Introduction
Mango plants (Mangifera indica L.) belong to the Anacardeiaceae family, a species of great economic importance, whose fruits are globally accepted for fresh consumption due to their characteristic smell, flavor, and nutritional aspects (Le et al., 2022 ). The main producing countries of this fruit are Mexico, Thailand, Brazil, Peru, and the Netherlands (FAOSTAT, 2020). In 2021, Mexico was the country with the highest mango exports with a production of 1'096,690.89 t (SIAP, 2021) which is mainly based on the cultivars of 'Tommy Atkins' (11, 314.99 t), manila (17, 702.31 t) and 'Ataulfo' (43,175.36 t); the 'Ataulfo' is distinguished by its excellent organoleptic characteristics. The main producing entities of the 'Ataulfo' mango are Chiapas (208,484.64 t) and Nayarit (110, 307.40 t) according to the report by SIAP (2021). Although various factors cause the loss of fruits in postharvest, it has been documented that the main cause is phytopathogenic fungi (Zhang et al., 2021). The fruits of tropical origin present problems during postharvest storage; estimating losses of 30 - 50 % due to fungi and bacteria (Danh et al., 2021; Choudhury et al., 2018). Most of the typical postharvest pathogenic fungi cause damage to the host tissue through enzymes or toxins that degrade the cell wall; subsequently, they absorb nutrients from the host's dead cells, causing tissue decomposition (Dukare et al., 2019). The main pathogenic fungi that occur in postharvest include Botrytis cinerea, Penicillium spp., Monilinia spp., Alternaria alternata, Rhizopus stolonifer, Trichothecium roseum, Fusarium spp., Colletotrichum spp. among others (Zhang et al., 2021). Colletotrichum spp. is the causal agent of anthracnose, a disease that attacks the 'Ataulfo' mango, affecting 60 % of the fruit production (Tovar-Pedraza et al., 2020).
The route of infection of Colletotrichum spp. is by direct penetration, remaining inactive and causing latent infections, interrupting the latency period when the fruit reaches the climacteric peak, which affects the quality parameters, decreasing its commercial value (Sivakumar et al., 2021). Anthracnose is controlled with chemical fungicides that are used indiscriminately during pre- and post-harvest, generating restrictions on its use due to its toxic effects, also inducing the development of resistance in the pathogen, which harms the environment and public health (Danh et al., 2021; Hu et al., 2014). Due to greater consumer awareness regarding chemical residues in food, the search for natural and environmentally friendly alternatives has been motivated; the use of edible coatings is one of the technologies that has emerged for the control of postharvest diseases as well as extending the shelf life of the products (Romanazzi & Moumni, 2022; Sapper & Chiralt, 2018). An edible coating is a thin continuous matrix that is applied to the food surface, creating a modified atmosphere in the fruit due to a barrier that occurs in gas exchange and water loss control, in addition to decreasing the respiration rate, enzymatic darkening and the release of volatile compounds into the environment, improving the appearance and maintaining the integrity of the fruit (Armghan et al., 2022; Oyom et al., 2022). Edible coatings are made of proteins, lipids, polysaccharides, and mixtures of these. Polysaccharides can be obtained from plants and marine species (crustaceans, red and brown algae), of which the industry most demands are cellulose, starches, alginates, polylan, chitosans, gums, and carrageenan (Kocira et al., 2021). Starch is the biopolymer that has drawn attention to the industry for its acceptable aesthetics, biodegradability, and thermoplastic properties; it contains considerable amounts of amylose, which positively affects its coating and edible film-forming properties, is inexpensive, and is abundant in nature. Starch-based coatings have organoleptic (odorless and tasteless), optical (transparent and colorless) properties, as well as a good gas exchange barrier (CO2 and O2 permeability) due to the arrangement of the hydrogen bond network (Singh et al., 2022; Oyom et al., 2022; Hassan et al., 2018). Oliveira et al. (2016) carried out an investigation (Short note) using four concentrations of cassava (Manihot esculenta) starch (1, 2, 3, and 4 %) as an edible coating to control anthracnose in papaya (Carica papaya); reporting that the coating made with 2 % cassava starch was the most effective in the control of anthracnose due to the lower cost, even considering that the four concentrations presented a 100 % control of anthracnose. Another study conducted by De Freitas et al. (2022) evaluated the effect of edible coatings based on cassava and corn (Zea mays) starch; and the combination of these at concentrations of 1, 2, 3, and 4 % for the control of anthracnose in avocado fruits (Persea americana Mill.). The fruits were covered by immersion and artificially inoculated with a conidial suspension of the pathogen that causes anthracnose. The researchers reported that the coating of cassava starch at 2 and 3 % combined with corn starch was efficient in restricting the increase in damaged areas in avocado fruits by anthracnose. Due to the above, the objective of the present investigation was to evaluate the microbiological activity of coatings based on starch extracted from stenospermocarpic banana 'Pera', soursop, and mango 'Ataulfo' fruit, applied to stenospermocarpic 'Ataulfo' mango fruits during postharvest storage.
Material and Methods
Plant material
The research was carried out in the microbiology analysis laboratory of the Food Technology Unit (UTA), of the Autonomous University of Nayarit (UAN) during the spring-summer cycle of 2021. Stenospermocarpic 'Ataulfo' mango fruits used in the present study were harvested at physiological maturity from the Atonalisco, municipality of Tepic, Nayarit, located at 234 masl with coordinates 21° 42' N, 104 ° 51' W and transported to the UTA of the UAN the same day of harvest. The fruits were washed with water and 2 % sodium hypochlorite (NaClO) for 10 min, rinsed with distilled water, and subsequently allowed to dry for 30 min at room temperature.
Isolation of the fungus causing anthracnose
The pathogens were isolated from 10 stenospermocarpic 'Ataulfo' mango fruits that were purchased at the Tepic, Nayarit food market at physiological maturity without visible mechanical damage or signs of disease. The fruits were washed with tap water and 2 % sodium hypochlorite (NaClO) for 10 min, rinsed with distilled water and subsequently allowed to dry for 30 min at room temperature and incubated at 28 °C and at a relative humidity (RH) greater than 90 %. Once the fruits developed signs of disease, 1 cm2 segments of affected and unaffected tissue (50 %) were taken. The segments were disinfected with 1 % NaClO by immersion (3 min) and washed three times with sterile water to remove NaClO residues; allowed to dry on sterile absorbent paper to remove the excess water. Subsequently, the tissue was placed in the center of Petri dishes with potato-dextrose agar (PDA Dibico MR) and then incubated for seven days at 28 °C, performing frequent reseeding to obtain and preserve the pure strains (Mulkay et al., 2010).
Inoculum preparation
A total of eight monosporic cultures were obtained. The initials CP with a subscript were assigned to each of the isolated strains, which were used as inoculum in the pathogenicity test. To each monosporic culture obtained from eight days of growth in PDA, 10 mL of sterile distilled water was added, to later be scraped and shaken until the disintegration of the colony in a shaker (Vortex-Genie II Mixer SI-0236, USA) at 600 rpm for 30 s. Once the suspension was obtained, it was decanted and filtered through sterile gauze into a sterile glass tube. A 10 µL aliquot of the spore suspension was placed per space in a Neubauer chamber.
The following equation was used to calculate the spore suspension at the desired concentration.
(…Eq. 1)
The concentration of the spore suspension was adjusted to 1x106 mL-1 spores when necessary (Oliveira et al., 2016). Likewise, in the in vivo microbiological evaluation, the same procedure was carried out to prepare the inoculum of the strain that presented the highest pathogenicity.
Pathogenicity test
The importance of the pathogenicity test or Koch's Postulates lies in the fact that the symptoms present in an artificially infected host should coincide with the symptoms (relevant morphological and biological traits) initially observed in a naturally infected host (Danh et al., 2021). In this bioassay, 36 stenospermocarpic 'Ataulfo' mango fruits with four replicates per pathogen were selected. The visually healthy fruits in a state of physiological maturity were washed and disinfected with a 2 % NaClO solution for two minutes and then rinsed and allowed to dry at room temperature. Two wounds were made to the fruit with a sterile punch of 0.4 mm in diameter. Subsequently, the fruits were inoculated with a conidial suspension of 1x106 spores mL-1 of each of the microorganisms previously isolated and 8 d after sowing in a PDA culture medium. Four fruits were inoculated for each organism and four fruits were used as control fruits. The fruits were placed at 28°C and an RH ≥ 90 %, adequate conditions for the fruiting of the pathogen. The pathogen that presented the greatest aggressiveness was selected, evaluating the damage qualitatively (observation) and quantitatively by daily measuring the diameter of the lesion using a Vernier (caliper) and documenting the development of the microorganism, as well as the severity of the symptom-based on the diameter of the lesion (Gañan et al., 2015). The area of damage caused by the pathogen was calculated using the following formula and was expressed in cm2:
(…Eq. 2)
Where A is the area of damage caused by the pathogen,
Morphological identification
For the morphological identification of the species with the highest pathogenicity, macroscopic and microscopic observations of the isolated culture were made. The pathogen was macroscopically identified by documenting the characteristics of the culture such as color, texture, and growth rate of the pathogen. For microscopic identification, structures such as conidia shape, formation of the appressorium and mycelium were observed, as well as any other structure that could identify the pathogen; for this, three microcultures were made by placing 20 µL of PDA medium on a slide that was inoculated with the pathogen once the culture medium had solidified. A gauze was placed at the bottom of the Petri dish adding 2 mL of sterile distilled water to create adequate conditions for the proliferation of the pathogen. The microcultures were incubated at 28 ˚C for 6 d; subsequently, the structures of the pathogen were observed in a Motic model BA310 microscope (Motic, British Columbia, Canada) with the 40X objective of the unrecognized fungus that was used for inoculation in the pathogenicity test and in vivo microbiological evaluation (Barrientos-Martínez et al., 2022).
In vivo microbiological evaluation
In the in vivo microbiological evaluation, 160 stenospermocarpic 'Ataulfo' mango fruits at physiological maturity were used, selecting the fruits by uniform size and homogeneous color, discarding those that presented physical or mechanical damage or some phytopathological affectation. Stenospermocarpic 'Ataulfo' mango fruits were washed and disinfested with 2 % NaClO, then rinsed and allowed to dry at room temperature. The visually healthy fruits underwent a 0.4 mm diameter wound in the epicarp with a sterile awl. The fruits were coated with 2 % (w/v) starch, extracted from banana 'Pera', soursop, and stenospermocarpic 'Ataulfo' mango. Once the fruits were covered, they were allowed to dry at room temperature for one hour. Next, the fruit were artificially inoculated. The wound site was inoculated with 10 µL of conidial suspension of the pathogen that presented the greatest severity of damage to the fruit at a concentration of 1x106 mL-1 conidia. Negative controls were treated with 10 µL of distilled water (Table 1). Once the fruits were inoculated, they were placed in a humid chamber at 28 ºC and a relative humidity > 80 % (Mulkay et al., 2010). The fruits were evaluated by observing and measuring the damage of the fruit in the cuticle and dark brown spots that appear due to physiological disorders produced by microorganisms. The evaluations were made according to the following scale: 0 = no visible damage; 1 = slightly visible (≤ 25 % of the cuticle); 2 = moderate (26-50 % of the cuticle); and 3 = severe (> 50 % of the cuticle) (Palou et al., 2007). The wound was measured in mm using a Vernier or caliper. The development of the pathogen and the appearance of signs of pathogenicity were observed and documented. The inhibition percentage was calculated using the following equation:
(…Eq. 3)
Where R1 is the radial growth of the pathogen in the absence of inhibition (positive control), R2 is the decreased radial growth of the pathogen compared to R1 which reflects the inhibition of the opposite colony; % I indicate radial colony growth that is inhibited in the presence of the opposite colony (Jacobs et al., 2003).
Statistical analysis
A multivariate analysis by the Bonferroni method with an α ≤ 0.005 was performed, using the Bartlett test for equal variances. The area damaged by the pathogen was considered a dependent variable in the analysis of variance (ANOVA). The STATA 11 software was used for the statistical analysis. The study consisted of a complete experimental design with a factorial arrangement (3x2), generating 6 treatments with two controls (positive and negative) with a total of 8 treatments. 20 fruits per treatment were used for this experiment; the treatments were distributed as shown in Table 1.
Treatments
Table 1 Treatments generated in this study.
| Treatment | Starch-based coating at 2 % | Inoculation of the pathogen CP3 |
|---|---|---|
| T1 | Banana ‘Pera’ | No |
| T2 | Banana ‘Pera’ | Si |
| T3 | Soursop | No |
| T4 | Soursop | Si |
| T5 | Stenospermocarpic Mango ‘Ataulfo’ | No |
| T6 | Stenospermocarpic Mango ‘Ataulfo’ | Si |
| T7 | Without coating (negative control) | No |
| T8 | Without coating (positive control) | Si |
Results and discussion
Isolation of the fungus causing anthracnose
The fungal strains that caused the greatest damage to stenospermocarpic 'Ataulfo' mango fruits were compared and selected according to the description of anthracnose disease lesions reported by Sivakumar et al. (2021). A total of eight monoconidial isolates were obtained, to which the nomenclature was assigned: CP2, CP3, CP4, CP5, CP6, CP7, CP8, and CP9.
Pathogenicity test
Anthracnose symptoms on a fruit appear as dark brown to black sunken lesions; likewise, when the fruit is exposed to high relative humidity, prominent pinkish-orange spores appear in the lesions (Sivakumar et al., 2021; Tovar-Pedraza et al., 2020).
Figure 1 shows the signs of disease that appeared from the fourth day on in fruits that were artificially inoculated, except CP3, being the pathogen that presented the greatest damage. The visual damage presented by the CP3 pathogen was moderate at 8 d after inoculation (26 - 50 % of the cuticle) according to Palou et al. (2007), presenting the signs of disease from the second day. The pathogenicity of the isolated fungus was confirmed, since the inoculated fruits presented typical symptoms of anthracnose disease (dark brown to black sunken lesions), similar to the pathogen that naturally infected the fruit, caused by the strain isolate assigned as CP3, fulfilling Koch's postulates. These results coincide with those presented by Camargo-Piñeres et al. (2021).
The results from the pathogenicity test are shown in Figures 2 and 3, strains CP2, CP4, CP5, CP7, and CP9 were located within group a, which behaved similarly to control fruits (CP1). In group b, the three strains with the highest pathogenicity were located: CP3, CP6, and CP8; finding a significant difference between CP6 and CP8 concerning CP3, CP3 showed the highest pathogenicity (CP8<CP6<CP3), which was re-isolated and preserved for later use.
The damages caused in the CP3, CP6, and CP8 strains are probably due to the climatic conditions to which the fruits were exposed once they were inoculated; same that favor the development and growth of the pathogen (25 - 28 °C and an RH > 90 %). In group c, CP6 and CP2 were located, which behaved similarly between them, presenting significant differences from the rest of the groups.
Figure 1 Pathogenicity test. CP nomenclature assigned to pathogenicity strains. Most pathogenic strains (CP+++), intermediate pathogenicity strains (CP++), and less pathogenic strains (CP+).
In Table 2, the Correlation between each of the phytopathogen microorganisms that damage the stenospermocarpic ‘Ataulfo’ mango.
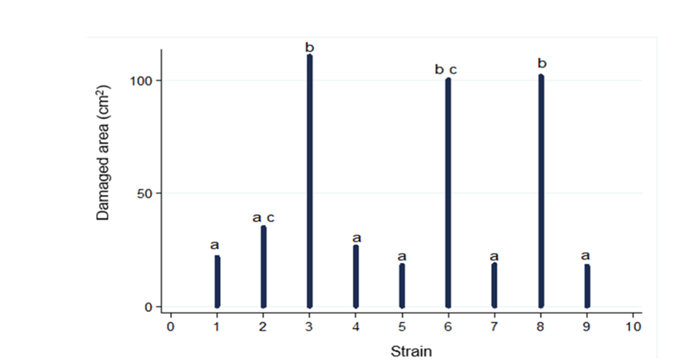
Figure 2 Fruit damage by pathogenic microorganisms inoculated in stenospermocarpic ‘Ataulfo’ mango fruit for pathogenicity test. Different letters indicate statistically significant differences (p ( 0.001).
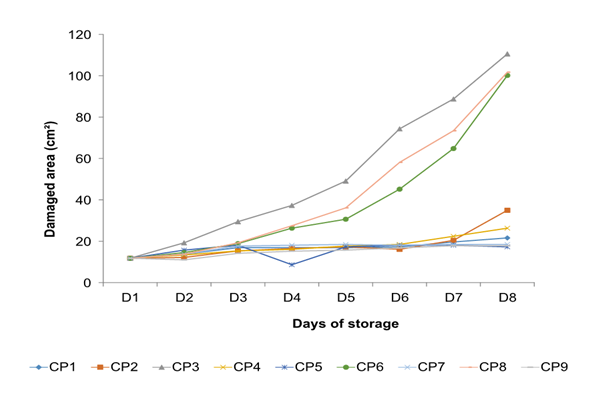
Figure 3 The behavior of pathogenic microorganisms inoculated in stenospermocarpic ‘Ataulfo’ mango fruit for the pathogenicity test per day of storage. The CP initials are the assignment given to each isolated pathogen.
Table 2 Correlation of area of damage by the strain of phytopathogenic microorganism using the Bonferroni method.
| Cepa | CP1 | CP2 | CP3 | CP4 | CP5 | CP6 | CP7 | CP8 |
|---|---|---|---|---|---|---|---|---|
| CP2 | 1.22719 (1.0) a | |||||||
| CP3 | 35.728 (0.014) | 34.5006 (0.021) | ||||||
| CP4 | 0.93662 (1.0) a | -0.29452 (1.0) a | -34.7951 (0.019) | |||||
| CP5 | -0.736313 (1.0) a | -1.9635 (1.0) a | -36.4641 (0.011) | -1.66898 (1.0) a | ||||
| CP6 | 22.4821 (0.775) | 21.2549 (1.0) c | -13.2457 (1.0) b | 21.5494 (0.983) | 23.2184 (0.639) | |||
| CP7 | 0.245437 (1.0) a | -0.98175 (1.0) a | -35.7823 (0.015) | -0.687225 (1.0) a | 0.98175 (1.0) a | -22.2366 (0.825) | ||
| CP8 | 26.1145 (0.289) | 24.8874 (0.407) | -9.6132 (1.0) b | 25.1819 (0.375) | 26.8509 (0.234) | 3.63247 (1.0) b | 25.8691 (0.309) | |
| CP9 | -2.06168 (1.0) a | -3.28886 (1.0) a | -37.7894 (0.007) | -2.99434 (1.0) a | -1.32536 (1.0) a | -24.5437 (0.447) | -2.30711 (1.0) a | -28.176 (0.158) |
*Values with different letters indicate statistically significant differences (P (0.05) using Bartlett's test for equal variances.
The damage caused by the most aggressive pathogen (CP3) in a stenospermocarpic ‘Ataulfo' mango is shown in Figures 4A and 4B.
Morphological identification
In the PDA culture medium, macroscopically, the isolate of the pathogen CP3, presented on the obverse (Figure 5Aa), a brown-pink center with a beige to cream central ring and dense cottony white aerial mycelium, while on the reverse (Figure 5Ab), the pathogen presented an appearance of a grayish-white center with black dots and concentric rings. Unicellular and cylindrical spores with rounded ends and some with slightly narrow ends were observed at the microscopic level. The measurements of the conidia ranged from 10 ~11.53 µm x 3 ~ 4.5 µm (n=25) (Figure 5B). The pathogen presented the sexual or perfect teleomorphic phase of Colletotrichum sp., dark brown, ovate, slightly irregular or lobed melanized appressoria (Figure 5Ca and 5Cb, respectively) and abundant and septate mycelium (Figure 5D). Additionally, CP3 has a high sporulation capacity, which increases its capacity for survival and rapid development of subsequent infections (Gutiérrez-Alonso et al., 2003).
Kim et al. (2009) isolated a total of 82 Colletotrichum species, identifying their cultural and morphological characteristics, 26 of the C. gloeosporioides isolates produced their teleomorph Glomerella cingulata in the PDA culture medium, coinciding with some of the main morphological characteristics of that study with those of the present investigation, inferring that the CP3 pathogen is a species of the genus Colletotrichum, the causative agent of anthracnose.
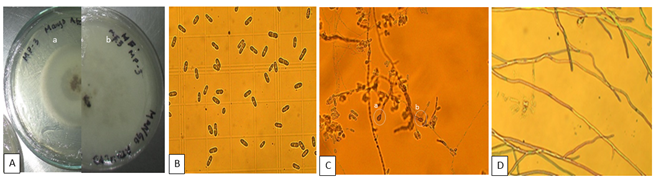
Figure 5 Macroscopic description of Colletotrichum sp. A) Isolated strain causing the greatest damage to stenospermocarpic ‘Ataulfo’ mango fruit. a) Obverse and b) reverse. Microscopic description of Colletotricum sp. B) Conidia C) Sexual or perfect teleomorphic stage of Colletotrichum sp. (Glomerella cingulata) b) appressorium D) Mycelium.
In vivo microbiology evaluation
In the analysis of variance, the damaged area caused by the pathogen that origin the anthracnose was considered as the dependent variable, finding significant differences between the groups (F = 13.1, p ( 0.001) where the Bartlett test for equal variances was applied to determine of specific groups. Treatments 1, 3, and 5 were found in group (a) (banana 'Pera' coating, soursop and stenospermocarpic mango 'Ataulfo' without pathogen) which behaved similarly to treatment 7 (negative control: fruits without coatings and without pathogen, respectively) observing minimal damage to them (Figure 5). In group (b), treatments 2, 4, and 6 were located (banana 'Pera', soursop, and stenospermocarpic mango 'Ataulfo' with the pathogen). Within this group, the damage caused by Colletotrichum sp. was remarkable. The treatments of group (c) T5 and T6 (stenospermocarpic 'Ataulfo' mango coating without and with the pathogen) behaved similarly between them. The damage intervals that occurred within these two groups were T5 = 12.88, 13.86, 14.23, and 23.17 cm2 of the damaged area on days 5, 6, 7, and 8, respectively, and T6 = 21.68, 29.61, 46.89 and 57.21 cm2 of the damaged area on days 5, 6, 7 and 8, respectively. That is, the T6 presented the lowest susceptibility to the pathogen at 66.91 % compared to the control T8 (positive control: fruits without coatings with the pathogen) which presented damages of 172.90 cm2 on the eighth day of storage while the treatment T2 and T4 inhibited 42.68 and 46.44 %, respectively. Finally, the fruits of T8 were located within group (d) (positive control: fruits without pathogen coating). These fruits suffered severe damage, presenting a significant difference from the rest of the groups. On the other hand, Figure 6 shows the damage in the fruits from the fourth day with a notable increase in damage from the sixth day in the fruits that were covered and inoculated; distinguishing less damage in the fruits of the treatments that were covered and that were not inoculated with the CP3 pathogen. Figure 7 shows the damage caused by anthracnose in stenospermocarpic 'Ataulfo' mango fruits that were treated with 2 % starch from stenospermocarpic banana 'Pera', soursop, and 'Ataulfo' mango fruits per day of storage in which they were stored, presenting moderate damage to the fruits in the presence of the pathogen and slightly visible damage to the fruits to which the coating was applied, but which were not inoculated, even though a wound was made in the epicarp of the fruits, which favors the favorable conditions for the pathogen to enter the fruit tissue (Figure 8).
Figure 6 Damage caused by Colletotrichum sp. in stenospermocarpic mango ‘Ataulfo’ fruit with a 2 % starch-based coating. Different letters indicate statistically significant differences (p ( 0.001).
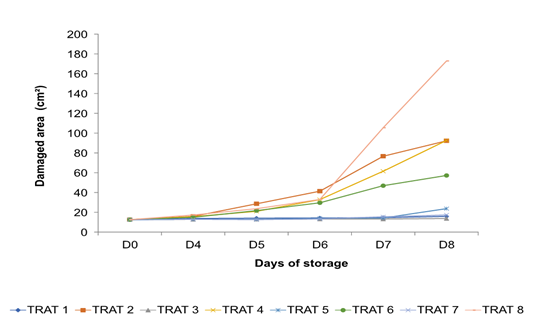
Figure 7 Damage caused by Colletotrichum sp. in stenospermocarpic mango ‘Ataulfo’ fruit with 2 % starch-based coating per day of storage.
Figure 8 Pathological damage by treatment: T1, T3 y T5 (based coating of banana ‘Pera’, soursop and ‘Ataulfo’ stenospermocarpic mango fruit without pathogen, respectively), T7 (negative control: fruit without coatings and without pathogen) and T8 (positive control: fruit without coating and inoculated with the pathogen).
In Table 3, the Correlation between each of the phytopathogen microorganisms that damage the stenospermocarpic ‘Ataulfo’ mango.
Table 3 Correlation of treatments of the area of damage by treatment using the Bonferroni method.
| Treatment | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|
| T2 | 29.2335 (0.001) | ||||||
| T3 | -1.15929 (1.0)a | -30.3928 (0.0) | |||||
| T4 | 24.6845 (0.0) | -4.54891 (1.0)b | 25.8438 (0.006) | ||||
| T5 | 1.22224 (1.0)a | -28.0112 (0.004) | 2.38154 (1.0)a | -23.4623 (0.039) | |||
| T6 | 16.0518 (0.545) | -13.1817 (1.0)b | 17.2111 0.387 | -8.63277 (1.0)b | 14.8295 (1.0)c | ||
| T7 | -0.268608 (1.0)a | -29.5012 (0.001) | 0.890687 (1.0)a | -24.9531 (0.009) | -1.49085 (1.0)c | -16.3204 (0.518) | |
| T8 | 46.5928 (0.00) | 17.3594 (0.333) | 47.7521 (0.000) | 21.9083 (0.041) | 45.3706 (0.00) | 30.5411 (0.0) | 46.8614 (0.0)d |
*Values with different letters indicate significant differences statistically (P (0.05) using Bartlett's test for equal variances.
Ali et al. (2020) searched for starch-based materials with antimicrobial effects, reporting that starch has been recognized as the most important of several polymers in the field of the food industry due to its biodegradability, biocompatibility, edible properties, and sustainability. However, they mention that presents limitations to be used in food packaging due to its reduced mechanical, barrier, and processing characteristics. The authors mention that to reduce these limitations, different plasticizers are mixed with the starch, in addition to the inclusion of materials such as clay, zinc oxide, titanium dioxide, and magnesium oxide, which can improve the microbial barrier and physical stability, chemical, mechanical and thermal properties of starch, alluding that oils extracted from plants provide good and safe antimicrobial properties. The authors conclude that different starch-based nanocomposite materials, which are available in nature, may be promising candidates in the field of the food packaging industry. Rodríguez et al. (2020) & Arauz (2000) reported that different analyzes have been proposed to help control anthracnose during postharvest; however, climacteric fruits such as mangoes, present a quiescent infection and exhibit an increase in their respiratory rate during their ripening period, which accelerates the production of ethylene, a mechanism that the pathogen uses as a signal to reactivate the process of infection with the fungus Colletotrichum spp. According to Torres-Leon et al. (2016), the mango fruit is rich in water, sugars, fiber, minerals, vitamins, and antioxidants; its seed contains bioactive compounds such as phenolic compounds (mangiferin, isomangifenin, homomangiferin, quercitin, kaempferol and anthocyanins), phenolic acids (gallic, protocatechuic, ferulic, caffeic, coumaric, ellagic, 4-caffeoylquinic) and antioxidant minerals (K, Cu, Zn, Mn, Fe, Se). In this regard, these researchers report an investigation on mango seeds grown in Egypt where they used high-performance liquid chromatography (HPLC) to determine phenolic compounds, identifying a high content of tannins (20.7 %) and vanillin (20.2 % respectively), acid gallic (6 %), coumarins (12.6 %), caffeic acid (7.7 %), ferulic acid (10.4 %) and cinnamic acid (11.2 %), concluding that the mango seed has antimicrobial activity attributed to its high capacity antioxidant, the content of various compounds and antioxidants. On the other hand, Yadav et al. (2022) in a review article report that mango peel is a good source of polyphenols, carotenoids, dietary fiber, and vitamins E and C. Researchers mention that the content of phenols, flavonoids, gallic acid, mangiferin, and antioxidant capacity is much higher (53.3 %) compared to the content of these compounds in the mango pulp and its content will depend on the cultivar, extraction method and stage of maturity in which the fruit is found. In the present investigation, three coatings based on starch at 2 % w/v were used, which were extracted from the tropical fruit of banana 'Pera', soursop, and stenospermocarpic mango 'Ataulfo', which reduced the incidence and severity of anthracnose in fruit of stenospermocarpic 'Ataulfo' mango during postharvest. It should be noted that in this study whole ‘Ataulfo' stenospermocarpic mango fruit at physiological maturity were used to obtain starch in the preparation of the edible coating of treatments T5 and T6. The starch-based coating extracted from stenospermocarpic 'Ataulfo' mango fruits (T6) applied to stenospermocarpic 'Ataulfo' mango fruits presented the best result in terms of inhibition of anthracnose, controlling the growth of anthracnose on the eighth day of storage by 66. 91 % and that, according to the scale proposed by Palou et al. (2007), the damage caused by the pathogen was from 0 to 1 (no visible damage to slightly visible damage).
Conclusion
The 2 % starch-based coatings extracted from banana ‘Pear’, soursop, and mango ‘Ataulfo’ diminished the damage caused by Colletotrichum sp. in stenospermocarpic mango ‘Ataulfo’ during postharvest storage. The starch-based coat extracted from stenospermocarpic mango ‘Ataulfo’ presented the lowest damage (66.91 %) in the fruits during postharvest storage, preserving the organoleptic and microbiology quality of the fruit, leading to fresh consumption.











 nueva página del texto (beta)
nueva página del texto (beta)


