Background
Delta-9-tetrahydrocannabinol (THC) was isolated for the first time in 1964 by a group led by Raphael Mechoulam ( Gaoni and Mechoulam, 1971 ). Studies on animals quickly suggested that this component was responsible for the psychotropic effects of marijuana. As a result, many studies sought to find the part of the brain affected by THC. Marijuana receptors were identified in 1988 ( Devane, Dysarz, Johnson, Melvin & Howlett, 1988 ) and, in 1992, the first endocannabinoid was described, known as anandamid (ananda means “inner blessing” in Sanskrit) or arachidonyl ethanolamide (AEA) ( Devane et al., 1992 ). It is currently accepted that the endocannabinoid system (eCBs) is widely distributed throughout the brain and participates in the modulation of learning and memory, the affective state, energy balance, sleep and the development of drug addiction.
This review seeks to describe the function of eCBs in behavioral processes and the structures that regulate it, such as the hippocampus, dorsal striatum, ventral striatum, amygdala, and prefrontal cortex (PFC), and the possible clinical implications of this.
Method
Literature of interest published in the last 17 years was consulted. Publications from 2000 to 2017 from PubMed/Medline, Scopus were included using the following terms: cannabis, THC, endocannabinoids, cannabinoid receptors, CB1, cannabis mental health, cannabinoid genes, cannabinoids and learning and memory, cannabinoids and mental health. Fourteen articles published between 1971 and 1999 describing for the first time the cannabinoid system were also included. In order to integrate the most useful information for this topic, the articles were selected through an evaluation and consensus among all the authors of this revision. Once all the sources had been included they were organized by topic.
Results
Genes that codify the cannabinoid receptors
In 1988, Howlett’s group ( Devane et al., 1988 ) suggested the existence of a specific receptor for cannabinoids known as “cannabinoid receptor 1” or CB1, which is mainly distributed throughout the central nervous system ( Burns et al., 2007 ; Egertová, Giang, Cravatt & Elphick, 1998 ; van Laere et al., 2008 ). CB1 was cloned by Matsuda, Lolait, Brownstein, Young & Bonner (1990) . The human gene that codifies it, CNR1, is in the chromosome 6q14-q15. Munro, Thomas and Abu-Shaar (1993) cloned a second receptor for cannabinoid, calling it CB2. CB2 is primarily distributed throughout the immunological and peripheral nervous systems, and to a lesser degree the central nervous system ( Xi et al., 2011 ). CB2 is codified by gene CNR2, which is located in the chromosome 1p36. The cannabinoids also interact with the transient receptor potential, V1 (TRPV1), which participates in the perception of pain and temperature. This receptor is codified by the gene TRPV1, located on the chromosome 17p13.3. They also affect the receptor attached to proteins G 55 (GPR55), which is codified by the gene HGNC, situated on chromosome 2 ( Palkovits et al., 2008 ).
Most neurons express the cannabinoid receptor
The technique of immunohistochemistry has been used to demonstrate that CB1 has broad distribution throughout the CNS ( Pettit, Harrison, Olson, Spencer & Cabral, 1998 ; Tsou, Brown, Sañudo-Peña, Mackie & Walker, 1998 ) (Figure 1). This has also been shown by Positron Emissions Tomography (PET) on humans ( Burns et al., 2007 ; Ceccarini et al., 2015 ). Among the locations with the most expressions of CB1 are the hippocampus, base nucleus, nucleus accumbens (NAcc), cerebral cortex, and amygdala.
Endocannabinoids modulate neurotransmission
CB1 is a metabotropic receptor of seven transmembrane domains attached to a Gi/o protein. The activation of CB1 inhibits the enzyme adenylate cyclase, by reducing the production of AMPc ( Bisogno, Ligresti & Marzo, 2005 ). It also activates the kinase protein activated by mitogens (MAPK), an enzyme related to cell proliferation and apoptosis ( Bisogno et al., 2005 ; Piomelli, 2003 ). Moreover, it modulates the activity of the N and P/Q type calcium canals (inhibiting the entry of Ca++) and of the Kir and A type potassium canals (facilitating the currents flowing out of K+), resulting in a global state of inhibition of the cell ( Kendall & Yudowski, 2017 ) (Figure 2). Likewise, Floran’s group has suggested that CB1 can form a heterodimer with the D2 dopaminergic receptor, allowing the increase of AMPc, and consequently exciting the neuron ( Muñoz-Arenas et al., 2015 ).
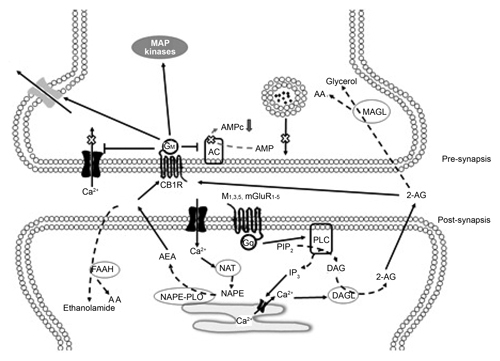
Figure 2. Diagram of a synapsis regulated by CB1. The literature suggests that endocannabinoids are released from the post-synaptic neuron in a form that is dependent on calcium and travel to the presynaptic region where CB1 is expressed (which can be the Glutamatergic or GABAergic presynapsis). The activation of CB1 in the presynapsis results in a decrease in the release of neurotransmitters, as the entry of calcium decreases and the outflow of potassium is facilitated.
The most consistent result observed when activating CB1 (with natural or synthetic agonists, inhibitors of the recapture or degradation of endocannabinoids) is a decrease in the release of neurotransmitters such as glutamate (Glut), gamma amino butyric acid (GABA), dopamine (DA) and acetylcholine (Ach). As a result of these effects, it has been suggested that the eCBs is a neurotransmission modulating system. One of the main characteristics of CB1 is that it is in the pre-synaptic regions ( Kendall & Yudowski, 2017 ). It is therefore possible to modulate small groups of neurons without altering the functioning of other adjacent neurons. This mechanism produces a delicate regulation of the neuronal processes, which are expressed in various nuances of behavior.
Inner blessing
In natural conditions, cannabinoid receptors are activated principally by endocannabinoids, a group of molecules of a lipidic nature. Some of the most frequently studied ones include AEA, oleamide (9-10-octadecenomide, ODA), 2-Arachidonoylglycerol (2-AG), Virodhamine (VIR), N-arachidonoyl dopamine (NADA) and Noladin-ether (N-E) ( Bisogno et al., 2005 ). The evidence collected to date indicates that the synthesis and release of endocannabinoids is an “on demand” process. That is, they are synthesized and released when required, and therefore not stored. This process is dependent on Ca++ and the site of release is the post-synaptic membrane ( Alger, 2002 ; Ohno-Shosaku, Maejima & Kano, 2001 ). This process has been studied for AEA and 2-AG, although there is as yet no evidence regarding ODA, VIR, NADA or NE. The AEA is formed in the lipidic membrane of the cells from N-arachidonoyl phosphatidylethanolamine (NAPE) ( Bisogno & Maccarrone, 2014 ). Bisogno & Macarrone (2014) suggest that the activation of NAPE occurs due to an increase in the intracellular concentration of calcium as a result of the activation of the cell by excitor neurotransmitters, for example, glutamate and the consequent neuronal activity. The formation of NAPEs occurs from arachidonic acid through the action of the catalyst enzyme N-acetyltransferase ( Bisogno & Maccarrone, 2014 ; Cadas, di Tomaso & Piomelli, 1997 ). Subsequently, owing to the action of a phospholipase D selective for NAPE, it is transformed into AEA. 2-AG is produced by the hydrolysis of diacylglycerol (DAG) thanks to the enzyme DAG-lipase. 2-AG can also be the product of phosphatidic acids (PA) in a reaction catalyzed by PA-phosphohydrolase. Regarding ODA, the synthesis procedure involves Cytochrome C, which uses ammonia as a source of nitrogen. Alternatively, it is synthesized by the enzyme peptidylglycine-alpha-monooxygenase (PAM), which uses oleolglycine as a source of oleic acid ( Howlett et al., 2004 ) (Table 1).
Endocannabinoids are synthesized as a result of the activation of the post-synaptic cell, particularly the activation of muscarinic receptors and metabotropic glutamatergic receptors. Once released, the endocannabinoids are a target of enzymatic mechanisms that maintain low levels of concentration. Fatty acid amidohydrolase (FAAH) is the most widely studied element in the process of cannabinergic inactivation ( Maccarrone & Finazzi-Agro, 2004 ). FAAH has been shown to be effective in hydrolyzing AEA and ODA, and there is evidence indicating that it also hydrolyzes 2-AG. FAAH is an integral protein of the membrane, and high levels of AEA have been observed in knockout mice of this enzyme ( Cravatt et al., 2004 ). Other processes of degradation have also been described for 2-AG, such as monoacylglycerol lipase (MAGL) ( Bisogno et al., 2005 ; Howlett et al., 2004 ).
There is currently discussion regarding the existence of a mechanism to recapture or transport endocannabinoids. It is believed to participate in the release and recapture of AEA (for a review, see Bisogno & Maccarrone, 2014 ; Howlett et al., 2004 ).
The cannabinergic system in the hippocampus
The hippocampus is one of the four brain structures with the highest number of cannabinoid receptors ( Pettit et al., 1998 ). It is perhaps the most extensively studied structure in terms of learning, declarative memory, and synaptic plasticity. In the hippocampus, CB1 is expressed in GABAergic ( Katona et al., 2000 ) and glutamatergic terminals. Their activation triggers a decrease in AMPc, a blocking of Ca++ canals and opening of K+ canals, and in general, inhibition of excitability ( Ohno-Shosaku et al., 2001 ).
The eCBs participates in short- and long-term plasticity. Regarding short-term plasticity, two processes have been observed, and depending on whether the synaptic terminal is glutamatergic or GABAergic, the inhibition of the excitation or the inhibition of the inhibition will occur, respectively ( Katona et al., 2000 , 2006 ).
The expression of long-term potentiation (LTP), a type of neuronal plasticity that has been proposed as part of the mechanisms of memory, is modulated by the eCBs. Carlson et al. (2002) demonstrated that the release of endocannabinoids by CA1 neurons during the suppression of inhibition induced by depolarization (DSI) facilitates the induction of PLP in a single cell. This could be an underlying mechanism of learning, such as the activation of “space fields” that occurs during spatial learning ( Carlson, Wang & Alger, 2002 ).
The release of endocannabinoids is facilitated by the brain-derived neurotrophic factor (BDNF) ( Khaspekov et al., 2004 ; Yeh, Selvam & Levine, 2017 ), whose final effect is to facilitate long-term depression (LTD) ( Heyser, Hampson & Deadwyler, 1993 ), which is a mechanism that not only facilitates memory processes, but also protects against neuronal death caused by excitotoxicity ( Katona et al., 2006 ). In the context of mental health, this is an important concept, since a patient with a major depressive disorder has a smaller BDNF in the hippocampus and smaller hippocampus itself. The selective inhibitors of the recapture of serotonin increase the synthesis and release of BDNF and restore, if only partially, the size of the hippocampus. It goes without saying that this improves depression in most patients.
Studies on the function of cannabinoids on cognitive processes have revealed that the administration of 9-THC, WIN-55,212-2 and CP-55,940 deteriorates working memory in rats ( Lichtman, Dimen & Martin 1995 ). Takahashi, Pamplona & Fernandes (2005) demonstrated that blocking CB1 through the inverse agonist SR141716A (rimonabant) facilitates the acquisition and consolidation of spatial memory. Meanwhile, the administration of WIN55, 212-2, a synthetic agonist for CB1, impairs the learning of the task of contextual conditioning to fear ( Arenos, Musty & Bucci, 2006 ; Pamplona, Prediger, Pandolfo & Takahashi, 2006 ). Varvel, Wise, Niyuhire, Cravatt & Lichtman (2007) demonstrated that an inhibitor of FAAH facilitates the extinction of spatial memory in Morris’s water maze test, an effect that is prevented by blocking CB1 with SR141716A. These results suggest that the anandamide facilitates the extinction of spatial memory through CB1. Likewise, it was shown that WIN55, 212-2 facilitates extinction in the water maze ( Pamplona & Takahashi, 2006 ).
These results support the important function of the eCBs in the modulation of mnemonic processes. Various experiments have shown that the activation of CB1 interferes with the formation of new memories when this activation takes place during the process of acquiring the task. The evidence suggests that it participates in facilitating extinction ( Pamplona et al., 2006 ).
The cannabinergic system in the striatum
CB1 has a high expression in the base nuclei, with the greatest concentrations in the black substance, the globus pallidus, and, importantly, in the striatum, though it has been observed that it is one of the regions with low concentrations of AEA ( Palkovits et al., 2008 ).
In the dorsolateral node, CB1 is expressed in glutamatergic and GABAergic neurons, in the pre-, post- and extra-synaptic regions. The activation of CB1 inhibits the release of GABA and glutamate, but not of DA ( Gerdeman & Lovinger, 2008 ).
It has been observed that the activation of CB1 in striatal neurons results in a decrease in their excitability ( Gerdeman & Lovinger, 2008 ). The highest amount of cannabinoid receptors is in the dorsolateral region. The main location for these receptors is in the terminals, suggesting that the action of endocannabinoids inhibits the release of the neurotransmitter.
Hernández-Tristán et al. ( Hernández-Tristán, Arévalo, Canals & Leret, 2000 ) report that activating CB1 with THC in rats increases dopamine levels in the amygdala and decreases noradrenaline levels in the hippocampus, but has no effect on the striatum. This study also observed an increase in the number of errors in the solution to the radial eight-arm maze. Most of studies reviewed indicate that the main effects of the eCBs in the striatum are through the glutamatergic and GABAergic systems.
In this group we have shown that blocking CB1 in the dorsolateral striatum of the rat induces perseverant behavior when performing a task in the T-maze. This perseverance prevents the subject from extinguishing the behavior ( Rueda-Orozco, Montes-Rodriguez, Soria-Gomez, Méndez-Díaz & Prospéro-García, 2008 ). Meanwhile, the N-E, through GPR55 in the striatum, facilitates procedure memory ( Marichal-Cancino, Fajardo-Valdéz, Ruiz-Contreras, Méndez-Díaz & Prospéro-García, 2016 ).
The cannabinergic system in the amygdala
In the amygdala it has been demonstrated that CB1 is principally in the GABAergic interneurons ( Katona et al., 2001 ). However, messenger ribonuclease acid (mRNA) has also been found in non-GABAergic cells, which suggests the participation of other neurotransmission systems, such as glutamatergic, widely represented in this structure.
Azad et al. (2003) have demonstrated that glutamatergic and GABAergic transmission in the basolateral nucleus of the amygdala is decreased by activating CB1. This decrease is caused by a pre-synaptic effect. These results are very similar to those found in other structures such as the hippocampus and the striatum.
Moreover, it has been observed that inactivating CB1, whether by pharmacological or genetic means, results in an extinction of aversive memories, but does not affect their consolidation ( Marsicano et al., 2002 ). This effect could be related to a rise in the excitability of GABAergic interneurons due to the absence of the cannabinergic system to inhibit them. An increase was also observed in the release of endocannabinoids during the extinction phase in the basolateral nucleus of the amygdala, which confirms the participation of the eCBs in this type of learning.
The cannabinergic system in the nucleus accumbens
Also known as the ventral striatum, the NAcc is conventionally related to motivational states and reinforcement. It is generally accepted that all abuse drugs increase the extracellular DA in the NAcc, and that this action contributes to its reinforcing properties. Cannabinoids also have effects on the activity of the NAcc ( Chen, Marmur, Pulles, Paredes & Gardner, 1993 ).
THC stimulates the release of DA in NAcc ( Chen et al., 1993 ), and, by activating D2 receptors, the latter stimulates the release of AEA ( Giuffrida et al., 1999 ). This effect can also be observed by antagonizing the D1 receptors. CB1 and D2 can form heterodimers that are functionally excitors ( Palkovits et al., 2008 ).
CB1 is extensively distributed throughout the terminals emanating from the excitatory innervation at the shell of NAcc, such as the amygdala, hippocampus and cerebral cortex. Studies have shown that cannabinoids, in common with other abuse drugs, inhibit the excitability of neurons at the shell of NAcc ( Soria-Gómez et al., 2007 ).
These data help us to understand how cannabinoids affect the reinforcement system and can explain the pharmacological effect of THC, and of all substances of use, which include addictive properties when interacting with other transmission systems.
The cannabinergic system in the prefrontal cortex (PFC)
It has been demonstrated that THC, administered systematically, increases the extracellular concentrations of Glut and DA in the PFC of rats, while GABA levels drop ( Pistis et al., 2002 ). On the other hand, Gessa et al. ( Gessa, Casu, Carta, & Mascia, 1998 ) report that the systematic administration of AEA and THC increases the release of ACh in rats’ PFC. However, it has been shown that this rise only occurs when the administration of cannabinergic agonists is systematic, not when it takes place in the PFC. This suggests that this effect is dependent on structures outside PFC.
Jentsch et al. (1997) demonstrated that, in rats, stimulating CB1 with THC impairs performance in a working memory task. This effect correlates with an increase in the production of DA in PFC ( Jentsch, Andrusiak, Tran, Bowers Jr. & Roth, 1997 ).
Although not much work has been done on the eCBs and the cerebral cortex, current evidence indicates that, as in other structure, the eCBs probably modulates the release of neurotransmitters and synaptic plasticity processes.
Endocannabinoids and mental health
“Endocannabinoids can act as a factor to recover from stress, as they relieve some of the responses induced by stressors and send messages such as: relax, sleep, forget, eat and protect oneself” ( Vinod & Hungund, 2005 ). Nonetheless, their malfunction can lead to abnormalities in behavior, intelligence ( Meier et al., 2012 ), and even the flow of thought, such as schizophrenia ( Ferdinand et al., 2005 ). This malfunction can be “artificial”, such as that displayed by genetic changes.
The World Health Organization (WHO) considers that marijuana has a negative influence on mental health, since it produces dependency, can induce psychosis, amotivational syndrome, and precipitate or exacerbate schizophrenia ( World Health Organization, 2016 ).
Despite the controversy, several studies show that the psychosis a marijuana user may display is due to an existing vulnerability to suffering from the illness and is not caused by the drug. There is increasing evidence that marijuana use triggers the first psychotic episode ( Ferdinand et al., 2005 ). These studies also show that individuals experiencing a psychotic episode induced by marijuana have first-degree relatives with schizophrenia.
All these studies can support the participation of the eCBs in the physiopathology of schizophrenia. It has been documented that there are at least nine alleles of CNR1. This classification is based on at least two polymorphisms: the polymorphism 1359G/A (one adenine is exchanged for a guanine) in the codon 453 in the codifying region of the CNR1. This is a synonym polymorphism (1359G/A that codifies for the amino acid threonine) and is therefore silent. The other polymorphism is the number of AAT repeats in the 3’ flank region ( Comings, 1998 ). Nine alleles have been described based on the number of repeats in a Japanese population ( Ujike et al., 2002 ). The alleles display 9, 10, 12-18 repeats. Of these alleles, the one with nine AAT repeats is associated with schizophrenia in that population. Other alleles have been associated with schizophrenia in a Spanish population ( Martínez-Gras et al., 2006 ). Nine alleles were also described, but with a different number of repeats. The shortest allele was seven repeats and the remainder were nine to 15 repeats. Alleles with 12 or 13 repeats are more frequent among schizophrenics, while that with 10 repeats is less frequent. Studies conducted in Mexico ( Ruiz-Contreras et al., 2011 ) have shown that there are eight polymorphisms in the Mexican sample studied. The most frequent alleles were 10 and 12-14 repeats. These alleles have not been linked to schizophrenia, but rather to efficiency in cognitive processes ( Ruiz-Contreras et al., 2011 ; Ruiz-Contreras et al., 2013 ). Moreover, post mortem studies carried out on patients with schizophrenia have demonstrated that they displayed a greater concentration of CB1 in the cortex of the left frontal cincture (64% above controls) ( Zavitsanou, Garrick & Huang, 2004 ). Berding et al. (2006) , using the Positron Emission Tomography technique (PET) and the radioligand 124I-AM281, showed that patients with schizophrenia contain a larger amount of CB1 in various regions of the brain, on the right-hand side, in the base nuclei, where the putamen and globus pallidus were the structures with the most CB1 ( Berding et al., 2006 ). The orbitofrontal cortex, the frontal cincture and the temporal cortex also displayed a sizable expression of CB1. There is a reduction of CB1 in the dorsolateral PFC ( Eggan, Hashimoto & Lewis, 2008 ). It has also been observed that AEA and palmitoylethanolamide (PEA) are higher in the cephalorachidean liquid of patients with schizophrenia, in comparison with that of normal subjects ( Leweke, Giuffrida, Wurster, Emrich & Piomelli, 1999 ) and that successful antipsychotic treatment reduces the seric levels of AEA ( Giuffrida et al., 2004 ). Moreover, CB2 and FAAH have been observed to have risen ( de Marchi et al., 2003 ). It is controversial that both the ligands and receptors are high, as normally when the ligand is high, the receptor is low (downward regulation) and vice versa. However, all these data suggest that schizophrenia coincides with a deregulation of the eCBs.
Discussion and conclusion
As we have been able to observe throughout this review, the eCBs is a growing field of research, with extensive therapeutic potential. Currently, the most consistent field as regards the CNS is modulation of synaptic plasticity processes. With a few differences, the effects that trigger the activation of CB1 in the various structures review are very similar and basically involve inhibiting the neuron that expresses them. Their effect is principally at a pre-synaptic level. This is a retrograde transmission system that is activated by excitability of the post-synapsis, where it is released and travels to the pre-synapsis, where it normally inhibits the release of the neurotransmitter.
The characteristics of the eCBs make it a system that can modulate communication, both within a structure and between several structures. It appears to act as a valve that is activated in response to excitation, to maintain or inhibit it. Its effects are resolved basically in a few microns and appear to be the mediator of an extremely precise dialogue between the cell that releases the endocannabinoid (post-synapsis) when it is overstimulated, and the neuron that releases the neurotransmitter and expresses CB1 (pre-synaptic). Through this apparently simple relationship, plastic processes are modulated that range from changes in neuronal function to symptoms displayed in mental illnesses such as schizophrenia and behavioral disorders such as drug addiction.
The cannabinergic system is currently the subject of numerous studies examining its therapeutic potential, including the treatment of psychiatric illnesses. Though the information is as yet incipient, we believe that research into this system will provide more answers regarding the relationship with mental and behavioral disorders than we have at present. This will enable the implementation of pharmacological strategies that will help control these gnoseologic entities.











 nueva página del texto (beta)
nueva página del texto (beta)




