Introduction
The Maritza/Evros/Meric river basin, which includes the Arda, Tunca, and Ergene tributaries, is one of the major river systems located in the eastern Balkans, with a total length of 550 km and total catchment area of 53000 km2. About 66% belongs to Bulgaria, 28% to Turkey, and 6% to Greece. The Meric is an international river that runs through an area of approximately 321 km in Bulgarian territory and flows into Aegean Sea. The Basin includes its main tributaries, i.e., the Arda and Tunca Rivers that mainly flow in Bulgaria and the Ergene River that flows entirely in Turkish territory. The basin provides suitable conditions for intensive irrigation and raising livestock (UNECE, 2009).
The Meric River forms the border between Greece and Turkey. Turkish authorities consider it to be an “international river” between Turkey and Greece and a “transboundary river” where it crosses the border between Bulgaria and Turkey. The water is used mostly for irrigation. It is also used at hydroelectric power plants. In addition, the Delta region provides good fishing for Turkey and Greece. The Delta is a very important stopover for birds that winter there. The Meric Delta is listed as a Class A International Wetlands (TCSV, 1989).
Many studies of the Bulgarian portion of river have been undertaken by Russev (1970), Russev et al. (1981), Detcheva (1981), Detcheva (1982), Russev and Janeva (1983), Kovachev (1985), Georgiev (2006), Stefanova et al. (2008), Rozdina et al. (2008), the oligochaeta studies by Dimitrov (1966), Uzunov (1976), Uzunov (1980), Uzunov (1981), Uzunov and Kovachev (1981), Uzunov et al. (1981), Uzunov and Kapustina (1993). Up to now, Özkan (1998) and Kalebaşı (1994) have studied the Turkish portion of the Meric River. There has been no study on macrobenthic fauna in the Turkish part of Meric River. To date, there have been no studies related to macrobenthic fauna and environmental parameters.
The objective of this study is to investigate the relationships between the physicochemical parameters and macrobenthic invertebrates in the Meric River. In addition, as part of the conclusions we offer a number of suggestions for the sustainable use of the river.
Materials and methods
The Meric River is the most important aquatic ecosystem in the Thrace Region of Turkey and the longest river in the Balkans. It originates in Bulgaria, delimits a small segment of border between Greece and Bulgaria, and then establishes the entire Greek-Turkish border. After crossing the Greek - Turkish border, one of its main tributaries, the Arda, flows into the Meric, and after crossing the Bulgarian-Turkish border, the Tunca, the other main tributary, joins the Meric near the Turkish city of Edirne. The river is between 150 and 300 meters wide (Emir, 1990). The transboundary basin of the Meric is shared by three European countries: Bulgaria, Greece, and Turkey. Bulgaria is upstream; Turkey and Greece are downstream (Fig. 1).
We conducted this study from January 2011 to December 2011 at eight stations. Samples were taken monthly. Some properties of selected stations on the river: Station 1: This station is located upstream from industrial facilities located in Kapıkule. The substrates are composed of mud. The river’s width at this station is about 160 meters. Station 2: This station is about 5 km away from the station 1. The substrates are composed of sandy-mud. The river’s width at this station is about 175 meters. Station 3: The Arda Stream joints the Meric. The substrates are composed of sand. The width at this station is about 180 meters. Station 4: At this station, the Tunca Stream joints the Meric. The substrates are composed of clay-detritus. The width at this station is about 200 meters. Station 5: Tatarköy village. There are many rice-growing areas around this location. This station is used for irrigation. The substrates are composed of stones. The river’s width at this station is about 220 meters. Station 6: Saclımüsellim village. This station is located 23 km from station 5. The substrates are composed of sand. The width at this station is about 200 meters. Station 7: Like station 5, this station has many rice fields. This station is located before the junction with the river Ergene. The substrates are composed of sand. The river’s width at this station is about 210 meters. Station 8: This station is located after the point of union with the river Ergene. The substrates are composed of clay and stone. The width of this station is about 250 meters.
At each site, water samples were taken periodically, at which time samples of benthic macroinvertebrates were taken. Benthic macroinvertebrate samples were taken at a depth of 1.5 meters.
Macrobenthic samples were taken from each station twice by using Ekman Birge grab (15 x 15 cm) and washed through on sieve series (1.19 mm, 0.595 mm, 0.297 mm mesh size). All collected samples were immediately fixed in 4% formaldehyde in the field and then transferred to 70% ethanol. In the laboratory, collected benthic macroinvertebrates were sorted and counted by using a stereomicroscope and then identified to the lowest possible taxon (species, genus, or families) by using a binocular microscope.
Identification of the Oligochaeta species was made by using keys in Brinkhurst and Jamieson (1971), Brinkhurst and Wetzel (1984), Kathman and Brinkhurst (1998), Milligian (1997), Timm (1999), and Wetzel et al. (2000). Chironomidae larvae were identified by using the keys in Seather (1980), Cranston (1982), Pinder and Reiss (1983).
At each station, the Meric River’s water temperature (using ordinary thermometer), electrical conductivity (using a conductivity meter), and pH (using a pH meter) were measured when benthic samples were taken. The latter were taken by a Ruttner sampler and carried to the laboratory in 2 L bottles with dissolved oxygen (using the classical Winkler method). BOD, SO42, PO42-, NO3-N, NO2-N, Cl, Mg, Ca, total hardness, salinity, H2S, and suspended solid material were analyzed by classical titrimetric and spectrophotometric methods. The water-quality level was determined according to official standards set by Turkey’s National Water Quality Standards for inland waters (SKKY, 2004). The Shannon-Wiener index was used to evaluate the species diversity of the river. The similarities between the stations and months were evaluated by the Bray-Curtis similarity index (Krebs, 1999). The relationships between Oligochaeta taxa and physicochemical parameters of the water were evaluated by using the Spearman Correlation index (Krebs, 1999).
Results
A total of 39 taxa consisting of an average of 851 individuals/m2 were collected at the Meric River stations during the study period. Samples were grouped as “Oligochaeta”, “Chironomidae” and “other benthic macroinvertebrates”. We found that 13 taxa belonging to Oligochaeta consist of 686 individuals/m2, 17 taxa belonging to Chironomidae consist of 145 individuals/m2, and 9 taxa belonging to other benthic macroinvertebrates consist of 20 individuals/m2. Brachiura sowerbyi (Beddard, 1982) that belongs to Oligochaeta and Pottashia alternis (Sahin, 1987) that belongs to Chironomidae were determined as new records for Turkish fauna. With regard to the rational distribution of these groups, Oligochaeta were the predominant group with 81%, followed by Chironomidae larvae with 17%, and other benthic macroinvertebrates 2% (Table 1).
Table 1 List of taxa and number of individuals (per square meter) of benthic macroinvertebrates that were recorded at stations along the Meric River (Turkish Thrace). Ave. = Average; Ab. = Abundance; Tot.Ab.= Total abundance.
| Station/Benthic macroinvertebrates | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Ave. | Ab. | Tot.Ab. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Olıgochaeta | |||||||||||
| Tubifex tubifex (Müller, 1774) | 0 | 9 | 11 | 13 | 402 | 4 | 20 | 15 | 59 | 8.60 | 6.93 |
| Limnodrilus hoffmeisteri (Claparède, 1862) | 819 | 124 | 176 | 691 | 976 | 346 | 257 | 413 | 475 | 69.24 | 55.81 |
| L. dekemianus (Claparède, 1862) | 44 | 2 | 2 | 54 | 0 | 0 | 0 | 18 | 15 | 2.19 | 1.76 |
| L. profundicola (Verrill, 1871) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0.14 | 0.11 |
| Potamothrix hammoniensis (Michaelsen, 1901) | 11 | 0 | 2 | 40 | 239 | 6 | 2 | 29 | 41 | 5.98 | 4.81 |
| Nais bretscheri (Michaelsen, 1899) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.14 | 0.11 |
| N. elinguis (Müller, 1773) | 0 | 0 | 15 | 179 | 0 | 24 | 200 | 0 | 52 | 7.58 | 6.11 |
| Aulophorus furcatus (Müller, 1774) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0.14 | 0.11 |
| Dero digitata (Müller, 1773) | 0 | 0 | 0 | 0 | 259 | 0 | 0 | 4 | 32 | 4.67 | 3.76 |
| D. obtusa (D’Ukem, 1855) | 0 | 0 | 0 | 0 | 26 | 0 | 0 | 0 | 3 | 0.44 | 0.35 |
| Ophidonais serpentina (Müller, 1773) | 0 | 0 | 0 | 0 | 39 | 0 | 0 | 0 | 4 | 0.60 | 0.47 |
| Lumbriculus sp. | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0.14 | 0.11 |
| Enchytraeidae | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0.14 | 0.11 |
| Total Oligochaeta | 878 | 135 | 206 | 981 | 1941 | 380 | 481 | 483 | 686 | 100 | 81 |
| Number of taxa | 5 | 3 | 5 | 7 | 6 | 4 | 5 | 6 | |||
| Chironomidae | |||||||||||
| Tanypus punctupennis (Meigen, 1818) | 0 | 0 | 2 | 2 | 50 | 3 | 0 | 0 | 7 | 4.82 | 0.82 |
| Pracladius (Holotanypus) sp. | 0 | 0 | 0 | 3 | 8 | 0 | 0 | 0 | 1 | 0.68 | 0.11 |
| Cricotopus (C.) bicinctus (Meigen, 1818) | 2 | 0 | 0 | 9 | 0 | 0 | 5 | 0 | 2 | 1.40 | 0.23 |
| Rheocricotopus fuscipes (Kieffer, 1909) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.68 | 0.11 |
| Chironomus anthracinus (Zetterstedt, 1860) | 2 | 0 | 11 | 11 | 48 | 7 | 4 | 0 | 10 | 6.90 | 1.17 |
| C. (C.) tentans (Fabricius, 1805) | 3 | 1 | 11 | 4 | 365 | 57 | 0 | 28 | 58 | 40 | 6.81 |
| C. plumosus (Linnaeus, 1758) | 2 | 0 | 0 | 5 | 28 | 6 | 0 | 0 | 5 | 3.45 | 0.58 |
| Stictochironomus sp. | 0 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 1 | 0.68 | 0.11 |
| Polypedilum aberrans (Chernovskij, 1949) | 26 | 0 | 27 | 153 | 116 | 16 | 18 | 0 | 44 | 30.34 | 5.17 |
| P. exsectum (Kieffer, 1915) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0.68 | 0.11 |
| Paratendipes albimanus (Meigen, 1818) | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 0.68 | 0.11 |
| Cryptochironomus defectus (Kieffer, 1913) | 12 | 2 | 8 | 18 | 0 | 0 | 2 | 0 | 5 | 3.45 | 0.60 |
| Einfeldia pagana (Meigen, 1838) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0.68 | 0.11 |
| Pottashia alternis (Sahin, 1987) | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 1 | 0.68 | 0.11 |
| Tanytarsus gregarius (Kieffer, 1909) | 3 | 0 | 0 | 24 | 5 | 0 | 2 | 2 | 4 | 2.8 | 0.47 |
| Virgotanytarsus arduensis (Kieffer, 1909) | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 2 | 1.40 | 0.23 |
| Rheotanytarsus sp. | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0.68 | 0.11 |
| Total Chiromidae | 52 | 3 | 59 | 255 | 620 | 91 | 44 | 33 | 145 | 100 | 17 |
| Number of taxa | 8 | 2 | 5 | 12 | 7 | 6 | 9 | 3 | |||
| Other benthic macroinvertebrates | |||||||||||
| Diptera | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 1 | 5 | 0.1 |
| Gastropoda | 0 | 0 | 0 | 0 | 33 | 0 | 0 | 7 | 5 | 25 | 0.5 |
| Bivalvia | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 1 | 5 | 0.1 |
| Ephemeroptera | 4 | 2 | 0 | 15 | 0 | 0 | 0 | 0 | 3 | 15 | 0.3 |
| Odonata | 18 | 2 | 2 | 19 | 6 | 0 | 2 | 4 | 6 | 30 | 0.7 |
| Coleoptera (larvae) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0.1 |
| Trichoptera | 5 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 1 | 5 | 0.1 |
| Hemiptera | 0 | 0 | 0 | 7 | 2 | 0 | 0 | 0 | 1 | 5 | 0.1 |
| Isopoda | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 5 | 0.1 |
| Total other benthic macroinvertebrates | 29 | 4 | 4 | 45 | 45 | 0 | 15 | 11 | 20 | 100 | 2 |
| Number of taxa | 4 | 2 | 2 | 5 | 5 | 0 | 2 | 2 | |||
The most abundant taxa on average was Limnodrilus hoffmeisteri (Claparéde, 1862), comprising 69.24%, while the least abundant taxa on average were Limnodrilus profundicola (Verrill, 1871), Nais bretscheri, (Michaelsen, 1899), Aulophorus furcatus (Müller, 1774), Lumbriculus sp., and Enchytraeidae 0.14%. In addition, L. hoffmeisteri is the most abundant species at station 5. We found that the abundance of Oligochaeta was very high at station 5 (total number of individuals was 1941 per m2), while station 2 had the least (total number of individuals was 135 per m2) (Table 1).
Oligochaeta were found to have the highest number in May. In February and October none were found. The number of taxa belonging to Oligochaeta was the highest in June (9 taxa) (Table 2). Oligochaetes were found to have the highest number of taxa at station 4 (7 taxa), while the least taxa were found at station 2 (3 taxa) (Table 1).
Chironomidae had 17 taxa and 17 % abundancy. Chironomus Camptochironomus tentans (Fabricius, 1805) was found to have the highest abundance (40%), while P. (Holotanypus) sp., Rheocricotopus fuscipes (Kieffer, 1909), Stictrochironomus sp., Pentapedilum exectum (Kieffer, 1915), Paratendipes albimanus (Meigen, 1818), Einfeldia pagana (Meigen, 1838), Potthastia alternis (Sahin, 1987), and Rheotanytarsus sp., were found to have the lowest abundance (0.68%). Chironomids were found to have the highest number of taxa at station 4 (12 taxa), followed by station 7 (9 taxa), and station 1 (8 taxa) respectively. C. tentans, Chironomus anthracinus (Zetterstedt, 1860) and Cryptochironomus defectus (Kieffer, 1913) were the most abundant and frequently recorded on sediments. Chironomidae was found to have the highest number in April. In January and February, none were found (Table 2). The number of taxa belonging to Chironomidae was the highest in May (11 taxa) (Table 2).
Other benthic macroinvertebrates had 9 taxa and 2% abundancy. Odonata belonging to this group was found to have the highest abundance (30%), while Diptera (Chironomidae larvae excepted), Bivalvia, Coleoptera larvae, Trichoptera, Hemiptera, and Isopoda were found to have the lowest abundance (5%). Other benthic macroinvertebrates were found to have the highest number at stations 4 and 5 (total number of individuals 45 per m2), following by station 1 (29 per m2), and station 7 (15 per m2). None were found at station 6 (Table 1). Other benthic macroinvertebrates were found to have the highest number in April (483 per m2), following by June (433 per m2), and July (308 per m2), respectively. In January and February, none were found. The number of taxa belonging to other benthic macroinvertebrates was highest in May (6 taxa) (Table 2).
Table 2 Monthly distribution of benthic macroinvertebrates and number of individuals (per square meter) in the Meric River (Turkish Thrace).
| Month/Benthic macroinvertebrates | Jan. | Feb. | Mar. | Apr. | May. | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oligochaeta | ||||||||||||
| Tubifex tubifex (Müller,1774) | 0 | 0 | 0 | 0 | 278 | 330 | 5 | 0 | 8 | 0 | 31 | 59 |
| Limnodrilus hoffmeisteri (Claparède,1862) | 0 | 0 | 97 | 481 | 2038 | 889 | 319 | 508 | 353 | 0 | 291 | 722 |
| L.udekemianus (Claparède,1862) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 86 | 5 | 0 | 0 | 22 |
| L. profundicola (Verrill,1871) | 0 | 0 | 0 | 0 | 69 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Potamothrixhammoniensis (Michaelsen,1901) | 0 | 0 | 11 | 155 | 295 | 11 | 15 | 4 | 5 | 0 | 0 | 0 |
| Nais bretscheri (Michaelsen,1899) | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| N. elinguis (Müller,1773) | 0 | 0 | 109 | 492 | 22 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulophorus furcatus (Müller, 1774) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Dero digitata (Müller, 1773) | 0 | 0 | 0 | 308 | 81 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| D. obtusa (D’Ukem,1855) | 0 | 0 | 0 | 0 | 0 | 39 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ophidonais serpentina (Müller, 1773) | 0 | 0 | 0 | 58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lumbriculus sp. | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Enchytraeidae | 5 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 5 | 0 | 217 | 1494 | 2783 | 1286 | 339 | 603 | 375 | 0 | 322 | 803 |
| Number of taxa | 1 | 0 | 3 | 5 | 6 | 9 | 3 | 4 | 5 | 0 | 2 | 3 |
| Chironomidae | ||||||||||||
| Tanypus punctupennis (Meigen, 1818) | 0 | 0 | 0 | 0 | 3 | 75 | 0 | 3 | 3 | 0 | 0 | 0 |
| Pracladius (Holotanypus) sp. | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 3 | 0 | 0 | 0 | 0 |
| Cricotopus (C.) bicinctus (Meigen, 1818) | 0 | 0 | 5 | 11 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Rheocricotopus fuscipes (Kieffer, 1909) | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Chironomus anthracinus (Zetterstedt, 1860) | 0 | 0 | 0 | 22 | 17 | 66 | 8 | 8 | 3 | 0 | 0 | 0 |
| C. (C.) tentans (Fabricius, 1805) | 0 | 0 | 0 | 308 | 230 | 94 | 30 | 8 | 8 | 0 | 13 | 8 |
| C. plumosus (Linnaeus,1758) | 0 | 0 | 0 | 30 | 19 | 0 | 3 | 8 | 0 | 0 | 0 | 0 |
| Stictochironomus sp. | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 7 | 0 | 0 | 0 | 0 |
| Polypedilum aberrans (Chernovskij,1949) | 0 | 0 | 0 | 94 | 14 | 129 | 200 | 30 | 69 | 3 | 0 | 0 |
| Pentapedilum exectum (Kieffer,1915) | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paratendipes albimanus (Meigen, 1818) | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cryptochironomus defectus (Kieffer, 1913) | 0 | 0 | 0 | 0 | 3 | 5 | 36 | 5 | 11 | 0 | 0 | 3 |
| Einfeldia pagana (Meigen, 1838) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
| Potthastia alternis (Sahin, 1987) | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tanytarsus gregarius (Kieffer, 1909) | 0 | 0 | 0 | 3 | 8 | 11 | 16 | 0 | 14 | 3 | 0 | 0 |
| Virgotanytarsus arduensis (Kieffer, 1909) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 0 | 0 | 0 |
| Rheotanypus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Total | 0 | 0 | 5 | 477 | 308 | 391 | 296 | 72 | 153 | 6 | 13 | 14 |
| Number of taxa | 0 | 0 | 1 | 8 | 11 | 7 | 7 | 8 | 9 | 2 | 1 | 3 |
| Other benthic invertebrates | ||||||||||||
| Diptera | 0 | 0 | 0 | 0 | 10 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastropoda | 0 | 0 | 0 | 0 | 28 | 25 | 0 | 5 | 0 | 0 | 0 | 0 |
| Bivalvia | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Ephemeroptera | 0 | 0 | 0 | 0 | 3 | 2 | 3 | 0 | 17 | 0 | 0 | 0 |
| Odonata | 0 | 0 | 0 | 3 | 3 | 7 | 6 | 3 | 36 | 7 | 6 | 5 |
| Coleoptera (larvae) | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichoptera (larvae) | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 5 | 0 | 3 | 0 | 5 |
| Hemiptera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
| Isopoda | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Total | 0 | 0 | 5 | 483 | 358 | 433 | 308 | 88 | 213 | 19 | 19 | 32 |
| Number of taxa | 0 | 0 | 1 | 2 | 6 | 5 | 3 | 3 | 3 | 2 | 1 | 3 |
According to the Shannon-Weiner index, the Meric River had H’ = 0.69 richness on average and station 4 was found to have the highest diversity H’ = 0.84, while station 2 had the lowest H’ = 0.47.
The results of Bray-Curtis index indicated that stations 6 and 8, stations 1 and 4, and stations 2 and 3 are the most similar to each other (82.50%, 80.25%, and 79.17% similarities, respectively). Stations 2 and 5, stations 5 and 7, stations 1 and 2 were determined to be the least similar (12.81%, 23.87%, 24.87% similarities, respectively). The Bray Curtis Similarity index indicated that September and July, July and November, September and November are the most similar to each other (92.15%, 89.56%, 85.79% similarities, respectively). February and April, February and June were determined to be the least similar (0.07%, 0.15% similarities, respectively) (Figs 2a-b).
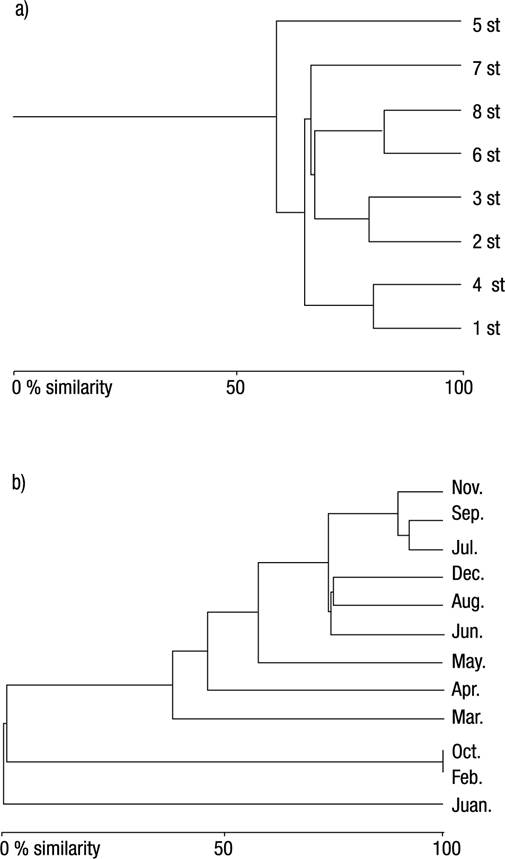
Figures 2a-b Bray Curtis Similarity Dendograms by sampling stations (a) and months (b) along the Meric River (Turkish Thrace) based on Oligochaeta species. st = station.
The average data on physicochemical parameters at each station are shown in Table 3. The lowest electrical conductivity was 214 μs/ cm (at station 8), while the highest was 1197 μs/cm (station 8). The average value measured was 502 μs/cm. The lowest pH measured was 7.50 (station 8), while the highest was 10.03 (station 1). The average value was 8.42. The lowest dissolved oxygen was 2.28 mg/L (station 2), while the highest was 8.94 (station 7). The average value of dissolved oxygen was 5.53 mg/L. The values of biological oxygen demand ranged between 6.75-78.7 mg/L. The average value of biological oxygen demand was 37.32 mg/L. The lowest sulphate registered was 0.641 mg/L (station 4), while the highest was 4.250 mg/L (station 8). The average value of sulphate was 1.841 mg/L. The lowest phosphate value was 0 mg/L (stations 3, 4, 6, and 7), while the highest was 0.273 mg/L (station 5). The average phosphate value was 0.068 mg/L. The nitrite values ranged between 0-0.641 mg/L. The average value of nitrite was 0.034 mg/L. The nitrate values ranged between 0-33.750 mg/L. The average nitrate value 5.846 mg/L. The lowest chloride was 3.99 mg/L (station 1), while the highest was 179.94 mg/L (station 8). The average chloride value was 34.99 mg/L. The lowest magnesium value was 0.96 mg/L (station 4), while the highest was 59.56 mg/L (station 6). The average magnesium value was 16.71 mg/L. The lowest calcium was 24.04 mg/L (station 5), while the highest was 90.58 mg/L (station 8). The average calcium value was 54.75 mg/L. The lowest value of total hardness was 3.2 FSº (station 4), while the highest was 41.2 (station 6). The average total hardness was 17.3 FSº. The lowest salinity was 0.02‰ (station 4), while the highest was 0.23 (station 8). The average value was 0.068‰. Hydrogen sulfide values ranged between 0-3.195 mg/L. The average value of hydrogen sulfide was 0.110 mg/L. Suspend solid material registered ranged between 70-1630 mg/L. The average value of suspend solid material was 345 mg/L (Table 3).
Table 3 The average physicochemical parameters of the Meric River (Turkish Thrace) during the study in 2011. W.T. = Water temperature; E.C. = Electrical Conductivity; D.O. = Dissolved oxygen; S.S.M. = Suspended Solid material; T.H. = Total hardness; BOD = Biological oxygen demand.
| Months/ Parameters | Jan. | Feb. | Mar. | Apr. | May. | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W. T. (ºC) | 4.31 | 5.25 | 9.31 | 13.8 | 20.3 | 25.5 | 28.3 | 26.3 | 23.4 | 12 | 6.3 | 7.6 |
| pH | 8.86 | 8.17 | 7.96 | 8.13 | 8.4 | 9.08 | 8.48 | 8.41 | 8.34 | 8.16 | 8.26 | 8.45 |
| E.C. (µS/cm) | 295.6 | 349.7 | 411.2 | 417.1 | 539.1 | 619.8 | 649.2 | 618.3 | 685.3 | 453.7 | 510.1 | 484 |
| D.O. (mg/L) | 4.73 | 5.31 | 5.09 | 3.01 | 4.92 | 5.16 | 4.99 | 7.27 | 6.99 | 6.28 | 6.82 | 5.87 |
| Salinity (‰) | 0.06 | 0.06 | 0.06 | 0.05 | 0.06 | 0.09 | 0.06 | 0.06 | 0.08 | 0.07 | 0.08 | 0.07 |
| Cl-(mg/L) | 27.4 | 27.8 | 33.4 | 23.4 | 30.9 | 35.2 | 42.3 | 30.8 | 53.9 | 32.3 | 41.1 | 40.9 |
| H2S (mg/L) | 0.000 | 0.000 | 0.026 | 0.346 | 0.266 | 0.399 | 0.000 | 0.213 | 0.079 | 0.000 | 0.000 | 0.000 |
| S.S.M. (mg/L) | 383.7 | 270 | 360 | 343.7 | 372.5 | 448.7 | 462.5 | 200 | 342.5 | 287.5 | 373.7 | 347.5 |
| Ca+2 (mg/L) | 44.28 | 43.37 | 47.19 | 55.5 | 56.7 | 58.01 | 41.87 | 57.51 | 63.82 | 58.21 | 71.63 | 59.71 |
| Mg+2 (mg/L) | 7.07 | 7.62 | 8.77 | 14.52 | 12.4 | 14.76 | 24.57 | 17 | 24.51 | 16.46 | 28.6 | 22.88 |
| T. H. (FSº) | 11.2 | 13.7 | 15.4 | 7.85 | 9.02 | 8.37 | 20.6 | 20.67 | 26.05 | 21.07 | 29.7 | 24.92 |
| NO2-N (mg/L) | 0.03 | 0.028 | 0.053 | 0.02 | 0.019 | 0.08 | 0.024 | 0.016 | 0.074 | 0.028 | 0.028 | 0.007 |
| NO3-N (mg/L) | 8.318 | 8.191 | 9.643 | 6.258 | 5.041 | 3.426 | 0.683 | 2.759 | 7.751 | 3.504 | 4.153 | 10.44 |
| PO4-3 (mg/L) | 0.041 | 0.041 | 0.075 | 0.053 | 0.061 | 0.044 | 0.02 | 0.064 | 0.085 | 0.052 | 0.069 | 0.227 |
| SO4-2(mg/L) | 1.044 | 1.218 | 1.567 | 1.037 | 2.07 | 1.908 | 2.383 | 1.798 | 2.242 | 2.045 | 2.873 | 1.952 |
| BOD (mg/L) | 15.83 | 19.47 | 21.2 | 11.46 | 21.66 | 40.41 | 40.55 | 62.85 | 61.77 | 53.96 | 54.25 | 44.65 |
Discussion
Oligochaeta was the dominant group of benthos in the river, while Chironomidae was the second dominant group of benthic fauna during the study period. Oligochaeta and Chironomidae larvae are usually abundant in benthos and play important roles in river ecosystems. The main reasons are that oligochaetes also feed on organic material in the water and are common in environments where large amounts of organic materials are present due to their ability to survive with low oxygen levels and other low-level conditions better than most other macrobenthos species (Yankson & Kendall, 2001). Like oligochaetes, chironomid larvae have also been used as indicators of organic pollution because they are often abundant in environments with low oxygen where organic material as a food resource is abundant (Coffman & Ferrington, 1996; Jenderedjian et al., 2007). The composition and distribution of Oligochaeta species depend on many factors such as water temperature, physical and chemical properties of the water, sediments, microfauna, and vegetation (Grigelis et al., 1981). Up to now, there has been no study of the Meric River regarding benthic fauna and their relationships with physicochemical parameters. For this reason, there are no other studies to compare with our work. No Oligochaetes were found in February and October. In February, rain and snow may have increased the water level in the river. Thus, sampling may not have been done exactly at the river bed. In October, snowfall caused the water level in the river to rise and sampling could not be done.
In Özkan’s study (1998), the average number of Chironomidae larvae was 481 per m2 for 65 taxa. In our study, we found that the average number of Chironomidae larvae was 145 per m2 for 17 taxa. If this study is compared with Özkan’s findings (1998), we see that both the individual and taxa number of Chironomidae larvae are declining. The main reasons for the decline of this species are habitat loss, pollution, meteorological factors, and changes in the structure of the river bottom.
Uzunov (1980) reported that there were 8 families for a total of 79 species along the Bulgarian segment of the Meric River. In the study of the Meric River by Uzunov et al. (1981), the following taxa were found: Oligochaeta (54), Gastropoda (7), Bivalvia (2), Isopoda (1), Ephemeroptera (45), Odonata (9), Heteroptera (10), Diptera (39). In Russev and Janeva’s study (1983), 46 Ephemeroptera species were found. In Uzunov and Kapustina’s study (1993), they found 54 Oligochaeta taxa.
According to SKKY (2004), the water temperature, pH, chloride, sulphate, phosphate, and nitrate values were found to be of first quality level. The value of dissolved oxygen was found to be at a second quality, whereas nitrite-N was found to be between second and third quality level. Biological oxygen demand was at fourth quality level. The relationships between the Oligochaeta species and the physicochemical parameters were evaluated by the Spearman correlation index. According to this index, the abundance of Limnodrilus hoffmeisteri (Claparède, 1862) showed positive correlations of water temperature (r = 0.627, p <0.05), electrical conductivity (r = 0.605, p <0.05), magnesium (r = 0.805, p <0.01), salinity (r = 0.717, p <0.01), chloride (r = 0.580, p <0.05), phosphate (r = 0.609, p <0.05), pH (r = 0.596, p <0.05). There was a negative correlation to nitrate (r = -0.600, p <0.05). The abundance of L. udekemianus showed positive correlations to hydrogen sulfide (r = 0.739, p <0.01), dissolved oxygen (r = 0.736, p <0.01), phosphate (r = 0.603, p <0.05), and biological oxygen demand (r = 0.709, p <0.01). The abundance of Nais elinguis (Müller, 1773) showed positive correlations to hydrogen sulfide (r = 1.000, p <0.01), and negative correlations to nitrate (r = -0.604, p <0.05). The abundance of Tubifex tubifex (Müller, 1774) showed positive correlations to calcium (r = 0.633, p <0.05), chloride (r = 0.640, p <0.05), and phosphate (r = 0.591, p <0.05). The abundance of Potamothrix hammoniensis (Michaelsen, 1901) showed positive correlations to water temperature (r = 0.697, p <0.05), and negative correlations to nitrate (r = -0.636, p <0.05).
Compared with previous studies of Kalebaşı (1994) and Ozkan (1998), the river water has become alkaline. Dissolved oxygen content had dropped.
Electrical conductivity is higher at station 8 than at the other stations. The Ergene River has been adversely affected by increasing population, industrial activities, heavy pesticides and fertilizer use in agriculture, and domestic wastes. Water hardness was found to be at an intermediate level. Since there are no previous studies of water hardness, we cannot make a comparison. Suspended solid material averaged 348 mg/L in the river. Suspended solids in a body of water are often due to natural causes. These natural solids include organic and inorganic materials such as silt and sediment. The majority of suspended sediment in water bodies comes from runoff and erosion. Some of the more common suspended solid pollutants are wastewater effluent, sewage, and airborne particulates.
Rivers are open, dynamic ecosystems whose physical, chemical, and biotic characteristics are greatly influenced by anthropogenic activities within their drainage basins (Moyaka et al., 2004).
The river, originating in Bulgaria, receives wastewater from industrial plants in the Kapıkule. At first, it merges with the Arda River, and later with the Tunca River. After passing through rice fields, it joins the Ergene River. The Ergene River has been adversely affected by increasing population, industrial activities, heavy pesticide and fertilizer use in agriculture, and domestic wastes. The Ergene River is the main source of pollution in the Meric Basin.
The river, an important irrigation source for the Thrace region, is a natural resource that must be used and preserved in a stable manner. As a result, both physicochemical properties and the results of tests on macrobenthic fauna indicate that the water quality of this river is organically polluted.
In recent years, due to population growth, rapid urbanization, and increasing discharge of solid wastes, water pollution problems are emerging.
Many industries still discharge untreated wastes and wastewaters into rivers. This should not be done if the goal is to assure sustainable use of the river water. Industrial wastewater contains pollutants. Only the necessary fertilizers and insecticides should be used at the appropriate dose. All the components that make up the river basin should be defined and managed together as a system. Because the Meric is both a trans-boundary and a border river, Turkey, Bulgaria, and Greece need to cooperate in order to develop and manage its waters. This would involve undertaking joint projects and studies. These and similar studies should be done regularly to monitor and determine how river water can be used sustainably to maintain its quality. The change of water quality in the river must be recorded.











 nueva página del texto (beta)
nueva página del texto (beta)




