1.Introduction
The large energy consumption, contamination, and decreased energy sources are a major problem for the world energy crisis 1. Similarly, the harmful emissions caused by fossil fuels also present a serious threat to our ecological balance. In order to resolve these issues, scientists are seeking newly affordable, effective, and environmentally friendly alternatives to energy resources like wind biofuels, photovoltaics, and thermoelectric energy conversion devices 2. Thermoelectric devices based on the Seebeck effect are responsible for transforming waste heat into usable electrical energy thus minimizing energy waste. These devices are consequently proving to be relatively economical and are a source of renewable energy3-6.
Materials such as perovskites have gained a great deal of attention due to their multifunctional character 7 and have therefore been a major subject of interest. Perovskites with ABO3 structure have been extensively studied for their physical and chemical properties 8-11, by either doping or simply duplicating the lattice sizes resulting in the creation of a double perovskite. Such systems can be more efficient when filling the cation’s site. Double perovskites are a very recent class of materials with a general A2BB’O6 formula where A site is occupied by a rare-earth or alkaline-earth metal, B and B’sites are occupied by different cations (transition/non-transition metals) depending on their charge and the ion size12.
Due to their remarkable properties such as half-metallicity, ferromagnetism, and high thermopower, the transition-metal-based double perovskites have received considerable attention in various technologically important fields, applied and fundamental areas of material science 13,14. Another significant factor in the application of organic-inorganic hybrid perovskites in solar thermoelectric generators is the conversion of sunlight to electricity14,15.
Acharya et al. 16 have evaluated
Sr2TiCoO6based double perovskites modified by aliovalent
substitution of Bi+3 in Sr-site for high-temperature thermoelectric
applications. Further, Saxena et al. 17 have reported the synthesis and thermoelectric properties
of Sr2TiCoO6and Sr2TiMoO6double
perovskites experimentally. They have also confirmed the cubic structure with
Nevertheless, to the best of our knowledge, no detailed studies on the recently synthesized Cubic Sr2TiCoO6double perovskites have been investigated in the literature using ab-initio calculations.
This research was carried out to investigate the structural, magneto-electronic, and elastic properties of Sr2TiCoO6 in its cubic structure from the first principles of DFT calculations, which are followed by the transport properties using semi-classical Boltzmann theory.
2.Computational details
The first principles-based spin-polarized full potential linearly augmented plane wave method (SP-FPLAPW) 19 as implemented in the Wien2k code 20 has been used to calculate highly precise ground state properties of the present material. Two different approximation methods, generalized gradient approximation (GGA) 21 and modified Becke-Johnson (mBJ) 22 were employed for the exchange-correlation potential. The basis set inside each muffin-tin sphere is split into core and valence states. The core states are treated within the spherical part of the potential and are assigned to have spherically symmetric charge density confined within the muffin-tin spheres. The cut-off parameter RMT Kmax in the basis set is chosen to be 7 23 where KMax is the plane wave cut-off and RMT is the smallest muffin-tin radius. The cut-off energy, which defines the separation among the core and the valence states, was set at -8.0 Ry.
400 k-points are used in the calculations. The total charge and energy convergence are taken to be 10-4 Ry and 10-4 a.u3 respectively. Elastic constants calculations have been done within the scheme developed by Charpin 24 as integrated into WIEN2K. The transport properties are calculated within the framework of semiclassical Boltzmann theory by using the BoltzTraP code under the constant relaxation time approximation 25. A dense mesh of 130000 k points was used to obtain accurate transport properties.
3.Results and discussions
3.1.Structural properties
The double perovskite Sr2TiCoO6crystallizes in an ideal
cubic structure as shown in Fig. 1, with
space group
Table I Calculated values of lattice constant a(Å), unit cell volume, bulk modulus B (GPa), pressure derivative of bulk modulus B’ and ground-state energies E0 (Ry) of Sr2TiCoO6 double perovskite.
| Present work | Other | |
| Method | GGA | Exp |
| a(Å) | 7.76 | 7.35 18 |
| V (Å3) | 788.3819 | - |
| B (GPa) | 164.0811v | - |
| B’ | 4.55 | - |
| E0: Minimum total energy per unit cell (Ry) | -18117.909431 | - |
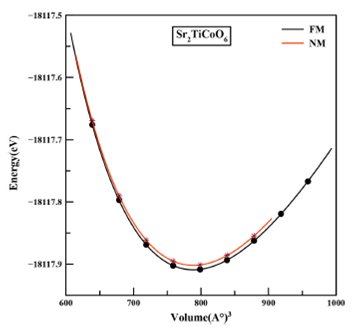
Figure 2 Structural optimization plots of Sr2TiCoO6 in ferromagnetic (FM) and non-magnetic (NM) phases.
The stability of the material was also checked by calculating the tolerance factor (τ) as:
Where rSr is the ionic radius of the Strontium atom, r0 is the ionic radius of the oxygen atom, rTi is the ionic radius of the Titane atom, and rCo is the ionic radius of the Cobalt atom. Materials with τ in the range of 0.9 to 1.0 have a perfect cubic structure, and τ greater than 1.0 results in a hexagonal structure 28. The value of the tolerance factor computed for Sr2TiCoO6is equal to 0.92.
3.2.Electronic and magnetic properties
The spin-polarized electronic band structures of Sr2TiCoO6along the high-symmetry Brillouin zone points have been studied using GGA and mBJ approximations, as presented in Fig. 3. In order to have the correct estimation of the band-gap in spin up channels which is usually underestimated by GGA, mBJ has been used. First, in the case of the spin-up channel, we can distinguish the Fermi level in the middle of the band-gap showing the semiconducting nature. While for spin-down states it is clear that the fermi level is completely occupied, presenting the metallic nature in both approximation methods. As seen from the band profiles using both approximated schemes, half-metallicity is achieved, and the studied material can find various applications in spin-based devices. The value of the gap using GGA is different from the one using mBJ, and it is equal to 1.18 eV, 2.14 eV, respectively, at symmetric points ‘X’ and ‘Γ’, generating an indirect band-gap. The gap increases as we go from GGA to mBJ.
The mechanism of magnetism in the double perovskite (DP) compound has been extensively discussed 29,30,31,32. In the crystal structure shown in Fig. 1, the magnetism in the material can be understood in terms of an ionic description where the Co5+ (3d3) and the Ti4+ (3d2) ions occupy alternating ionic positions along the three axes of the larger cube with consecutive anti-parallel spin alignment, suggesting a total magnetic moment of 1 μB per formula unit (f.u.) for the system. However, the half-metallic states and consequently the magnetic moments are critically dependent on the perfect ordering of the Co and Ti sites. In order to unravel the origin of the magnetism in the Sr2TiCoO6 series, we have first investigated the electronic density of states (DOS) of this compound. Figure 4 shows the DOS as obtained in spin-polarized DFT calculations within GGA-mBJ method. The peaks below -6.2 eV to about -4.5 eV are a mix between the Co-eg d and Co-t2g d states. While the peaks below -1.2 eV refer mostly to oxygen contributions. The peaks crossing the Fermi level and ranging from -1.2 eV to about 0.3 eV belong mainly to Co-t2g d states and some slightadmixture of oxygen p states. The presence of approximate cubic symmetry of the octahedral coordination of oxygen atoms around the transition metal sites results in a splitting of the d levels into d-t2g and d-eg orbitals. The Co-t2g peaks are partially filled in the minority spin channel, while the Co-eg, Ti-t2g, and Ti-eg bands remain empty.
The occupied part of the bands near the Fermi level in the majority spin channel [see Fig. 4] is mainly composed of Co d states, which hybridize with the oxygen p states. The narrow bands lying directly above the Fermi level covering an energy range of about 0.8 to 2.2 eV are mostly from Co-eg and Ti-t2g contributions, while the Ti-eg bands are high in energy.
Furthermore, the calculated individual, interstitial, and total magnetic moments have also been calculated with GGA and mBJ. Our compound has a ferromagnetic nature with a total magnetic moment of nearly 1 μB which is the summation of the partial moments from various atoms and the interstitial sites. Sr, Ti, and O atoms show a very small contribution to the total magnetic moment, which is almost negligible as displayed in Table II. The main contributions to the total magnetic moment come from the Co atom, where its partial moment is of 0.718 μB (GGA) and 0.6662 μB (mBJ). The FM nature for Sr2TiCoO6is predominately due to cobalt atoms. The main source of magnetization in this compound is from unfilled Co-3d orbitals. The positive value of the magnetic spin moment is due to cobalt, whereas the low negative value is attributed to Titane. Comparing to other experimental reported results it is shown that antiferromagnetic-type coupling dominates over weak ferromagnetic coupling in the STC system 33.
Table II Calculated partial, Interstitial and total magnetic moment and band gap (eV) for Sr2TiCoO6 within GGA and mBJ (in Bohr magneton μB).
| Compound | Method | Magnetic Moment (µB) | Band Gap (eV) | |||||
| Interstial | Sr | Ti | Co | O | Total | |||
| Sr2TiCoO6 | GGA | 0.0523 | 0.0029 | -0.0708 | 0.718 | 0.0486 | 0.9972 | 1.18 |
| mBJ | -0.0394 | -0.0026 | -0.0989 | 0.6662 | 0.0796 | 1 | 2.14 | |
3.3.Elastic and mechanical properties
Elastic properties not only provide a decent and dynamic suggestion of the system but also give us better knowledge of the material strength for its technological applications. These constants give concrete and necessary comprehension of solid mechanical stability under different forces.
In this study, we investigated the mechanical and elastic properties of Sr2TiCoO6. The elastic constants were obtained by calculating energy about the tetragonal and rhombohedral strain 34. Only three independent elastic constants (C11, C12, and C44) are used for materials with cubic symmetry. The values of these elastic constants under ambient conditions were determined and shown in Table III. For cubic structures under ambient condition the stability criteria is: (C11 - C12 > 0, C11 > 0, C44 > 0, (C11+2C12 > 0, C12 < B < C11, where C11 stands the compressive resistance along the direction of 𝑋-axis. Also, the value of C11 indicates a larger value as compared to other values, which suggests the incompressible essence of the material, C12 indicates transverse strain, C44 is shear modulus and B represents bulks modulus. The calculated values of elastic constants strictly follow the generalized mechanical stability criteria35.
Additionally, these elastic parameters are related to other mechanical properties through empirical expressions displayed as 36.
Where E and B are Young’s and Bulk’s moduli respectively. v is a Poisson’s ratio and 𝐴 is an anisotropy ratio which are listed in Table III. Bulk modulus describes the stiffness of a material, the higher the value of B, the higher its stiffness resistance is. The calculated value of bulk modulus of Sr2TiCoO6is largely showing strong resistance to volumetric change caused by applied stress. The shear modulus (G), which characterizes the calculated plastic twist of material, has been obtained from the Voigt-Reuss-Hill approximation 37,38 using the arithmetic mean of Voigt, GV, and Reuss GR, presented in Table III and calculated as:
Table III Elastic constants C11, C12, C44 in (GPa), Bulk modulus B (GPa), Shear Modulus G (GPa), Young’s modulus E (GPa), Poisson’s ratio v, Zener anisotropy factor A. Pugh’s ratio (B=G), Cauchy’s pressure (C12-C44), and melting temperature Tm (K) for Sr2TiCoO6.
| Mechanical Properties and Melting Temperature | Alloy Sr2TiCoO6 |
| C11 | 402.1 |
| C12 | 95.6 |
| C44 | 126.3 |
| B | 197.77 |
| GV | 137.08 |
| GR | 135.85 |
| G | 136.46 |
| E | 334.05 |
| v | 0.22 |
| B/G | 1.44 |
| C12-C44 | -30 |
| A | 0.82 |
| T m | 2929.81 |
The value of Young’s modulus (E) was obtained from the bulk modulus (B) and the shear modulus (G) 8. As (E) defines the strength of the material, the higher the value of (𝐸), the higher its strength is. The obtained value of (E) was calculated to be 334.05 GPa, which is large enough, and therefore Sr2TiCoO6acts as a hard material.
Poisson’s ratio ‘v’ is used to evaluate the ductility and brittleness of a compound. The material has a ductile nature if its value is greater than 0.26; otherwise, the material is considered to be brittle. Table IIshows that Poisson’s ratio for Sr2TiCoO6was found to be lower than 0.26, which confirms its brittle nature. Poisson’s ratio ‘𝜈’ also gives knowledge about the estimation of bonding nature 39,40 in a material. According to this limit, the type of material bonding will be ionic if ≈0.25, covalent if ≈0.10, and metallic if ≈0.33. For our compound, 𝜈 is found close to 0.25 and thus major bonding in these materials is ionic.
The Pugh’s ratio 41 is another
stability criterion to define whether the material is ductile or brittle. Its
numerical or index value is
The brittle nature was also verified from Cauchy’s pressure relation 42 defined as (C12-C44). A positive value indicates that the material is ductile otherwise the nature is identified as brittle. For Sr2TiCoO6it is clear that Cauchy’s pressure is negative which emphasizes its brittle nature.
Zener’s anisotropy factor (A) describes the degree of the elastic anisotropy of the solid. For an isotropic material, the anisotropy factor (A) is equal to one, while any different value shows anisotropy 43. From Table III, it was found to be 0.82 (less than 1), signifying that the material has elastic anisotropic nature.
There are no theoretical or experimental data on the elastic properties of this compound for which we can compare our work. We believe that in the future, our work can encourage further research in this direction.
The melting temperature is also a further thermodynamic quantity which has been calculated using the following empiric expression 44 as described below.
The corresponding observed value for the present alloy is 2929.81 ± 300 K and thus suggests that the material has a strong capability to maintain its crystal structure over a large range of temperatures.
3.4.Thermoelectric properties
Thermoelectric materials (TE) have the potential to convert waste heat into usable energy 45. Because of their energy convergence management, these materials are currently being studied at faster levels rather than other technologically appropriate materials.
To explore the electronic transport properties of
Sr2TiCoO6layered perovskite, we have made use of
semi-classical Boltzmann theory as employed in BoltzTraP code 25. In order to have potential
thermoelectric properties in a material, it needs to have a large value of
electrical conductivity (
In this article, the thermoelectric parameters in the range of temperatures from 100 K to 1000 K have been reported, in Fig. 5(a). The electrical conductivity per relaxation time (σ/ τ) as a function of temperature for Sr2TiCoO6is represented, we can observe that electrical conductivity increases with increasing temperature until reaching a maximum value of 2.5488 X 1018 (Ωms)-1 at 1000 K. Electrical conductivity is about 19.189 x 103 at low temperatures (100 K). The variation of the Seebeck coefficient (S) as a function of temperature is shown in Fig. 5b). It is clearly apparent from the plot that the Seebeck coefficient (S) decreases to a minimum of 393.15 μV/K with an increase in temperature. The Seebeck coefficient is positive, meaning 𝑝−type conduction. At 100 K, a maximum value of 3068.24 μV/K was obtained. Figure 5c) depicts the response of electronic thermal conductivity (κe/τ) within 100 K and 1000 K for Sr2TiCoO6. The figure clearly shows that the nature of (κe/τ) is increasing with increasing temperature. The value of (κe/τ) increases from 18.09 W/mKs at 100 K to 5.58 x 1014 W/mKs at 1000 K. In order to estimate the thermoelectric efficiency of Sr2TiCoO6, we calculated the fluctuation of the power factor over a large temperature range as shown in Fig. 5d). We can observe that PF increases slightly with rising temperatures, from 1.806 x 1011 (W.m-1.K-2s) at 100 K until reaching the value of 3.939 x 1011 (W.m-1.K-2s) at 1000 k. The thermoelectric values found for Sr2TiCoO6are definitely higher to those reported experimentally for BaxSr2-xTiCoO6 with x = 0.0, x = 0.10, x = 0.15, and (d) x = 0.2 46. The obtained results from the first principles calculation reveal that Sr2TiCoO6is a promising thermoelectric material.
4.Conclusion
The first-principles calculations have been performed to predict the structural parameters, elastic, magneto-electronic, and transport properties of Sr2TiCoO6 double perovskite. GGA and TB-mBJ potential were used for exchange-correlation. Sr2TiCoO6 is stable in a cubic structure with a lattice constant of 7.76 Å. The lattice constant from our calculations is found to agree well with the experimental data. The calculated elastic constants have been found to follow the mechanical stability criteria. Cauchy pressure, Pugh’s and Poisson’s ratio altogether confirm that Sr2TiCoO6is brittle in nature. Also, the material was determined to have a high melting temperature value equal to 2929.81 ± 300 K. The electronic results display an indirect half-metallic nature, semiconducting in spin-up channels and metallic in spin-down channels.
Total and individual magnetic moments indicate that the Co atom is responsible for ferromagnetism. Transport coefficients such as Seebeck coefficient, electrical conductivity, thermal conductivity, and power factor have been measured by using BoltzTraP. The envisaged overall properties validate that this alloy is a promising candidate for applications in thermoelectric and spintronic applications.











 nova página do texto(beta)
nova página do texto(beta)






