Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Tecnología y ciencias del agua
versión On-line ISSN 2007-2422
Tecnol. cienc. agua vol.11 no.3 Jiutepec may./jun. 2020 Epub 10-Jun-2024
https://doi.org/10.24850/j-tyca-2020-03-09
Articles
Zinc and cadmium removal by bacteria: Study of factors associated to the process
1Facultad de Biología, Universidad de La Habana, La Habana, Cuba, lizandra@fbio.uh.cu
2Facultad de Biología, Universidad de La Habana, La Habana, Cuba, armando@fbio.uh.cu
3Facultad de Biología, Universidad de La Habana, La Habana, Cuba, irina@fbio.uh.cu
4Glendale Community College, Glendale, EUA, lui2152145@maricopa.edu
5Facultad de Biología, Universidad de La Habana, La Habana, Cuba, mcruz@fbio.uh.cu
6Instituto de Tecnología y Ciencia de Materiales, Universidad de La Habana, La Habana, Cuba, salleyne@imre.uh.cu
7Instituto de Tecnología y Ciencia de Materiales, Universidad de La Habana, La Habana, Cuba, ody@imre.uh.cu
6Instituto de Tecnología y Ciencia de Materiales, Universidad de La Habana, La Habana, Cuba, liva@imre.uh.cu
9Facultad de Biología, Universidad de La Habana, mecarballo@fbio.uh.cu
The capacities of microorganisms to capture the metals present in aquatic ecosystems constitute an alternative for the conservation of natural resources. Metallic ions in aquatic ecosystems are considered important inorganic pollutants of the environment due to their mobility and toxicity to living organisms. In the present work, it was evaluated the biosorption capacities of zinc and cadmium by bacterial strains of Proteus mirabilis and Bacillus subtilis in aqueous solutions, the influence of different variables such as culture physiological age, cell concentration, pH, contact time and pre-treatment of cell biomass with physical and chemical methods. The capture of metals by bacterial cultures did not present differences in the different physiological stages evaluated. Biomass enough to reach high capture values was 2 g.L-1. Also, it was determined the greatest influence in the process lies in the pH utilized, the contact time, and effect of each treatment to bacterial biomass. The results show effectiveness of tested microbial biomasses in the biosorption of zinc and cadmium and their potential for environmental sanitation.
Keywords: Biosorption; microbial biomasses; zinc; cadmium; biotic and abiotic factors
Las capacidades de los microorganismos de captar metales presentes en ecosistemas acuáticos constituyen una alternativa para la conservación ambiental y de los recursos naturales. En el presente trabajo se evaluó la capacidad de biosorción de cinc y cadmio en soluciones acuosas por cepas de las especies bacterianas Proteus mirabilis y Bacillus subtilis, así como la influencia de diferentes variables, como la edad fisiológica del cultivo, la concentración celular, el pH, el tiempo de contacto y el pretratamiento con métodos físicos y químicos de la biomasa celular en el proceso de remoción. En los estadios fisiológicos evaluados no se observó diferencia en la captura de los metales por los cultivos bacterianos y 2 g.L-1 de biomasa fueron suficientes para alcanzar altos valores de captura. Se determinó que la mayor influencia en el proceso la ejerce el pH empleado, el tiempo de contacto y el efecto de cada tratamiento a la biomasa bacteriana. Los resultados muestran la efectividad de las biomasas microbianas investigadas en la biosorción de cinc y cadmio, y sus potencialidades para el saneamiento ambiental.
Palabras clave: biosorción; biomasas microbianas; cinc; cadmio; factores bióticos y abióticos.
Introduction
Environmental pollution arises largely as a result of human actions, mainly by industrial and agricultural activities (Beltrán-Pineda & Gómez-Rodríguez, 2016); cause wastewater discharges with insufficient treatment to eliminate its pollutant load (Chowdhury, Jafar, Al-Atta, & Husain, 2016). These effluents are discharged to ecosystems causing reach high concentrations, especially near the discharge site, which causes environmental deterioration (Nour-Abdel-Ghani & Ghadir-El-Chaghaby, 2014). Among main pollutants are heavy metals, which unlike chemical pollutants of organic origin, these do not degrade by biological or chemical route and persist long time in aquatic and terrestrial environments (Salgado-Bernal, Pérez, Carballo, Martínez, & Cruz, 2015). The metal ions accumulation in the organisms from different links of food chain (Mehta & Kumar, 2017), their mobility and toxicity in ecosystems, make their elimination a priority worldwide (Nour-Abdel-Ghani & Ghadir-El-Chaghaby, 2014).
Some heavy metals are essential for development of microorganisms, plants, and animals. They play important roles in biochemical reactions and are essential for the growth and development. However, when they occur in high concentrations, can form nonspecific compounds with cytotoxic and lethal effects. While some metals have biological importance, others such as cadmium, chromium and mercury, are toxic (Beltrán-Pineda & Gómez-Rodríguez, 2016). Zinc is not highly toxic and is considered an essential element for life, but adverse effects have been found at high concentrations (Kvasnová, Hamarováb, & Pristašc, 2017), between 100-500 mg/day (Volesky, 1994). However, cadmium toxicity is widely known for the destruction of active enzyme sites (Essa, Al-Abboud, & Khatib, 2017) and alterations of the nervous system in humans, even at low concentrations (Chauhan, Solanki, & Nehra, 2017), until 10 mg/day (Kjellstrom & Nordberg, 1985).
In order to ensure the protection and integrity of water resources contaminated with heavy metals, restrictions on discharge of contaminated water are increasingly stringent (Nour-Abdel-Ghani & Ghadir-El-Chaghaby, 2014). In addition, environmental research is intensified with utilization of microbial diversity. Their structural and metabolic potentials as bioremediating agents are used for wastewater treatment and restoration of contaminated environments (Irawati, Riak, Sopiah, & Sulistia, 2017). The analysis of the environmental factors during the interactions microbial biomass and the different metal ions is necessary due to their influences in effectiveness process. These include temperature, pH, inoculum concentration (biosorbent), and metals (Li, Peng, Yingying, Lu, & Fan, 2016).
Although research has been carried out that evaluates biotic and abiotic conditions that influence on metals capture (Andreoni, Finoli, Manfrin, Pelosi, & Vecchio, 1991; Augusto-da-Costa & Pereira, 2001; Boyanov et al., 2003), these are still insufficient considering the particularities of each microbial biosorbent, as well as metallic species. In this regard, to contribute to characterization of metal removal process and to decrease these pollutants in the environment, in the present study, the zinc and cadmium removal by Proteus mirabilis and Bacillus subtilis and the effect of different environmental factors in the process were evaluated.
Materials and methods
Biological material and culture conditions
Bacterial species Proteus mirabilis and Bacillus subtilis were used from collection of the Department of Microbiology and Virology, Faculty of Biology, at University of Havana. Microbial cultures were obtained in nutrient broth, under incubation conditions of 120 rpm, 30 ± 2°C for 24 hours. Biomass was collected by centrifugation at 3 200 g for 15 minutes.
Zinc and cadmium removal by bacterial biomass
Aqueous solutions of zinc and cadmium at a concentration of 1 mM, pH 6.0,
were prepared from the salts of CdCl2.4H2O (Cadmium
Chloride Tetrahydrate) and ZnSO4.7H2O (Zinc Sulfate
Heptahydrate). Both were contacted individually with microbial biomass at
concentration of 2 g.L-1. Microorganism-metal suspension was
maintained at 100 rpm, 28 °C for 24 hours and pH 6.0 adjusted with 0.1 M HCl
or 0.1 M NaOH, as required. The supernatant was collected by centrifugation
at 3200 g for 20 minutes, for analysis of residual metals by Phillips PU
9100X Atomic Absorption Spectrophotometry with air-acetylene flame. The
metal solutions were used as control at the concentration established in
each case, without adding biomass and maintaining the same experimental
conditions in all samples. For quantification of metals was used lamp
current 12 mA at λ = 228.8 nm and 10 mA and a λ = 230.9 nm for cadmium and
zinc respectively. The amount of metal captured per grams of biomass
(mg.g-1) was determined according to the equation
Effect of factors on zinc and cadmium removal by bacteria
Physiological age of bacterial culture
The microbial growth in nutrient broth of each bacterium was determined at 28°C at 100 rpm for 24 hours. During this time, readings were realized every two hours on a spectrophotometer at ʎ = 640 nm against an uninoculated medium as a blank. The metals capture was carried out with microbial biomass collected in two physiological states corresponding to 6 and 24 hours for Bacillus subtilis and Proteus mirabilis at 8 and 24 hours of growth.
Cell concentration
Wet biomass collected from each bacterial culture obtained in nutrient broth, was contacted with metals solutions at three different concentrations: 2, 3, and 4 g.L-1. As control was used cell concentration corresponding to 2 g.L-1.
Pretreatment of biomass
Bacterial cultures collected by centrifugation at 24 hours of growth were inactivated by dry heat at 60 °C in an oven for 12 hours. The treatment action on cell viability was verified by planting of treated biomass on nutrient agar. Inactivated cells were treated separately with solutions of 0.1 M HCL for 3 hours and 0.1 M KOH for 2 hours. After these times, until the pH reached a value of 6.0. The samples were washed with distilled water. After each treatment, the biomass was washed with double-distilled water and dried in an oven at 60 °C for 12 hours for contact with zinc and cadmium. As control of the experiment was used living cells untreated microbial biomass.
Biometric analysis
All experiments were performed by triplicate. The data were tested for normality and homogeneity of variance through the Kolmogorov-Smirnov and Bartlett tests, respectively. Subsequently, the Student test was applied for comparison of two means and simple classification ANOVA and Tukey test posteriori for more than two means, referring to removal levels (q) at a significance level of 0.05. The Microsoft Excel computer program and the Statistic 8.0 statistical software package for Windows were used for statistical processing.
Results
Zinc and cadmium removal capacity by bacteria
Proteus mirabilis and Bacillus subtilis have ability to remove metal ions from the solution. Values ranging between 16.42 ± 0.069 and 22.36 ± 0.246 mg.g-1 (Figure 1).
Influence of culture physiological age on zinc and cadmium removal
Bacterial biomass captured the zinc and cadmium ions in two growth stages evaluated, not significant differences in capture levels in the both physiological stages (Figure 2, A and B).
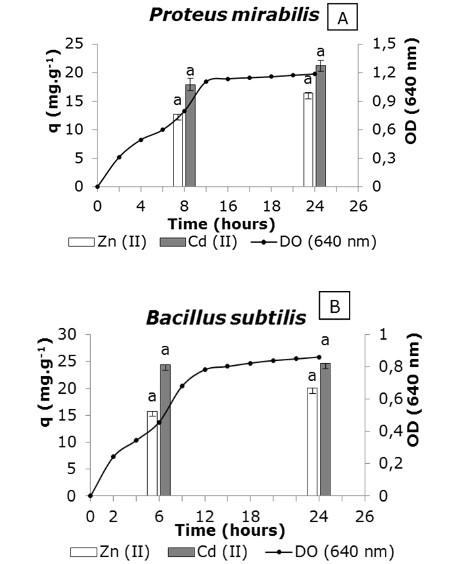
Figure 2 Influence of the physiological age of the culture on the removal of Zn (II) and Cd (II) from aqueous solution; 28 ± 2 °C; pH 6.0; metal in solution 1 mM ; wet biomass 2 g.L-1; stirring at 100 rpm, 24 h. Error bars represent standard deviation of three repetitions per physiological stage. T-student was applied for each metal analyzed. Different letters on the bars indicate significant differences between the values of q in the different physiological stages for p < 0.05.
Effect of cell concentration
Data obtained from zinc and cadmium ions capture showed a different behavior between both bacteria. Proteus mirabilis-zinc ions interaction does not show significant differences among all cell concentrations; at the same way it was observed for cadmium in the two lowest concentrations. At 2 g.L-1 of cell concentration, Bacillus subtilis resulted the least number of cells to achieve the greatest removal of both metals (Figure 3).
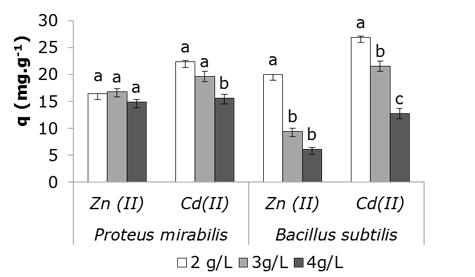
Figure 3 Effect of cell concentration on the capture capacity of Zn (II) and Cd (II) by bacterial biomass; 28 ± 2 °C; pH 6.0; metal in 1 mM; solution; wet biomass; stirring at 100 rpm, 24 h. (±) represent standard deviation of three repetitions per cell concentration. Simple classification ANOVA was applied for each microorganism analyzed. Different letters in the table indicate significant differences between the values of q of the different concentrations for p < 0.05, according to the statistical test Tukey a posteriori.
Effect of pH on metals removal
pH effect on the removal of metals by bacterial biomass is shown in Figure 4. At pH 5.0 the lowest capture values of both cations were obtained by bacteria, however a favorable effect is observed at near neutral values.
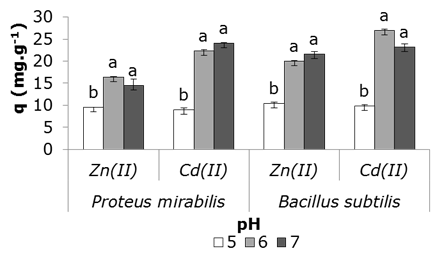
Figure 4 Effect of pH on Zn (II) and Cd (II) removal by bacterial biomass; 28 ± 2°C, metal in solution 1 mM; wet biomass 2 g.L-1; stirring at 100 rpm, 24 h. Error bars represent standard deviation of three repetitions per pH. Simple classification ANOVA was applied for each microorganism analyzed. Different letters on the bars indicate significant differences between the values of q of the different pH for p < 0.05, according to the statistical test Tukey a posteriori.
Effect of contact time on metal removal
In general, the removal of both metals is a rapid process in the first hours and subsequently reaches equilibrium (Figure 5, A and B). Around 6 hours is enough for Bacillus subtilis and Proteus mirabilis to obtain the maximum capture values ions and balance biomass-metals.
Removal of metals by biomass pretreated by chemical and physical methods
In general, there was an increase in the capture values of both ions by pretreated biomass, respect to untreated biomass (control), at least one treatment (Figure 6). Zinc effect is evidenced mainly in Bacillus subtilis, where the three treatments applied led to higher capture values than those achieved by the control and statistically confirmed results. A different response was detected for cadmium, marked by variability in effect of each treatment during ion removing ion. Bacillus subtilis was highlighted, a significant effect on the cadmium capture and treating biomass with KOH.
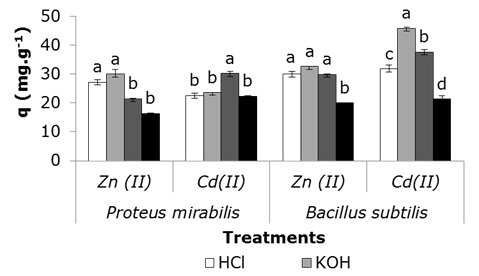
Figure 6 Removal of Zn (II) and Cd (II) from aqueous solution by bacterial biomass subjected to different treatments; 28 ± 2 °C, metal in solution 1 mM; wet biomass 2 g.L-1; stirring at 100 rpm, 24 h. Error bars represent standard deviation of three repetitions per treatment. Simple classification ANOVA was applied for each microorganism analyzed. Different letters on the bars indicate significant differences between the values of q of the different treatments for p < 0.05, according to the Tukey a posteriori statistical test.
Discussion
Chemical composition of the cell envelopes of both bacteria can be a way to explain their ability to remove zinc and cadmium ions. According to results (Figure 1), we allow to infer that polyanionic nature of Proteus mirabilis cell wall, characterized by the outer membrane of lipopolysaccharides and phospholipids, offers abundant functional groups such as carboxyl, phosphates and hydroxyls for the union of zinc and cadmium (Sharma et al., 2016). Amino, hydroxyl, and sulfates are other active sites in this structure involved in the bonding of these metals (Goswami, Manikandan, Pakshirajan, & Pugazhenthi, 2017). Having these ligands allows metals to be in polar region of the membrane or within the peptidoglycan layer, all of which can support the behavior of this bacterium against the metals tested. However, Bacillus subtilis contact with cations may be due to the presence of teichoic and teichuronic acids, associated with the derivatives of N-acetyl muramic and N-acetyl glucosamine sugars, which are a source of phosphate and carboxylic groups that offer negative charge to the cell surface. In this way, electrostatic bonding between the cell and metal ions is favored and consequently the capture of metals (Sharma et al., 2016).
Another properties inherent to prokaryotes are high surface-volume ratio (Uthra & Kadirvelu, 2017), metabolic characteristics (Kvasnová et al., 2017) and genetics that facilitate the adsorption and absorption of metals dissolved in aqueous solution (Chauhan et al., 2017), which support interactions can be established between Proteus mirabilis, Bacillus subtilis and metal ions. When considering absorption, as a possible mechanism present in both bacteria, the capture of zinc and cadmium ions could also occur by intracellular accumulation. This mechanism mediated by transport protein systems incorporates metals into the cytoplasm has been referred to other bacterial species in the capture of different metal ions (Beltrán-Pineda & Gómez-Rodríguez, 2016; Hansda, Kumar, & Anshumali, 2016; Muñoz, Espínola, & Ruiz, 2017).
Capture Levels of zinc and cadmium reached by Proteus mirabilis and Bacillus subtilis, meet selection criteria informed in the literature. It is proposed to compete with conventional technologies, in the metals removal, the active agent, but microorganisms, must have a capture capacity greater than 15 mg.g-1 (Brierley, Kelly, Seal, & Best, 1985; Cañizares-Villanueva, 2000). This could ratify bacteria constitute one of the microbial groups of interest in the study of metal removal (Ramya & Thatheyus, 2017). Similar results have been reported for the microbial species Pseudomonas mendocina (Ps-1) (Carballo et al., 2017), Pseudomonas aeruginosa (Uthra & Kadirvelu, 2017), and Klebsiella sp. 3S1 (Muñoz et al., 2017).
The removal of zinc and cadmium by B. subtilis and P. mirabilis in two growth phases evaluated (Figure 2), suggests the joint participation of intracellular mechanisms and extracellular accumulation of metals. This is deduced by the ability to capture both metals during the different growth phases, both in an advanced exponential growth phase and in the stationary phase. An active metabolism is involved to a bioaccumulation process, while extracellular capture depends on the interactions between metal cations and active cell surface groups, which can occur throughout the cell cycle. Similarly, similar results obtained in previous works have been explained (Ghaima, Mohamed, Al-Meshhdany, & Abdulhassan, 2017).
However, it is important to keep in mind extracellular accumulation can depend or not on either an active metabolism, considering different physiological stages of microbial cultures; it could occur alterations on functional groups, present in the cell wall, involved in the processes of joining metal to biomass, as it has been referred to in other researches (Fan, Okyay, & Rodrigues, 2014; Ghaima et al., 2017). The different microorganism behaviors in analysis of this biotic factor allow us to state the relationship between the metals capture and crop age will depend on type of microorganism and its genetic characteristics, as well as the metallic species.
According to results obtained in the evaluation of the effect of the cell concentration on the capture capacity in the bacteria evaluated (Figure 3), we can think that an increase in the concentration of cells could cause interference between the metal and biomass binding sites , which can inflict a decrease in cation entrapment. Similar results have been reported by different authors in the removal of metal ions by bacterial biomass (Limcharoensuk et al., 2015; Choińska-Pulit, Sobolczyk-Bednarek, & Łaba, 2018). This may explain what happened in the analyzed samples of Bacillus subtilis.
Zinc and cadmium decrease in capture values by both bacteria evaluated (Figure 4) at pH 5.0, confirm biosorption depends on the protonation or deprotonating of functional groups on the cell wall. In these concentrations, hydronium ions (H3O+) increase and high ionic mobility present favors competition between them and the metal cation for metal-binding functional groups in the microbial biomass. The predominance exists at pH value of positive charges on the cell surface prevents the union of positively charged metal species. It has been previously reported (Li et al., 2016). Other studies have reported similar results in the removal of metals by different bacterial biomass (Fan et al., 2014; Limcharoensuk et al., 2015).
Increases in the capture of zinc and cadmium ions by Proteus mirabilis and Bacillus subtilis at or near neutral pH values may be due to the presence of fewer free H+ protons in the solution, which consequently decreases competition between these and metal ions. The existence of a lower protonation in the active sites of the biomass, leads to a predominance of negatively charged groups on the cell surface, which causes an intensification of the electrostatic forces involved in the metal capture process (Ghaima et al., 2017). Thus, biomass surface ligands allow reaction with the cations to be greater and number of ions bound to the biomass increases. Similar behaviors have been detected in the removal of metals by different microbial biomass (Limcharoensuk et al., 2015; Li et al., 2016; Carballo et al., 2017).
Evaluation results of removal dynamics about ions by bacterial biomass (Figure 5) are on correspondence to characteristics of these processes in cations capture by microorganisms. It occurs mainly quickly in the first contact times biomass-metal. This particularity is very importance for the design and operationality of the process. As it happens in the present work, other authors indicate existence of two phases in the process of capturing metals. However, they emphasize time in which the equilibrium is reached can change from minutes to days, it will depend on the type of biosorbent, metallic species, and other factors (Nwidi & Agunwamba, 2015; Ling, Huang, Li, Liu, & Cheng, 2016; Uthra & Kadirvelu, 2017; Zhang, Hu, & Lu, 2017).
Favorable action of dry heat treatment applied to Bacillus subtilis and Proteus mirabilis, for the latter, with the exception of zinc removal by P. mirabilis (Figure 6), in comparation to control may be given by the removal of impurities present in the cell surface, therefore a greater exposure of functional groups of union to metals. Both cell death of bacteria, proven by the absence of growth in nutrient agar after treatment, conditioned a metabolically inactive state of these treated cells, in which transport systems would allow the bioaccumulation of the metal are absent; therefore, a greater capacity of biosorption on the cell surface is possible. Inactivated biomass, treated with HCl and KOH, there were variations in their properties of bonding metals. This result coincident reported by other authors (Hansda et al., 2016). The chemical agents applied in the present work can enhance electrostatic interactions, as facilitate the formation of optimal conditions for ion exchange and increase binding sites for metal cations. In this way, previous results have been supported (Zeraatkar, Ahmadzadeh, Talebi, & Moheimani, 2016).
Cell wall is fundamentally involved in the biosorption process and its modification can enhance capture of metal ions (Barange, Srivastava, Srivastava, & Palsania, 2014; Hansda et al., 2016). Other authors report effectiveness of treatments will depend among other factors of the metallic species and microorganism (Mota et al., 2016). As well as mechanism or sub-mechanism governs capture of ions by microbial biomass (Kiran, Rani, & Kaushik, 2016). However, they have been well supported in the literature as an alternative to increase metal removal (Shoaib, Aslam, & Aslam, 2013).
Conclusions
Microorganisms are used to metal biosorption and represent an alternative in the search for an economical and environmentally friendly solution. Zinc and cadmium capture values by microbial biomass showed the potential of Bacillus subtilis and Proteus mirabilis for elimination of both metals present in aqueous solutions. Also, it was shown natural potentialities can be favored by characterizing different factors associated with removal of metal ions and modification of biomass with physical and chemical treatments. Among the factors most affected the improvement of removal levels are biomass-metal contact time, pH, and treatments applied. Inactivated cells constitute a favorable condition for the use of both bacteria without environmental risk and in particular for health. It is enouhg 6 hours to achieve maximum capture of zinc and cadmium, which is very important along the way to make the application of this bacterial biomass in biotechnological processes economically more viable.
Referencias
Andreoni, V., Finoli, C., Manfrin, P., Pelosi, M., & Vecchio, A. (1991). Studies on the accumulation of cadmium by a strain of Proteus mirabilis. FEMS Microbiology Ecology, 85, 183-192. [ Links ]
Augusto-da-Costa, A. C., & Pereira, F. (2001). Bioaccumulation of copper, zinc, cadmium and lead by Bacillus sp., Bacillus cereus, Bacillus sphaericus and Bacillus subtilis. Brazilian Journal of Microbiology, 32, 1-5. [ Links ]
Barange, M., Srivastava, A., Srivastava, J. K., & Palsania, J. (2014). Biosorption of heavy metals from wastewater by using microalgae. International Journal of Chemical and Physical Sciences, 3(6), 67-81. [ Links ]
Beltrán-Pineda, M. E., & Gómez-Rodríguez, A. M. (2016). Biorremediación de metales pesados cadmio (Cd), cromo (Cr) y mercurio (Hg) mecanismos bioquímicos e ingeniería genética: una revisión. Revista Facultad de Ciencias Básicas, 12(2), 172-197. [ Links ]
Boyanov, M. I., Kelly, S. D., Kemner, K. M., Bunker, B. A., Fein, J. B., & Fowle, D. A. (2003). Adsorption of cadmium to Bacillus subtilis bacterial cell walls: A pH-dependent X-ray absorption fine structure spectroscopy study. Geochimica et Cosmochimica Acta, 67(18), 3299-3311. [ Links ]
Brierley, C. L., Kelly, D. P., Seal, K. J., & Best, D. J. (1985). Materials and Biotechnology. In: Higgins, I. J., Best, D. J., & Jones, J. (eds.). Biotechnology. Principles and Applications, 2nd ed. (pp. 163-212). Oxford, UK: Blackwell Science Ltd. [ Links ]
Cañizares-Villanueva, R. (2000). Biosorción de metales pesados mediante el uso de biomasa microbiana. Revista Latinoamericana de Microbiología, 42, 131-143. [ Links ]
Carballo, M. E., Martínez, A., Salgado-Bernal, I., Pérez, L., Cruz, M., Liva, M. B., Allende, S., Rodríguez, M. M., & Garza, Y. (2017). Standardization of variables involved in cadmium and zinc microbial removal from aqueous solutions. Biotecnología Aplicada, 33, 1221-1225. [ Links ]
Chauhan, M., Solanki, M., & Nehra, K. (2017). Putative mechanism of cadmium bioremediation employed by resistant bacteria. Jordan Journal of Biological Sciences, 10(2), 101-107. [ Links ]
Choińska-Pulit, A., Sobolczyk-Bednarek, J., & Łaba, W. (2018). Optimization of copper, lead and cadmium biosorption onto newly isolated bacterium using a Box-Behnken design. Ecotoxicology and Environmental Safety, 149, 275-283. [ Links ]
Chowdhury, S., Jafar, M. A., Al-Atta, O., & Husain, T. (2016). Heavy metals in drinking water: Occurrences, implications and future needs in developing countries. Science of the Total Environment, 569-570, 476-488. [ Links ]
Essa, A. M. M., Al-Abboud, M. A., & Khatib, S. I. (2017). Metal transformation as a strategy for bacterial detoxification of heavy metals. Journal of Basic Microbiology, DOI: 10.1002/jobm.201700143. [ Links ]
Fan, J., Okyay, T. O., & Rodrigues, D. F. (2014). The synergism of temperature, pH and growth phases on heavy metal biosorption by two environmental isolates. Journal of Hazardous Materials, 279, 236-243. [ Links ]
Ghaima, K. K., Mohamed, A. I., Al-Meshhdany, W. Y., & Abdulhassan, A. A. (2017). Resistance and bioadsorption of cadmium by Pseudomonas aeruginosa isolated from agricultural soil. International Journal of Applied Environmental Sciences, 12(9), 1649-1660. [ Links ]
Goswami, L., Manikandan, N. A., Pakshirajan, K., & Pugazhenthi, G. (2017). Simultaneous heavy metal removal and anthracene biodegradation by the oleaginous bacteria Rhodococcus opacus. 3 Biotech, 7(37), 2-9. [ Links ]
Hansda, A., Kumar, V., & Anshumali. (2016). A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World Journal of Microbiology Biotechnology, 32(170), 1-14. [ Links ]
Irawati, W., Riak, S., Sopiah, N., & Sulistia, S. (2017). Heavy metal tolerance in indigenous bacteria isolated from the industrial sewage in Kemisan River, Tangerang, Banten, Indonesia. Biodiversitas, 18(4), 1481-1486. [ Links ]
Kiran, B., Rani, N., & Kaushik, A. (2016). FTIR spectroscopy and scanning electron microscopic analysis of pretreated biosorbent to observe the effect on Cr (VI) remediation. International Journal of Phytoremediation, DOI: 10.1080/15226514.2016.1183577. [ Links ]
Kjellstrom, T., & Nordberg, G. F. (1985). Kinetic model of cadmium metabolism. In: Fridberg, L., Elinder, C. G., Kellstrom, T., & Nordberg, G. F. (eds.). Vol I. Cadmium and health, a toxicological and epidemiological appraisal (pp. 179-197), Boca Raton, USA: CRC Press. [ Links ]
Kvasnová, S., Hamarováb, L., & Pristašc, P. (2017). Zinc bioaccumulation by microbial consortium isolated from nickel smelter sludge disposal site. Nova Biotechnologica et Chimica, 16(1), 48-53. [ Links ]
Li, X., Peng, W., Yingying, J., Lu, L., & Fan, W. (2016). Removal of cadmium and zinc from contaminated wastewater using Rhodobacter sphaeroides. Water Science and Technology, DOI: 10.2166/wst.2016.608. [ Links ]
Limcharoensuk, T., Sooksawat, N., Sumarnrote, A., Awutpet, T., Kruatrachue, M., Pokethitiyook, P., & Auesukaree, C. (2015). Bioaccumulation and biosorption of Cd2+ and Zn2+ by bacteria isolated from zinc mine in Thailand. Ecotoxicology and Environmental Safety, 122, 322-330. Recuperado de http://dx.doi.org/10.1016/j.ecoenv.2015.08.013. [ Links ]
Ling, W., Huang, S., Li, X., Liu, M., & Cheng, Y. (2016). Bio-remediation of acephate-Pb (II) compound contaminants by Bacillus subtilis FZUL-33. Journal of Environmental Sciences, 45, 94-99. [ Links ]
Mehta, A., & Kumar, S. (2017). Heavy metals as a threat to aquatic environments. International Journal of Current Microbiology and Applied Sciences, 6(6), 386-389. [ Links ]
Mota, R., Rossi, F., Andrenelli, L., Bernardes, S., De Philippis, R., & Tamagnini, P. (2016). Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: Interactions between metals and RPS binding sites. Applied Microbiology and Biotechnology, DOI: 10.1007/s00253-016-7602-9. [ Links ]
Muñoz, A. J., Espínola, F., & Ruiz, E. (2017). Biosorption of Ag (I) from aqueous solutions by Klebsiella sp. 3S1. Journal of Hazardous Materials, 329, 166-177. [ Links ]
Nwidi, I. C., & Agunwamba, J. C. (2015). Selection of biosorbents for biosorption of three heavy metals in a flow metals in a flow-batch reactor using removal efficiency as parameter. Nigerian Journal of Technology, 34(2), 406-413. [ Links ]
Nour-Abdel-Ghani, T., & Ghadir-El-Chaghaby, A. (2014). Biosorption for metal ions removal from aqueous solutions: a review of recent studies. International Journal of Latest Research in Science and Technology, 3(1), 24-42. [ Links ]
Ramya, D., & Thatheyus, A. J. (2017). Microscopic investigations on the biosorption of heavy metals by bacterial cells: A review. Science International, 6(1), 11-17. [ Links ]
Salgado-Bernal, I., Pérez, J. E., Carballo, M. E., Martínez, A., & Cruz, M. (2015). Aplicación de rizobacterias en la biorremediación del cromo hexavalente presente en aguas residuales. Revista Cubana de Ciencias Biológicas, 4(2), 20-34. [ Links ]
Sharma, S., Rana, S., Thakkar, A., Baldi, A., Murthy, R. S. R., & Sharma, R. K. (2016). Physical, chemical and phytoremediation technique for removal of heavy metals. Journal of Heavy Metal Toxicity and Diseases, 1(2), 1-15. [ Links ]
Shoaib, A., Aslam, N., & Aslam, N. (2013). Trichoderma harzianum: Adsorption, desorption, isotherm and FTIR studies. Journal of Animal and Plant Sciences, 23(5), 1460-1465. [ Links ]
Uthra, K., & Kadirvelu, K. (2017). Biosorption of nickel using mixed cultures of Pseudomonas aeruginosa and Bacillus subtilis. Defence Life Science Journal, 2(4), 442-447. [ Links ]
Viera, R., & Volesky, B. (2003). Biosorption: A solution to pollution? International Microbiology, 3, 17-24. [ Links ]
Volesky, B. (1994). Advances in biosorption of metals: Selection of biomass types. Microbiology Reviews, 14, 291-302. [ Links ]
Zeraatkar, A. K., Ahmadzadeh, H., Talebi, A. F., & Moheimani, N. R. (2016). Potential use of algae for heavy metal bioremediation, a critical review. Journal of Environmental Management, 30, 1-15. [ Links ]
Zhang, H., Hu, X., & Lu, H. (2017). Ni (II) and Cu (II) removal from aqueous solution by a heavy metal-resistance bacterium: Kinetic, isotherm and mechanism studies. Water Science and Technology, DOI: 10.2166/wst.2017.275 [ Links ]
Received: July 26, 2017; Accepted: September 10, 2019











 texto en
texto en 





