Introduction
Diatoms are of great ecological significance for the functioning of aquatic and terrestrial ecosystems, and are useful environmental and ecological indicators (e.g. Smol & Stoermer, 2010). They are also key oxygenic photosynthesizers and prolific producers of extracellular polymeric substances (EPS) in most benthic habitats today (Krumbein et al., 2003; Pentecost, 2005). In fluvial environments, diatoms are also key primary producers and abundant on calcareous tufas (also called spring-associated limestones) worldwide (e.g. Stevenson et al., 1996; Gradzinski, 2010). Because diatoms sequester dissolved CO2 through photosynthetic C fixation, which results in a shift in pH, and because they produce large quantities of EPS that can agglutinate and accrete particles, they may play an important role in tufa formation.
The Iberian Range in Spain harbors a great variety of fluvial tufa systems that have been accumulating since the Pleistocene (Vázquez-Urbez et al., 2012). In particular, the Mesa River (MR) and the nearby Piedra River, have been of historical importance since ancient times (e.g. Corral-Lafuente, 2000). Despite this fact, the eukaryotic component of these ecosystems is poorly known, even though the microbial diversity of other tufas in the Iberian Range have been partially documented (Beraldi-Campesi et al., 2012).
In this paper, we aim to document the diatom genera present on substrates distributed along the Mesa River, and detect any possible relationship with environmental conditions, using sedimentary and hydrochemical variables that are known to influence tufa deposition. This preliminary report will increase our understanding of these geobiological systems and will allow comparisons with diatoms from other tufa systems and neighboring areas where diatoms have been used for monitoring environmental changes (e.g. Flor-Arnau et al., 2008).
Materials and methods
Environmental context of the Mesa River. The Mesa River (MR) is one of several rivers in the Iberian Range, NE Spain (Fig. 1) that display thick tufa deposits (Vazquez-Urbez et al., 2010, 2012; Auque et al., 2013). The MR is a tributary of the Jalon River that later enters the Ebro River near the city of Zaragoza (Fig. 1). It flows from southwest to northeast and cuts through Mesozoic and Tertiary rocks. Mesozoic (Lower Jurassic and Upper Cretaceous) carbonate units hold the aquifers that feed the river and are responsible for the calcium bicarbonate composition of the water. The regional climate is Mediterranean continental, with strong seasonal changes in temperature and precipitation. Mean annual precipitation varies from ~20 to ~55 mm and occurs mostly in spring (April-May) and autumn (September-October). Mean annual air temperature varies from ~5 to 25 ºC (4-5 ºC in December and January and 23-25 ºC in July). Mean discharge of the MR reaches 49 hm3/year with marked variability (~2 m3/s in May and ~1 m3/s in August; see Auque et al., 2013). Several natural springs occur along the MR, most notoriously near Mochales and Jaraba (Fig. 1). Water temperature at or close to resurgence points is rather constant through the year, about 13-14 °C in the river at site 1 (Mochales) and between 20-32 °C in the low-thermal springs near Jaraba (Pinuaga et al., 2004; Sanchez-Navarro et al., 2004). Mean underground water discharge in Jaraba is also constant through the year (570 to 647 l/s; Pinuaga et al., 2004). During dry seasons, the river discharge depends mainly on underground in puts (Auque et al., 2013). All these climatic and physicochemical variables drive the overall process of calcite precipitation.
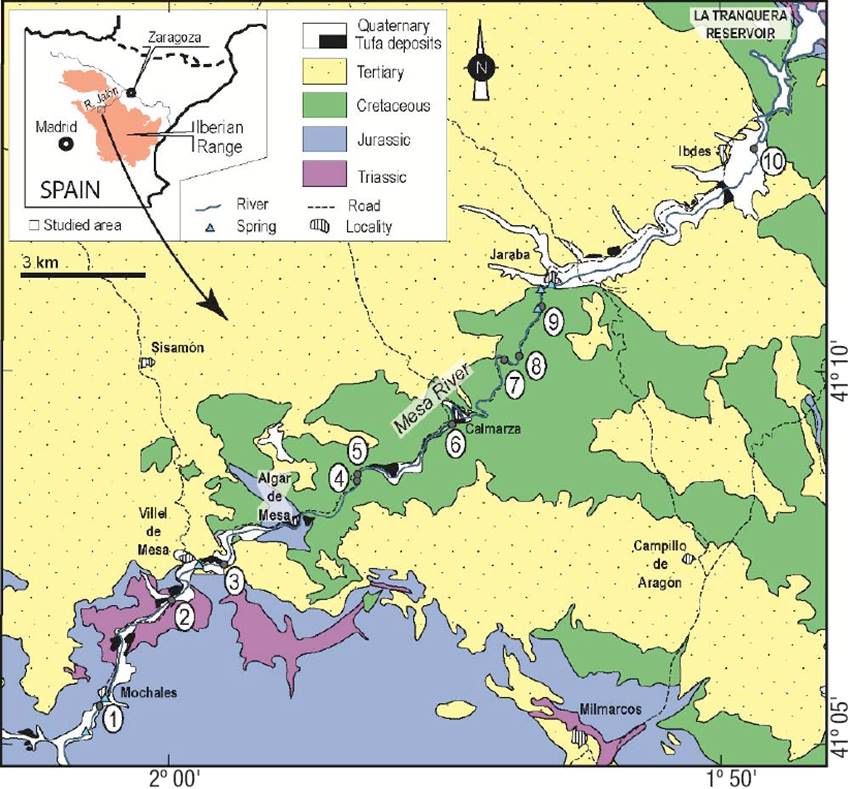
Figure 1: Geographic distribution of sampling sites (numbered dots) and geological context of the Mesa River. The river flows from SW to NE.
Facies characterization. Different depositional environments were characterized as sedimentary facies based on the type of substrate (in plan view and cross section), water depth, water flow, and type of flora (according to the main component on the surface: mosses, algae, cyanobacteria).
Diatom sampling and identification. Ten sites distributed along the MR (Fig. 1, Table 1) were selected according to bed configuration, sedimentary facies, and physical parameters (e.g. slope, depth, and water velocity; see below), representing the main sub-environments in the river. Sites ranged from empty grounds and gravel beds, to areas densely populated by plants, bryophytes, and macroscopic colonies of algae and cyanobacteria.
Table 1: Genera of benthic diatoms found along the Mesa River. Presence is indicated by ‘X’. Photos of each genus are shown in Figs 2-3. Facies: A = moss-dominated, porous tufa; B = dense, laminated tufa; C = tufa free gravel. See text for sedimentary facies details. Richness is expressed as the number of diatom genera found in the samples.
At each site, 3 to 5 pieces (~1-3 cm3) of the soft, recently-formed tufa surface were cored from limestone tablets previously placed at each site (see Vazquez-Urbez et al., 2010 for explanation), and combined into a single sample. Combined samples were immediately stored in a 20% ethanol-formalin solution for transport to the laboratory and further frozen at -10 °C until inspected under a microscope.
For microscopic observations of diatom frustules, tufa (CaCO3) fragments were dissolved in a 50% HCl solution, washed with distilled water in 50 ml vials, and centrifuged to obtain a pellet. Pellets were washed in distilled water and centrifuged many times before aliquots were taken for observation. This was done on a brightfield, phase-contrast, and dark-field microscope (Olympus BH2) equipped with an Olympus DPII digital camera. Abundance of different genera per sample was noted but not quantified, as frustule counts could be highly biased by this method without exhaustive sampling of larger areas. Observations per sample were concluded when no new morphotypes were discovered in the aliquots. All identifications were made upon comparisons with the literature (Hustedt, 1930; Smith, 1950; Bourrelly, 1968; Round et al., 1990). Taxonomic names were updated from the Algaebase database (Guiry & Guiry, 2015). Statistical analyses (Poisson regression, etc.) were processed for hydrochemical data using the R statistical software (R Core Team, 2014).
Results
Diatom genera distribution. A total of 25 diatom genera were detected in the 10 studied sites (Table 1; Figs 2-3). Most of them were pennate and only two were centric (Class Coscinodiscophyceae, Melosira Agardh and Biddulphia Gray; Table 1). Not all the identified genera were present at all sites. In general, the number of diatom genera increased from sites 1 through 4, oscillated from sites 5 to 8, and abruptly decreased at sites 9 and 10 (Table 1).
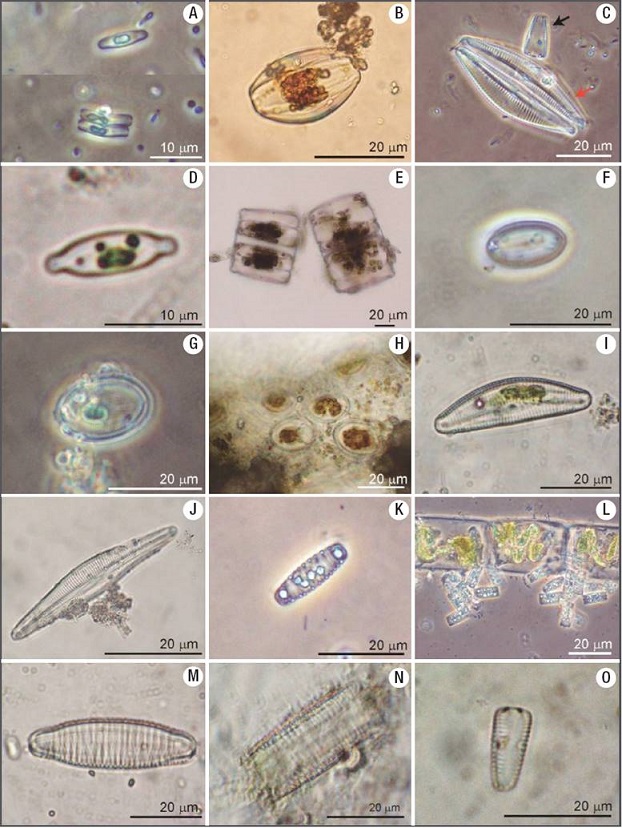
Figures 2A-O: Diatom genera found in the Mesa River. A) Achnanthidium. B) Amphora. C) Cymbella (red arrow) and Gomphonema (black arrow). D) Aneumastus or Cosmioneis. E) Biddulphia. F) Cavinula. G) Cocconeis. H) Cocconeis on algal filament. I-J) Cymbella. K) Denticula. L) colonies of Diatoma on algal filament. M) Diatoma. N) Diatomella. O) Gomphoneis.
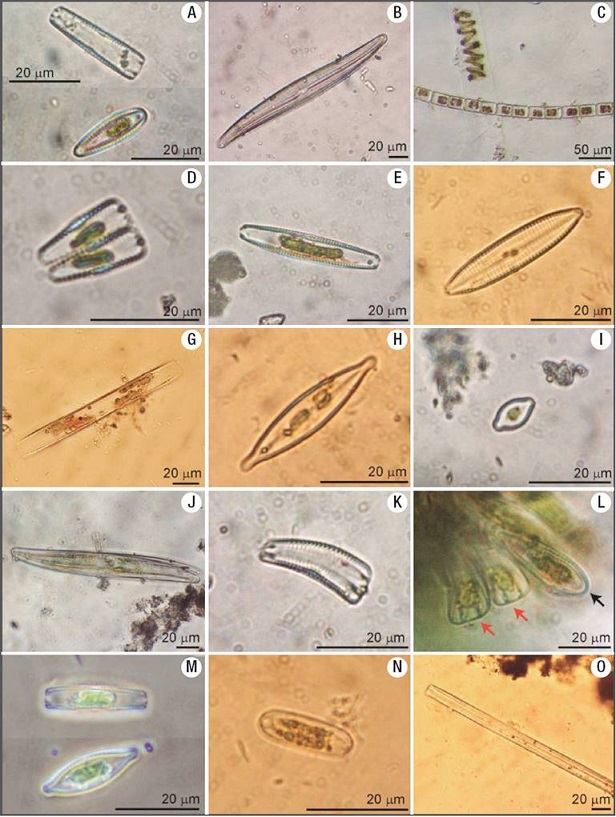
Figures 3A-O: Diatom genera found in the Mesa River. A) Gomphonema. B) Gyrosigma. C) Melosira next to an algal filament. D) Gomphonema. E-F) Navicula. G) Nitzschia. H) Navicula. I) Placoneis or Cosmioneis. J) Pleurosigma. K) Rhoicosphenia. L) periphytic Rhoicosphenia in perivalvar view (red arrow) and valvar view (black arrow). M) Stauroneis. N) Surirella. O) Synedra.
Among the pennate diatoms, 3 genera were araphid (class Fragilariophyceae) and 20 were raphid (class Bacillariophyceae; Fig. 4). Diatom genera are presented in Fig. 4 according to their frequency of appearance in the samples, from bottom (frequent) to top (rare); the most frequent genera were: Amphora Ehrenberg ex Kützing, Cocconeis Ehrenberg, and Navicula Bory de Saint-Vincent 1822 (Figs 2-3), which were detected in 7 sampling sites. Cymbella Agardh and Diatoma Bory de St-Vincent (Fig. 2) followed in frequency and were detected in 6 sites. Gyrosigma Hassall and Rhoicosphenia Grunow (Figs 2-3) were present in 5 sites. Genera present in 4 or less sites (Fig. 4; figle 1) were Achnanthidium Kützing, Gomphonema Ehrenberg, Stauroneis Ehrenberg, Denticula Kützing, Gomphoneis Cleve, Meridion Agardh, Nitzschia Hassall, Synedra Ehrenberg, Aneumastus Mann et Stickle, Biddulphia S. F. Gray, Cavinula Mann et Stickle, Cosmioneis Mann et Stickle, Diatomella Greville, Melosira C. Agardh, Pinnularia Ehrenberg, Placoneis Mereschkowsky, Pleurosigma Smith, and Surirella Turpin (Table 1; Figs 2- 3).
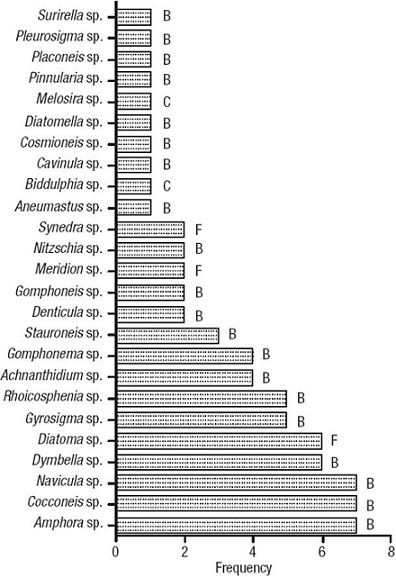
Figure 4: Frequency distribution of diatom genera from 10 sampling sites. B = Bacillariophyceae; F = Fragilariophyceae; C = Centric.
Although Cyanobacteria were visibly conspicuous on the tufa substrate, our samples rendered few specimens (Microcystis, Gloeocapsa, and Nostoc). Nevertheless, other cyanobacteria are known to exist at this river (Beraldi-Campesi et al., 2012). Algae within the Chlorophyta (Cladophora, Closterium, and Spirogyra), the Charophyta (Coleochaete), the Rhodophyta (Batrachospermum) and the Xanthophyta (Vaucheria), were commonly observed as part of the benthic microflora of the MR and found in our samples as well (some examples are shown in Figs 5 and 6). All these organisms were forming large, macroscopic colonies on the tufa, non-calcified submerged rocks, or on plant debris. Among these, Cladophora was ubiquitous, and its filaments were usually coated with thick layers of calcite (Figs 5K-N).

Figures 5A-U: Examples of benthic organisms co-existing with diatoms. A) Colony of the planktonic cyanobacterium Microcystis, although usually planktonic, these colonies become attached to the substrate at shallow exposures of the tufa. B) Close up of A showing the mucilage cover around the entire colony, which keeps it from disaggregating. C) Colony of the cyanobacterium Gloeocapsa showing stratified sheaths in a dense mucilaginous matrix. D-E) Arrangement of trichomes within a colony of Nostoc. F) Single filament within a Nostoc colony, around which abundant mucilage has been secreted. G) Ramified trichome of Cladophora. H) Filaments of Cladophora with and without growing bulbs. I) Zoom on a filament of Cladophora to appreciate cellular details and sparse calcite adhered to its sheath. J) Cladophora mingled with Vaucheria filaments. K) Filaments of Cladophora completely coated with calcite. L) Zoom on a Cladophora bulb without calcitic coat. M) A bulb of Cladophora in the process of being coated with calcite. N) Partially-coated Cladophora filaments. O-R) Unbranched, overlapped filaments of Spirogyra This alga was rarely found coated with calcite. S) Large cell of Closterium next to a Spirogyra filament. T) Basal cells of a Coleochaete colony. U) Terminal cells of a Coleochaete colony.
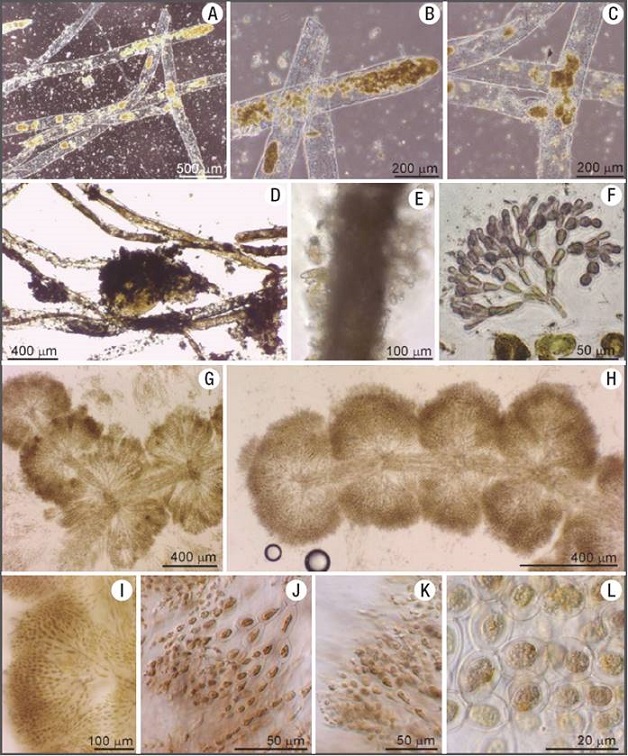
Figures 6A-L: Examples of benthic organisms co-existing with diatoms. A) Filaments of Vaucheria. B-C). Zoom on filaments of Vaucheria showing details of the cellu lar contents. Note almost no calcitic coatings around them. D) Filaments of Vaucheria in the process of being coated with calcite. E) Filament of Vaucheria completely coated with calcite and epiphytic diatoms (Gomphonema) on its surface. F) Fragment of a Batrachospermum terminal cells. G-H) Cellular arrangement of Batrachospermum showing a central stem and lateral branches. I-L) Cellular morphology and arrangement within a macroscopic branch (shown in G-H) of Batrachospermum.
From the collected samples and field observations we could see that diatoms were especially abundant on algae and on mosses, which covered most of the benthic surfaces together with cyanobacteria. For instance, colonies of Amphora, Cocconeis, Diatoma, Melosira, and Symploca were found growing preferentially where chlorophyceans (e.g. Coleochaete, Spirogyra) were most abundant. The most conspicuous diatom genus in our survey, Cocconeis, was also epiphytic on other diatoms, green algae, and cyanobacteria (Biddulphia, Cladophora, and Nostoc, respectively), on which calcification (micritic coatings) was visually pervasive. The stalk-forming Rhoicosphenia and Gomphonema were also conspicuous on filamentous algae and mosses, where also micritic particles accumulated around them. These biotic interactions were not exclusive, and mixtures could be seen at sites where chlorophyceans, rhodophyceans, and xanthophyceans (all carrying epiphytic diatoms) were growing together in large patches on the substrate.
Environmental context and sedimentary facies. The tufa deposits we studied displayed different sedimentary characteristics (e.g. variations in thickness, porosity, and presence/absence of lamination), depending on the local environmental conditions (water flow velocity, depth, slope, luminosity, shade, etc.) and the associated flora (e.g. algae, bryophytes, etc.). In sites with rapid calcification, these floras seemed to be quickly entombed within the tufa structure. Diatoms were notorious within calcified communities and seemed to be integral components of the tufa structure (Fig. 7). Three main sedimentary facies were identified along the MR (see below). Two facies (A and B, see Table 2) developed on small waterfalls and rapids with relatively shallow and fast-flowing water (~100 cm/s), and have been characterized for rapid tufa formation (~1.3 cm/yr; Vázquez-Urbez et al., 2010). A third facies (C), consisted mainly on gravel-dominated grounds, with poor or no carbonate deposition (Table 2). Facies A and B often occurred together in lit areas (e.g. sites 4, 5, 6, and 8), and were thicker than in shady ones (e.g. sites 2 and 7; See Fig. 1 and Table 1 for location of sites). The diatom genera distribution within each of these facies is reported in Table 1. Facies descriptions are as follows:
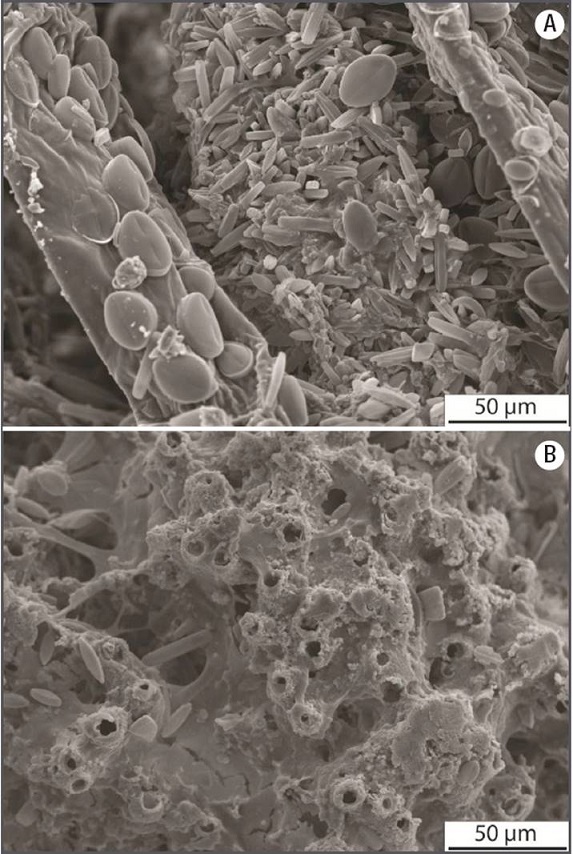
Figures 7A-B: Scanning Electron Microscope images of samples from facies A and B. A) facies A showing calcified filaments of green algae and mosses covered with diatoms. Note large Cocconeis Frustules on an algal filament (to the left). B) facies B showing calcite tubes (empty cyanobacterial sheaths) found within dense, laminated facies. Note the abundant exo-polysaccharides (EPS) filling interstitial spaces, where diatoms resembling Aneumastus or Cosmioneis are also present.
Facies A: This was the most widespread facies (Figs 8A-B) consisting of soft, very porous deposits, composed of completely or partially coated (calcified) filamentous cyanobacteria, and/or filamentous, siphonous, and parenchymatous algae (e.g. Spirogyra , Vaucheria , and Batrachospermum respectively), and bryophytes (Fig. 8B). Filamentous algae and mosses were visually more abundant than cyanobacteria in this facies. Both micrite and spar calcite crystals were present on these organisms, and pores and voids were sometimes filled with calcite, as in other examples of the same area (Arenas et al., 2000), indicating relatively quick diagenetic processes. A variety of benthic diatoms were observed as periphyton on bundles of filamentous algae in this facies (Fig. 7).
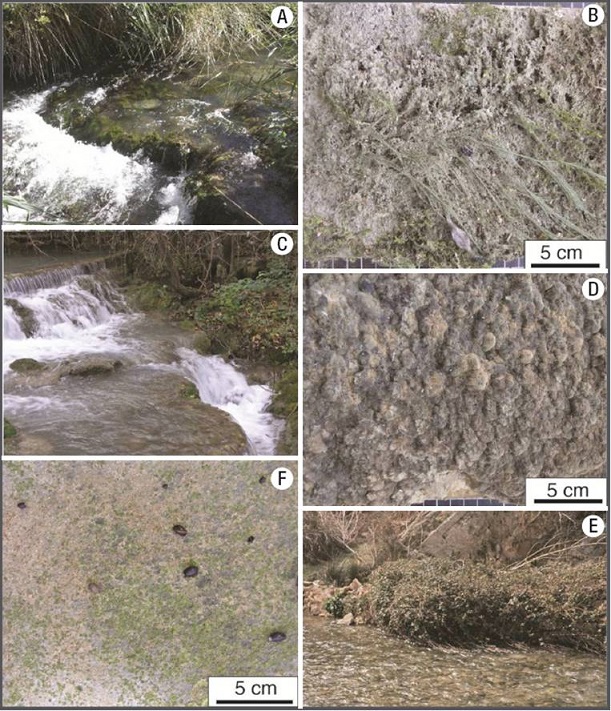
Figures 8A-F: Field view of the major sedimentary facies distributed along the Mesa River, and close ups of limestone tablets recovered at representative sites with those facies. A-B) facies A (moss- and algal-dominated, soft and highly porous sediment) at site 4. C-D) facies B (dense, laminated tufa) at site 2. E-F) facies C (gravel-dominated, poor tufa development) at site 9. Note snails of different sizes grazing on primary producers, which erode the tufa surface.
Facies B: This facies consisted of hard and dense, calcitic, laminated deposits (also called stromatolitic tufa; Figs. 8C-D). Cyanobacteria were more common than algae in this facies. Sub-millimeter to mm-thick laminae were composed of calcite tubes forming palisades and bunches subperpendicular to the surface. The tubes were hollow (inner diameter ~7 µm) and consisted of ~5-7 µm -thick micrite and spar calcite walls (shown in cross section in Fig. 7B), and were linked by calcite crystals and mucilaginous substance, on which diatoms were attached. The size and morphology of the tubes suggest that filamentous microbes acted as templates for the nucleation of calcite.
Facies C: This facies consisted of poorly calcite-coated substrates (Figs 8E-F) in less shallow water. The bedrock was dominated by gravel and cobble deposits on gently steep or quasi-horizontal beds, in medium to high-velocity flow conditions (60-100 cm/s). These sites were close to springs and thus received variable groundwater inputs. Thin patches of cyanobacteria-dominated communities, scarce filamentous algae, mollusks, and some insect nests were common biotic features of these facies. These components were almost devoid of calcite impregnations or coatings (e.g. sites 1, 3, 9, and 10).
Discussion
Diatoms and tufa formation. Pennate diatom genera were abundant and had a wide distribution within the MR samples, while centric diatoms were represented only by two genera. This has also been observed in other tufas and travertines of the world (Pentecost, 2005; Brinkmann et al., 2007; Arp et al., 2010; Gradzinski, 2010).
Most of the identified diatom genera have been reported from other freshwater streams of the world (Pentecost, 2005; Brinkmann et al., 2007; Arp et al., 2010). Among these, genera such as Amphora, Cocconeis, and Navicula had the widest distribution in the MR samples. In contrast, Aneumastus, Biddulphia, Cavinula, Cosmioneis, Diatomella, Melosira, Pinnularia, Placoneis, Pleurosigma, and Surirella were only present in one sample from the MR. Only 17 genera identified here (Achnanthidium, Amphora, Cocconeis, Cymbella, Denticula, Diatoma, Gomphoneis, Gomphonema, Gyrosigma, Melosira, Meridion, Navicula, Nitzschia, Pinnularia, Rhoicosphenia, Surirella, and Synedra), corresponded to 68 genera found in the Ebro basin (Flor-Arnau et al., 2008), which is a neighboring drier basin to the north of the Iberian Range. Even though our study was limited for close comparisons by the taxonomic resolution, it seems that these tufa environments bear a unique ‘oasis’ of diatom genera within the region. Nevertheless, complementary molecular fingerprinting studies would help to refine the identity and distribution of diatoms in these areas.
In the MR, 80% of the pennate diatoms displayed raphe, which is related to the motile benthic habit of the Bacillariophyceae (Round et al., 1990; Chafetz et al., 1991; Arp et al., 2010). A motile capability would perhaps favor pennate raphids over the non-motile pennate araphids in places where continuous and fast carbonate precipitation occurs (~5 mm/yr on average and up to 13 mm/yr in some places; see Vázquez-Urbez et al., 2010). It is likely that the fast carbonate deposition is in part responsible for changes in growth speed, coverage, and distribution of benthic microorganisms, including diatoms. It is reasonable to think that eukaryotic and prokaryotic communities at the surface of these (visually abundant) tufas, are very dynamic, in part due to the environmental pressure of permanent calcification and entombment. Another ecological alternative observed in non-calcareous, siliciclastic environments is that the distribution of motile versus non-motile diatoms may be due to their growth habits, in which non-motile diatoms tend to form bulk colonial aggregates, while motile diatoms are more widely dispersed (Hudon & Legendre, 1987).
Diatoms can be seen in fresh fractures of recently-formed tufa (upper 10 cm of actively-growing tufa) and likely play a role in the mediation of these sedimentary structures by enhancing carbonate precipitation and influencing the morphological development of the structure. Tufa formation can go on abiotically through CO2 outgassing (Merz-Preiβ & Riding, 1999; Chen et al., 2004; Vazquez-Urbez et al., 2010), but some organisms (bacteria, algae, fungi, bryophytes, plants) can mediate tufa development by a) trapping and binding particles with accretionary movement in and on a sticky, EPS-rich surface; b) serving as nucleation sites where calcite crystals accommodate according to the pre-existing 3D arrangement of the colonies, which develops particular structures and microfabrics; c) by removing dissolved CO2 during photosynthesis; and d) by altering the local equilibrium in favor of carbonate precipitation (Rogerson et al., 2008; Pedley et al., 2009; Dittrich & Sibler, 2010; Shiraishi et al., 2008, 2010).
SEM and light microscopy observations, however, have shown that diatoms of the MR are not necessarily coated with calcite, despite being attached to algae or solid substrates where micrite and calcite platelets are part of the bulk mass (Figs 6E, 7). Although diatoms (e.g. Cocconeis-like morphologies; Fig. 7) can be seen embedded in the already-formed tufa, they seem to avoid calcite precipitation directly on their extracellular surroundings. This phenomenon has been observed in other similar substrates as well (Merz-Preiβ & Riding, 1999; Arp et al., 2001; Gradzinski, 2010). This is perhaps because of the composition of their EPS, but could also be derived from a constant EPS replacement given their motile nature, in contrast with cyanobacteria and green algae that calcify in situ given their sessile nature. In this regard, it should be further determined if combined factors, including seasonality, also influence the way in which calcite precipitates around microbial colonies. For instance, poor precipitation on diatom biofilms during the winter (e.g. Arp et al., 2001) may reverse during the summer when there is a much higher rate of calcite precipitation (Vázquez-Urbez et al., 2010). Carbonate precipitation may also change due to variations in community composition, which in turn influence the amount and type of organic substrates on which CaCO3 can bind and start calcite nucleation (e.g. Lebron & Suarez, 1996). Although determining the particular role of the MR diatoms in the processes of tufa formation requires additional studies, the biotic components in the river certainly contribute to developing particular structures and textures, and determine much of the volume and porosity of the MR and other tufa deposits of the world (Rogerson et al., 2008; Pedley, 2009; Pedley et al., 2009; Gradzinski, 2010; Shiraishi et al., 2010; Vázquez- Urbez et al., 2010). Usually, the organisms that produce more biomass exert a major influence on the inner texture and fabric developed in tufas. This is evident in soft, moss-rich, porous tufa that can grow > 10 mm/year in these rivers, compared to 5 mm/year or less in microbial, biofilm-dominated tufas (Vázquez-Urbez et al., 2010).
Interestingly, this effect of the biology on the inner texture of the tufas also occurs in other chemical sedimentary deposits where microbes are present (e.g. travertines, stromatolites, thrombolites, and silica sinters; see Cady & Farmer, 1996; Jones & Renaut, 1996; Jones et al., 1998; Riding, 2000; Konhauser et al., 2001; Pentecost, 2005; Jones et al., 2007, 2008). In the MR, the presence of diatoms frustules within the tufa structure (and within most chemical sedimentary deposits), likely influences the diagenetic processes of lithification and remineralization early after lithification (which is quite fast in chemical deposits). Other means of influence may involve the amount of organic matter they contribute to the system, a part of which is expected to be entombed within the tufa structure (Fig. 7). It remains unclear, however, how this organic matter and silica frustules affect the diagenetic processes of the tufa over time. Nevertheless, fluids and minerals likely evolve through time and alter the primary structure of the tufa, as well as the amount and composition of organic matter and metabolic byproducts, along with the recycling of the opaline silica frustules trapped within the sedimentary structure during intermediate stages of tufa formation (e.g. Kastner et al., 1977; Hein et al., 1978; Barker et al., 1991; Michalopoulos et al., 2000).
Sedimentary and hydrochemical variables. Data from previous studies on the hydrochemistry of the MR (Auque et al., 2013) were used for correlation with the presence of diatom genera using an Anova Poisson regression (Fig. 9). Some of the physicochemical variables (alkalinity [HCO3 -], K+, Ca2+, pCO2, and TDIC) varied negatively and significantly with respect to diatom genera richness, while pH and CaCO3 were also significant but varied positively (Fig. 9). In sites with low (< 5) number of genera present (sites 1, 9, 10), these variables attained high values, whereas in sites with a higher number of genera (> 5) their values were low, especially sites 6 and 8, which had the highest richness of all.
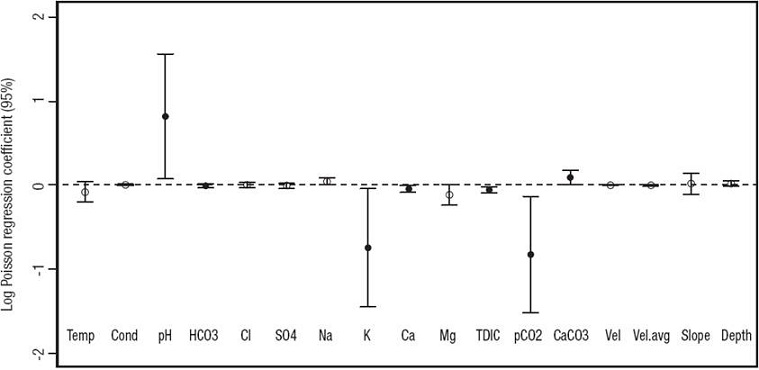
Figure 9: Poisson regression of physicochemical data (Auque et al., 2013) and diatom richness values. Only black dots indicate variables that correlated significantly with richness. Values for pH and CaCO3 correlated positively. Values for HCO3-, K+, Ca2+, TDIC, and pCO2 correlated negatively. The log regression was constrained to the 95% confidence interval.
The number of genera present in samples from the beginning and the end of the river was low, in sites where springs and human establishments (especially the largest, Jaraba and Ibdes) also occur (Fig. 1). Nevertheless, other sites close to (less populated) towns (e.g. sites 3 and 6) had a relatively high numbers of diatom genera, causing uncertainty as to whether human establishments had a direct influence on diatoms or not. Further considerations on the degree of human activity in these towns and its impact on the quality of nearby water, including outputs from agriculture and other activities, should be assessed before assuming a negative correlation between these two variables.
In the MR, deep-underground, somewhat thermal, and cool-water surface processes drive calcite precipitation at a large scale. Calculated saturation index (SIc) for calcite were consistent with abundant tufa formation with values above 0.77 (Auque et al., 2013) that seem sufficient to overcome the carbonate kinetic precipitation barrier (e.g. Jacobson & Usdowski, 1975; Dandurand et al., 1982; Suarez, 1983; Drysdale et al., 2002; Malusa et al., 2003; Lojen et al., 2004). The springs along the river (sites 1, 3 and 9) were the exception, as the SIc decreases below 3.20 (Auque et al., 2013) and almost no tufa formation took place at those sites (only facies C develops there). These sites were directly affected by nearby groundwater discharges (especially at the Jaraba thermal waters) at equilibrium or near equilibrium with respect to calcite. Mixing of groundwater inputs and surface water seemed to promote a clear decrease in the SIc values, as well as an increase in the pCO2 partial pressure, the HCO3 -, and TDIC contents. Therefore, the occurrence of several groundwater discharge points along the MR is likely a main factor controlling the tufa sedimentation rates, as groundwater inputs promote the increase in the partial pressure of CO2 and the decrease in the SIc values, precluding tufa formation near those groundwater discharge points. Downstream of these points, CO2 degassing increases the SIc values and, after a certain distance, saturation index values again reach the minimum threshold for tufa formation (see Auque et al., 2013 for further details).
Furthermore, the number of diatom genera decreased with higher concentrations of HCO3, K+, Ca2+, pCO2, and TDIC (Fig. 9), and increased with pH. In this respect, higher Ca2+ and HCO3 - concentrations in the water are assumed to derive from less CaCO3 precipitation for sites 1, 9, and 10, which coincidently record the lowest tufa deposition rates and the lowest numbers of diatom genera (Facies C). These sites were strongly influenced by groundwater inputs, which supplied dissolved CO2 to the flowing water, therefore inhibiting or lowering CaCO3 precipitation (Auque et al., 2013). The drastic decrease in numbers of diatom genera at site 9 and downstream may be influenced by the high underground discharge of the Jaraba springs (570 to 647 l/s). By contrast, sites with high numbers of diatom genera were away from spring discharges and had different sedimentary facies. For example, in sites with higher numbers of diatom genera (sites 4, 6, and 8), soft, porous tufa with abundant calcified filamentous algae and mosses has developed (facies A; Fig. 8 A-B). In contrast, sites 5 and 7 displayed both facies A and B, but developed in more shady areas (less photosynthetic activity), particularly site 7. It is possible that the dominance of diatoms in places with low CaCO3 precipitation, is also influenced by their biotic interactions, such as their epiphytic habit (higher richness where mosses and algae were more abundant), which are ultimately determined by the different physical and chemical conditions at each site. Despite the wide range of environmental conditions that a single species of diatom can tolerate (e.g. Fischer, 1979; Sánchez-Castillo 1993), the hydrochemistry along the MR has remained fairly constant throughout the last decade (Auque et al., 2013). Yet for some diatom species, a wide range of environmental conditions may not significantly affect changes in morphology (Stevenson et al., 1996). Therefore, assessments based on morphological traits to the level of genera should be taken with caution when using them as proxies for interpreting past or present environmental parameters, because discrete morphological changes may not be detected to the species level. Tufas exist since ancient times (Brasier, 2011) and thus are potential paleoenvironmental reservoirs of information about the physicochemical conditions at the time of deposition (Pedley & Rogerson, 2010), which may help us better understand the ecology of these environments through time. More studies on the diversity of the microflora living in these unique, freshwater sedimentary systems are needed. The use of biotechnological and bioinformatic tools are needed to explore such biodiversity. At least for diatoms, however, the recognition of the morphological expression of such biota should never be neglected.











 nueva página del texto (beta)
nueva página del texto (beta)



