentration that we used (0.177 mg/L). The bioconcentration of chomium in Daphnia magna and Argyrodiaptomus falcifer (Daday, 1905) was approximately 20 and 10-fold lower (respectively) than that found in L. papuana (Table 4). In addition, the concentration to which L. papuana was exposed (0.177mg/L) was about the 50% of that used with those two species (0.350 mg/L); however, our study was carried out in 24 h and those mentioned above in two days. The crab Zilchiopsis collastinensis (Pretzmann, 1968), showed a bioconcentration 10-fold lower than the one found in L. papuana, which were exposed 14 and 5 days respectively (Table 4). Moreover, the crab was exposed to a concentration 28-fold times higher (5 mg/L) than what was used in L. papuana. Thus, comparison of bioconcentration and adverse effects of Cr among aquatic species suggests that some species had a strong ability to resist the Cr VI exposure and/or to remove it from the body, involving Glutathione S-transferases and metallothionein proteins in the detoxification process; antioxidant enzyme systems have been reported in cases of recovery from Cr VI damage (Yuan et al., 2017). Similarly, previous studies with organisms of the Lecanidae family mention that these rotifers have a thick lorica that acts as a barrier against metal ions, which can be deposited in the lorica of Lecane rotifers; thus, bioconcentrating more chromium than other species (Table 4). The ecological niche occupied by species might have played a significant role in the accumulation; Lecane as a surface feeder and planktivorous species, can accumulate chromium from water, mud, debris, and detritus in addition to macrophytes and algae (Joadder, 2014). Binding of chromium in the sediment soil, depends on oxidation status, which is altered by pH, and microbial processes (Sanyal et al., 2017). Lecane papuana due to its high sensitivity to Cr VI can be considered a biomonitor species; biomonitor species can indicate the presence of contaminants even when they are not detected in a specific environment (Gagneten & Imhof, 2009). The sensitivity of an organism depends on several toxicokinetic mechanism; bioaccumulation of a substance is the process that confers the organism with more resistance to adverse effects (Muggelberg et al., 2017). In the case of L. papuana it has been documented elevated BCF´s (which explain its tolerance) when compared with other species and contaminants (Rivera-Dávila et al., 2021).
The Maximum permissible levels of chromium in water of diverse uses in Mexico is at least 0.5 mg/L, and the values that we obtained for chronic LOEC and NOEC for Cr III were 0.163 and 0.0816 respectively, which are around 3 and 6 times lower than 0.5 mg/L in Mexican Regulations. The chronic LOEC for Cr VI was 0.0885 mg/L and the NOEC was 0.0443 mg/L, which are 5.6 and 11.3 times lower than the maximum concentration allowed in the Mexican Regulation. These findings mean that L. papuana could be harmed in chronic exposures to chromium, in spite of compliance with the current environmental legislation.
There are few studies of chromium carried out with aquatic invertebrates (including cladocerans and rotifers), and the differences in the ranges for acute and chronic values (such as LC50) is great. There is a need to analyze these values and the protocols employed by different researchers. A first step is to use actual values instead of nominal ones (or at least indicate the percentage of similarity between actual and nominal values). The use of acute tests were acute values are obtain in absence of food somehow reduces the differences in ranges. The differences among species exposed to chromium could be due that this metal can act in different ways, in different species. Chromium has a role in glucose, fat and protein metabolism, participating in the insulin action. It links directly to macromolecules; fragments the DNA chains and acts in the peroxidation of lipids, generating free radicals and modifying the routes of cell signals. All these processes can contribute to the toxicity and carcinogenicity of chromium compounds (Gagneten & Imhof, 2009). Moreover, depuration of accumulated chromium in aquatic organisms differ markedly among the different taxa and the process generally has a complex pattern of elimination, showing large differences among metals and invertebrate groups (Rainbow, 2007).
CONCLUSION
In this research, acute, chronic, and bioconcentration toxicity tests were evaluated with the rotifer L. papuana, which is a sensitive organism to effects of both chromium species. We evaluated the susceptibility of this organism at concentrations that are of environmental concern; we discussed the acute rotifer sensitivity comparing our results with those of other aquatic invertebrate species. In general, L. papuana is one of the most sensitive species for both Cr III and Cr VI. In general, both chromium species showed high BFCs values. Our results support the idea that L. papuana is a suitable organism for toxicity assessment in tropical and subtropical environments, in acute, chronic, and bioconcentration tests. Maximum allowable chromium concentration in Mexican Regulations are higher than LC50 values obtained for this rotifer; and mostly above international values for protection of aquatic biota.
Acute and chronic effects on L. papuana, including acute and lethal body burdens and bioconcentrations factors aid answering questions on the fate, mechanisms of toxicity, and potential environmental effects of chromium in organisms.











 nueva página del texto (beta)
nueva página del texto (beta)



