Introduction
The use of bismuth oxide (Bi2O3) in the industry is quite broad because it is an important semiconductor with excellent optical and electrical properties such as high refractive index, high dielectric permissiveness and good photoconductivity, Bismuth oxide is found in six different polymorphic structures: two of them are the most stables phases (α and δ with crystalline system monoclinic and cubic centered on the faces, respectively); the principal applications of this phases of bismuth oxide are as catalysts due to its low bang gap, high temperature superconductors, fuel cells, sensors, ionic conductors, high temperature superconductors, functional ceramics and optical coatings. While β (tetragonal), γ (body centered cubic), ε (orthorhombic) and ω (triclinic) are considered metastables phases of the material and their most important applications are in the manufacture of high quality optical fiber, doped bismuth oxide fibers, photoelectric materials, clear ceramic glass, oxide varistors, electrical sensors and doped high temperature superconductors [1],[2],[3],[4].
In the same way, the metallic bismuth (Bi) has several industrial applications, as a component in alloys for low-melting welds, it is used to produce polonium in nuclear reactors and tetrafluorohydrazine, also in oxidation catalysts, in high temperature superconductors and has given way to the production of lead-free soldering with tin, bismuth and silver alloys. This metal is unique due to its electrical properties, it could be a thermoelectric material at room temperature, also it has a large number of industrial applications among which are: component of low melting point alloys, bismuth compounds have been used for many years to treat medical disorders, dental prostheses and medicines, in fact, currently it is used in some commercial drugs such as peptobismol. The metallic bismuth has three allotropic forms, the most stable is the rhombohedral system, the transition to cubic system is carried out at temperatures close to the melting point at 271 °C, hexagonal system is obtained at a lower temperature than the transition of the cubic system.
Carbon is an important chemical element, it is an essential component of the petroleum, natural gas and mineral carbon. The compounds of carbon are classified as organic materials in his five allotropic forms, which are, diamond, graphite, graphene, nanotubes and fullerenes. The diamond has a sp3 hybridization, and due to its structural arrangement is the strongest material in the world, for this reason, it is used as cutting and abrasive material. The graphite has a sp2 hybridization with a hexagonal crystalline structure, the electrons can travel freely through the material, and therefore, it is an excellent electricity conductor, and due to its layered arrangement it is used as lubricant. The graphene is a very hard, resistant, flexible and a very light material, it is formed by layers of six rings in hexagonal arrangement, it is a good conductor of heat and electricity and remains in very stable conditions when it is subjected to big pressures, the most common way to get graphene is breaking the forces of Van der Waals that join the layers of graphite. The carbon nanotubes are a specie of rolled graphite sheets, which consist of rings formed by 6 carbon atoms. It has very varied electrical properties according to their geometry and the number of layers that form the nanotube, in addition to its mechanical resistance. The fullerenes are obtained by subjecting graphite to laser radiation. A fullerene consists of a spherical network of atoms connected in rings of 5 and 6 members, the rings are arranged in such a way that there are no similar members together, that is, they are always separated by 6-membered rings. Each carbon atom is attached to three more atoms by covalent bonds, in a trigonal planar structure [5],[6].
Microwave's electromagnetic radiation is in a range of frequencies from 0.3 GHz to 300 GHz with wavelengths in a range of 1 m to 1 mm, these characteristics cause microwaves be included in the radiofrequency waves [7],[8]. One of the most important microwave's applications is the domestic microwave oven, which operates at a frequency of 2.45 GHz, in a power level of 500 to 1200 W. Electromagnetic waves have a magnetic component and an electric component. The electrical component of an electromagnetic wave can interact with the materials in three ways, being absorbed, reflected or transmitted. In function on this interaction we can find the following groups of materials: a) absorbent materials of microwaves that are the most important classification, because they are able to absorb electromagnetic waves and as a consequence of this release heat, these materials are commonly known as dielectric materials, and among them we can mention the glass, paper, bakelite, etc., b) reflective materials of microwaves that do not allow the passage of the electromagnetic radiation through them, therefore they reflect it, these are materials conductors with free electrons, between which we can find mainly metals and metallic alloys, c) transmitter material of microwave are characterized by being transparent to this type of electromagnetic radiation, that is, they allow the passage of the waves through them with a small attenuation and are known as insulating materials, some examples are: quartz and ceramics [9],[10],[11].
The interest in phase transformation of bismuth trioxide has become important in recent years, because each phase has different properties and with them can be used the same material in a great variety of applications, for example the change of bismuth oxide phase improve the conductivity properties of the material, Later, Harwig and Gerards studied the conductivity and they found to increase 3 orders of magnitude at the α to δ transition [12].
In this investigation, a series of experimental procedures were carried out with the objective of obtain structural changes in the bismuth trioxide to achieve a phase change of the material and with it, different properties, using microwave's electromagnetic radiation on blends of graphite and bismuth trioxide.
Bismuth trioxide and carbon (in its allotropic form of graphite) were used to carry out a phase change in α-Bi2O3, in addition to obtaining bismuth structures, from blends of these materials (C-Bi2O3) and by the application of electromagnetic energy of the microwave, below is a brief summary about the electromagnetic radiation in general, the generation of microwaves inside a conventional microwave oven and the interaction of this type of radiation with the materials [13]. After the change of bismuth trioxide to a metastable phase, the increase of the capacitance was studied in composites of Al2O3-C-Bi2O3, analyzing the effect of the blended time, composition of C/α-Bi2O3 and time irradiation.
Experimental details
Graphite powder obtained from commercial rods (SPI Supplies Division, Code 7782-42-5) having a purity of 99.99% and Bi2O3 Sigma-Aldrich with a purity of 99.999% (CAS 1304-76-3) were used as reactants.
The blending of the C/α-Bi2O3 reagents was carried out in an agate mortar. Once the reagent mixture is ready, it is introduced into a quartz cell, which is 1 centimeter in diameter and 6 centimeters in length, trying to distribute it by covering as much of it as possible, then the cell is placed on a ceramic support inside of the microwave oven and the irradiation time is programmed. A Panasonic microwave oven model NE-1258R with power level ranging from 120 to 1200 W and frequency of 2.54 GHz was employed.
The synthesized samples were divided into three groups according with the variable of study: time of blending of reactants, microwave's power level and the variation of the weight ratio C-Bi2O3 and time of irradiation.
The first group of experiments consisted of two samples, in which the time employed for the synthesis in the microwave oven at 1200 W of power level was 30 s and the weight ratio was constant in equal parts (1:1) adding 0.1 g of each reactant, the only modification between this samples, was the time of blending the reactant mixture before the microwave irradiation, employing first the blending during 1 min and for another sample 2.5 min of blending as is showing in the Table 1, section of influence of time of blending of the reaction. For the second group of experiments the weight of the reactants was 0.1 g for bismuth oxide - 0.1 g of graphite and time of irradiation was 30 s, in this group, the microwave's power level was studied in samples which the power level was established in 50%, 80% and 100% in a microwave which maximum value is 1200 W. The group of experiments is shown in the Table 1, section of i Influence of the microwave's power level in the synthesis. Finally, a third group of experiments was formed by seven samples (Table 1, section of the effect of weight ratio and time of irradiation), for all the samples the composition of graphite was 0.1 g and the power level of microwave was 1200 W. The first three samples were synthesized during 1 min in the microwave and weight ratio C-Bi2O3 of 1:10, 1:5 and 1:1. The next three samples have the same characteristics in composition in weight of reactants, but the time of irradiation is increased, at 2 min and a half. The last sample is 1:10 weight ratio and 5 min of irradiation.
Table 1 Parameters of synthesis.
| Influence of the time of blending of the reaction | |||
|---|---|---|---|
| Weight ratio | Weight C/α-Bi2O3 (g) | Time of blending (min) | Time of irradiation (s) |
| 1:1 | 0.1/0.1 | 1 | 30 |
| 1:1 | 0.1/0.1 | 2.5 | 30 |
| Influence of the microwave’s power level in the synthesis | |||
| Weight ratio | Weight C/α-Bi2O3 (g) | Microwave power level (%) | Time of irradiation (s) |
| 1:1 | 0.1/0.1 | 50 | 30 |
| 1:1 | 0.1/0.1 | 80 | 30 |
| 1:1 | 0.1/0.1 | 100 | 30 |
| Effect of the different weight ratio and time of irradiation | |||
| Weight ratio | Weight C/α-Bi2O3 (g) | Time of irradiation (min) | Microwave power level (%) |
| 1:10 | 0.1/0.01 | 1 | 100 |
| 1:5 | 0.1/0.02 | 1 | 100 |
| 1:1 | 0.1/0.1 | 1 | 100 |
| 1:10 | 0.1/0.01 | 2.5 | 100 |
| 1:5 | 0.1/0.02 | 2.5 | 100 |
| 1:1 | 0.1/0.1 | 2.5 | 100 |
| 1:10 | 0.1/0.01 | 5 | 100 |
Sample characterization
The characterization by X ray diffraction was performed in a Rigaku Miniflex 600 diffractometer, with a Cu Kα (λ = 1.54 Å) lamp at 40 kV and 15 mA. All the measurements were performed in symmetric geometry (θ-2θ), with scans of 3 to 100 degrees at a velocity of 3 °C/min. The characterization by scanning electronic microscopy was performed in a microscope JEOL, model JSM-7800F with a resolution of 1.2 nm. at 1 kV of acceleration and 0.8 nm at 15 kV.
Capacitance measurements
After the phase transformation by microwave, an Al2O3/C/ß-Bi2O3 composite for each sample was elaborated, alumina was employed as support due to it doesn't have any value of capacitance in it pristine state. 0.39 g of alumina was blended with 0.01 g of the sample of graphite and bismuth oxide obtained after microwave irradiation, the homogeneous mixture was placed in a press and it was applied a force of 2 ton. This composite was placed between two aluminum sample holders for SEM of 12.5mm diameter, 3.2 mm χ 8 mm type pin and wrapped in parafilm to avoid air currents and fastened with metal clips. Resistance and capacity of each composite was measure with a Digital LCR meter model LCR700 in resistivity and capacitance mode, respectively, all the samples were measured at 1 kHz of frequency. All this system is shown in Figure 1. The measurements of the electric parameters were determined in cylindrical capacitors of 2mm thickness and 13 mm of diameter for all the samples.
Results and discussion
X-ray diffraction analysis was performed by Match to know the phase of bismuth oxide in each sample. First, the characterization of the reagents was achieved, the results show α-Bi2O3, which belong to the crystallographic chart 27 0053, the result correspond to a monoclinic system with cell parameters of a=5.848 Å, b=8.166 Å, c=7.51 Å and angle ß=113° and a space group P21/c (14). While the graphite 2H coincides with the crystallographic chart 89-7213, this result belongs to the hexagonal system with space group P63/mmc (194) and cell parameters a=2.464 Å and c=6.711 Å. These results are shown in Figure 2.
Effect of blending time
The results of the next two samples corresponds to the study of the reactant's blending time previous to the irradiation stage, all of them have a weight ratio 1:1 and they were irradiated with microwaves during 30 s.
XRD analysis of the two samples at different time of blending of the reactants before irradiation (Figure 3a and 3b, black lines), demonstrated that the initial samples were formed by hexagonal graphite and monoclinic bismuth oxide agree with PDF charts 89-7213 and 27-0053 respectively, after irradiation of 30 s, the sample blended during 1 min previously, change the phase of the reactants from α-Bi2O3 to a product of ß-Bi2O3 (Figure 3a, blue line), the results corresponds to 65-1209 PDF chart of tetragonal Bi2O3 with lattice of a= 7.7425 Å, c=5.6313 Å with space group P-421-c(114), Figure 3a (red dotted line) also shows the reflections of hexagonal graphite in the planes (002) and (101); SEM micrograph of Figure 3a let observe the distribution of the particles in the mixture, the dark particles belong to the graphite and the clearest ones corresponds to bismuth oxide with tetragonal system and irregular morphology. In the sample blended during 2.5 min before irradiation the change was to ß-Bi2O3 and metallic bismuth as is shown in Figure 3b, where the XRD pattern demonstrated the presence of a mixture of phases in the green line, where a phase change for bismuth oxide from monoclinic to tetragonal Bi2O3 with lattice of a=7.7425 Å, c=5.6313 Å with space group P-421-c(114), metallic bismuth with rhombohedral crystalline system with red lattice of a=4.53 Å, c=11.81 Å and alpha bismuth oxide didn't react is observed, also the red dotted line correspond to the main reflections of 2H graphite, pink ones correspond to tetragonal bismuth oxide and the dotted blue lines for metallic bismuth, agree with charts 89-7213, 65-1209 and 85-1329 respectively The product obtained after irradiation is a mixture of irregular particles of graphite, monoclinic and tetragonal Bi2O3 and metallic bismuth. SEM micrographs show a graphite structure major than 10 μπι with structures of 1 micrometer of bismuth oxide with morphology type needle. In both cases were demonstrated that the time of 1 min of blend before irradiation is enough to achieve a phase change in the bismuth oxide, while the graphite doesn't have any structural or morphological change, the rest of the peaks correspond to α-Bi2O3 not transformed.
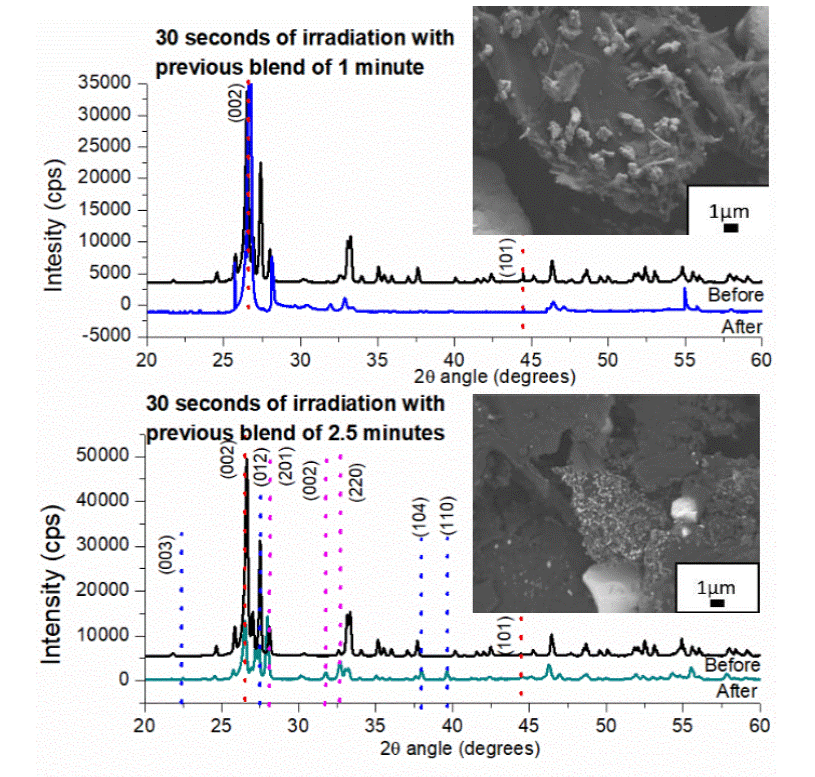
Figure 3 XRD patterns of the initial sample with a previous mixture of a) 1 min and b) 2.5 min; (black-line) before and (color-line) after 30 s of microwave irradiation. Along with SEM image of irradiated sample.
The resistance and capacitance were measured in both samples after irradiation, the results with numeric values and their behavior are shown in Table 2. An increase in the capacitance is observed in both cases, however, the sample blended prior to irradiation for only 1 min shows a 97.37% increasing, it is due the phase transition of monoclinic to tetragonal system in the bismuth oxide compound, in the second sample the value of capacitance only increases 46.73% and this is attributed to the characteristic phase change of the bismuth oxide system and the presence of metallic bismuth that prevents the increase of the capacitance. From the results obtained in this experimental section was verified that the ideal blending time before irradiating with microwaves is 1 min to avoid the formation of metallic bismuth.
Microwave's power level effect
Once the optimum blending time prior to irradiation is determined (previous section), the next study corresponds to the power level effect of three samples, they were carried out with a weight composition of equal parts of reagents (0.1 g each one), mixture of 1 min before irradiation and time of 30 s inside the microwave, the difference between each one of these samples is the power level of the microwave oven used for each synthesis which are 600, 960 and 1200 W respectively. The X-ray diffraction analysis of the sample irradiated at 600 W of microwave's power level demonstrated no change in the crystalline structure of the Bi2O3 (Figure 4a), SEM micrographs show the product of reaction of the samples at this condition, where is observed bismuth oxide agglomerates of 30 - 50 μπι on graphite particles of 30 μm; the sample irradiated at 960 W, as in the previous sample no change in the crystalline structure of Bi2O3 was observed, (Figure 4b). Finally, at 1200 W, the irradiated sample shows a change of morphology as is observed in the micrograph of the Figure 4c, where type needle micro structures of 1 micrometer average was obtained. XRD pattern demonstrated also a structural transformation, where a mixture of bismuth oxide phases was obtained, majority a change of phase from alpha to beta bismuth oxide was determined by this technique, pink dotted lines in Figure 4 show this transformation, however, characteristic reflections of metallic bismuth were found too, corresponding to blue dotted line in Figure 5c, agree with chart 85-1329.
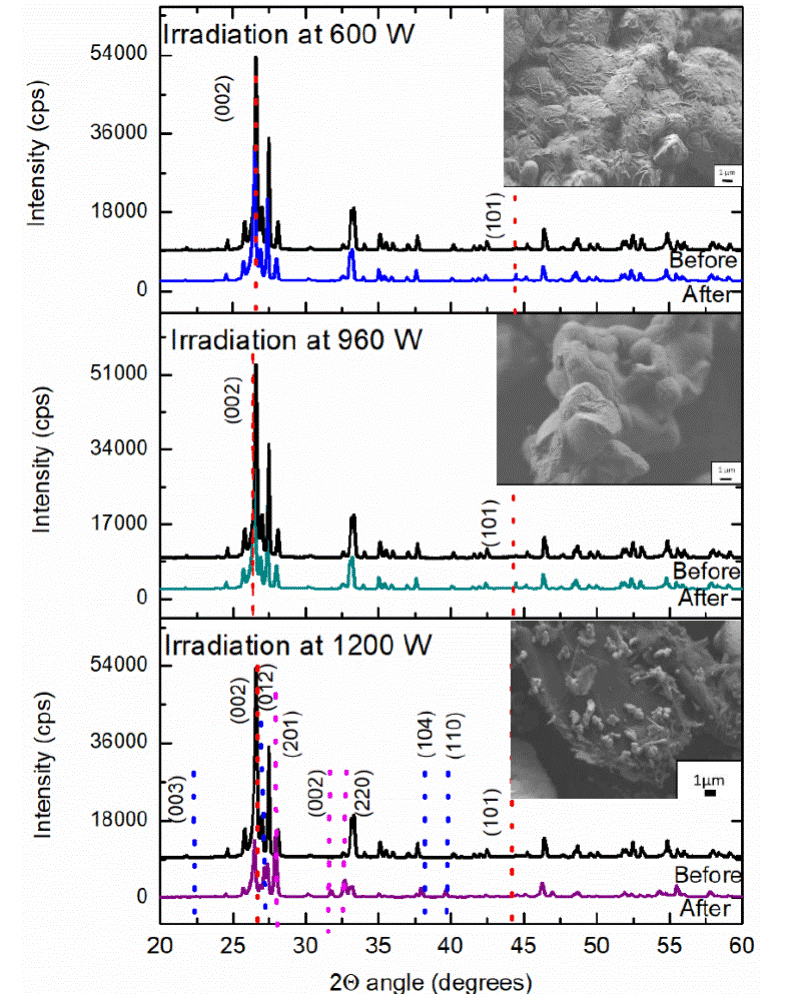
Figure 4 XRD patterns of the initial sample (black line) before, and (color line) after 30 s of microwave irradiation at a) 600 W, b) 960W, and c) 1200 W. Along with SEM image of the irradiated sample.
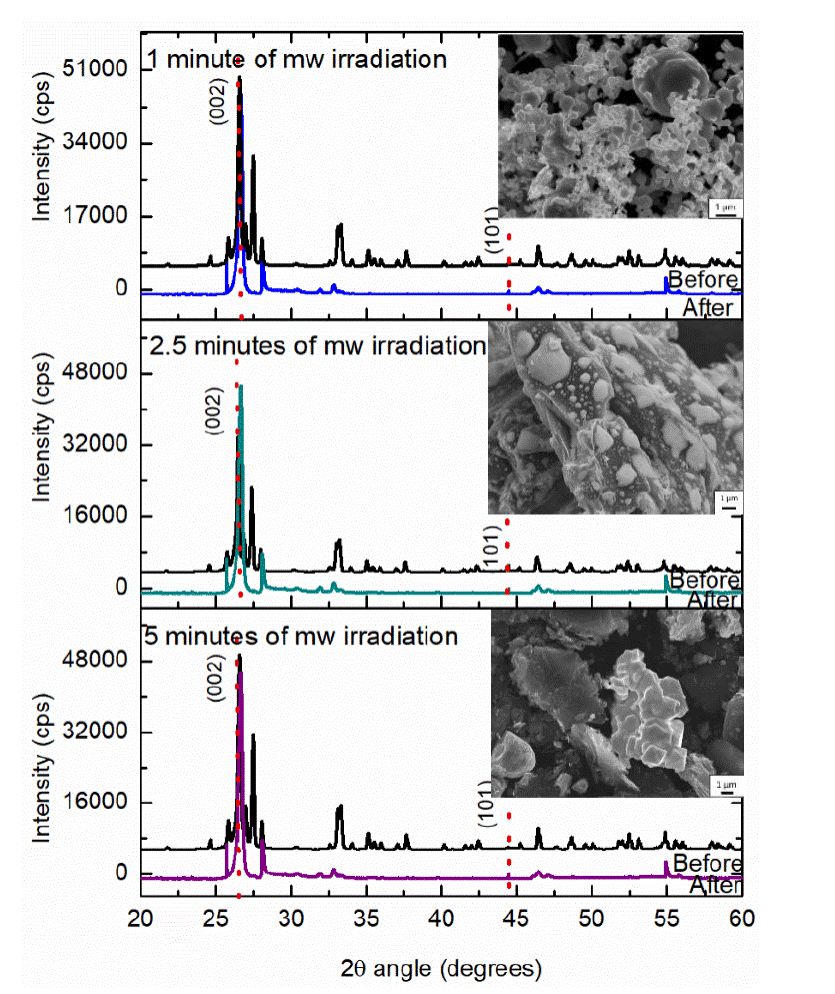
Figure 5 XRD patterns of the initial sample (black line) before, and (color line) after microwave irradiation at 1200 W during a) 1 min, b) 2.5, and c) 5 min. Along with SEM image of the irradiated sample.
Capacitance of each sample, before and after irradiation during 30 s at 600, 900 and 1200 W of microwave's power level was measured, the results are showing in Table 3, where values of resistance decreases after irradiation in each sample and capacitance increase in the three samples. At 600 and 960 W, the change is not significant due to no change in phase was obtained, or it was in very little portion to be measured by XRD. The sample irradiated at 1200 W demonstrated the power level enough to get a phase transition from a- Bi2O3 to ß- Bi2O3 and metallic bismuth, for this reason, all the experiments was made at 1200 W and other variables were modified in order to obtain only bismuth oxide phase change.
Table 3 The resistance and capacitance of the samples with different irradiation power level.
| Power level (W) | Resistance (MΩ) | Capacitance (pF) | Percent capacitance increase (%) | ||
| Before MW | After MW | Before MW | Before MW | ||
| 600 | 7.52 | 2.40 | 24.32 | 25.04 | 2.97 |
| 960 | 6.646 | 2.00 | 27.22 | 28.34 | 4.11 |
| 1200 | 6.32 | 1.90 | 29.70 | 58.62 | 97.37 |
Effect of irradiation time
The first sample for this experimental section was formed by 0.01 g of α-Bi2O3 and 0.1 g of graphite and blended during 1 min before irradiation, after, this sample was irradiated during 1 min at 1200 W of microwave's power level, X Ray Diffraction shows a blend of the reactants in Figure 5 a (black line), the analysis demonstrated that the initial sample is formed by the monoclinic phase of bismuth oxide and hexagonal graphite, while the green line shows the phase transformation from a or monoclinic to ß or tetragonal bismuth oxide, with lattice parameters of a=7.7425 Å, c=5.6313 Å and space group P-421-c(114) agree with PDF chart 65-1209. Graphite doesn't have any modification in his crystalline structure. The micrographs of the samples synthesized with different time of irradiation demonstrated not homogeneous distribution and morphology; the samples irradiated during 1 min show particles of graphite of approximately 3 to 5 μm surrounded by Bi2O3 microspheres of 500 nm to 1 μm of size (Figure 5a). The sample irradiated for 2.5 min, shows the same results from a to β bismuth oxide by XRD (Figure 5b) and the micrograph shows graphite particles surrounded by bismuth oxide with appearance of melted material. And finally, at 5 min of irradiation with microwaves, XRD results, shows the characteristic transformation from a to ß bismuth oxide, and the peaks of the bismuth oxide in the diffractogram are overshadowed by the graphite signal; this sample shows a structure with pyramidal morphology as is shown in Figure 5c.
The capacitance and resistance of the samples of this group of experiments are shown in the Table 4, where the effect of the time of irradiation was studied. The section of the XRD results shows the change of monoclinic to tetragonal phase of bismuth oxide in all the samples, then, the change of the capacitance in each sample could be due to its morphology and size, in the sample irradiated for 1 min the structures of bismuth oxide are smaller than the other ones and they are closer between them with a capacitance value of 209.8%, if the sample is irradiated during 2.5 min, the result is a group of structures with no defined morphology bigger than the previous one and with an increasing of capacitance value of 88.28% respect to the no irradiated sample, finally, the third sample of this group was irradiated during 5 min and increase 57.12% the capacitance in this system, the structure is more compacted and is the biggest of this three samples, with bismuth oxide structures of size from 2 to 5 μπι average, which proves that at bigger contact area there is a greater capacitance as it was demonstrated for other researchers [14].
Table 4 The resistance and capacitance of the samples with different microwave’s irradiation time.
| Irradiation time (min) | Resistance (MΩ) | Capacitance (pF) | Percent capacitance increase (%) | ||
| Before MW | After MW | Before MW | Before MW | ||
| 1 | 7.48 | 1.44 | 22.80 | 70.65 | 209.80 |
| 2.5 | 7.52 | 1.56 | 24.83 | 65.58 | 164.11 |
| 5 | 7.66 | 0.83 | 24.91 | 54.79 | 119.95 |
Effect of the quantity of reactant Bi2O3
The next serial of experiments was synthesized at 1200 W and irradiated for 1 min, for the sample with weight composition of 0.1 g of graphite and 0.01 g of Bi2O3 the results obtained by XRD demonstrated the transition of a to ß Bi2O3 in the first sample and the presence of phase transition of bismuth oxide and metallic bismuth in the rest of the samples. For the first sample, the micrograph shows semi-spherical structures with 500 nm. of size surrounding graphite spheres of 3 - 5 μι^ as can observe in Figure 6a. The next sample (0.1 g of graphite and 0.02 g of Bi2O3) presents similar results in the morphology to the previous one, however, Figure 6b shows the diffractogram of the blend of reactants (black line) and the characteristic change of phase of the monoclinic Bi2O3 to tetragonal Bi2O3 and the metallic bismuth growth (green line), the lattice parameter and space group of the metallic bismuth and ß-Bi2O3 coincide with the crystallographic charts 85-1330 and 65 1209 respectively, for both samples, the micrographs obtained show graphite particles from 5 to 20 μm surrounded by smaller spherical particles from 500 nm to 2 μm composed of Bi2O3 or metallic bismuth agree with XRD results. Finally, for the sample with equal quantity in weight of each reactant (0.1 g), the XRD diffractograms allowed to identify the currency of metallic Bi and the change of monoclinic Bi2O3 obtaining as result a tetragonal crystalline system; the characteristics signals of metallic Bi match with the information of the PDF chart 85-1330, where the rhombohedral crystalline structure is reported, with a space group R-3m (166) and lattice parameters: a = 4,535 Å and c = 11,814 Å. Micrographs obtained by SEM (Figure 6c) show particles of metallic bismuth or Bi2O3 with irregular shape and approximately size of 1 micrometer.
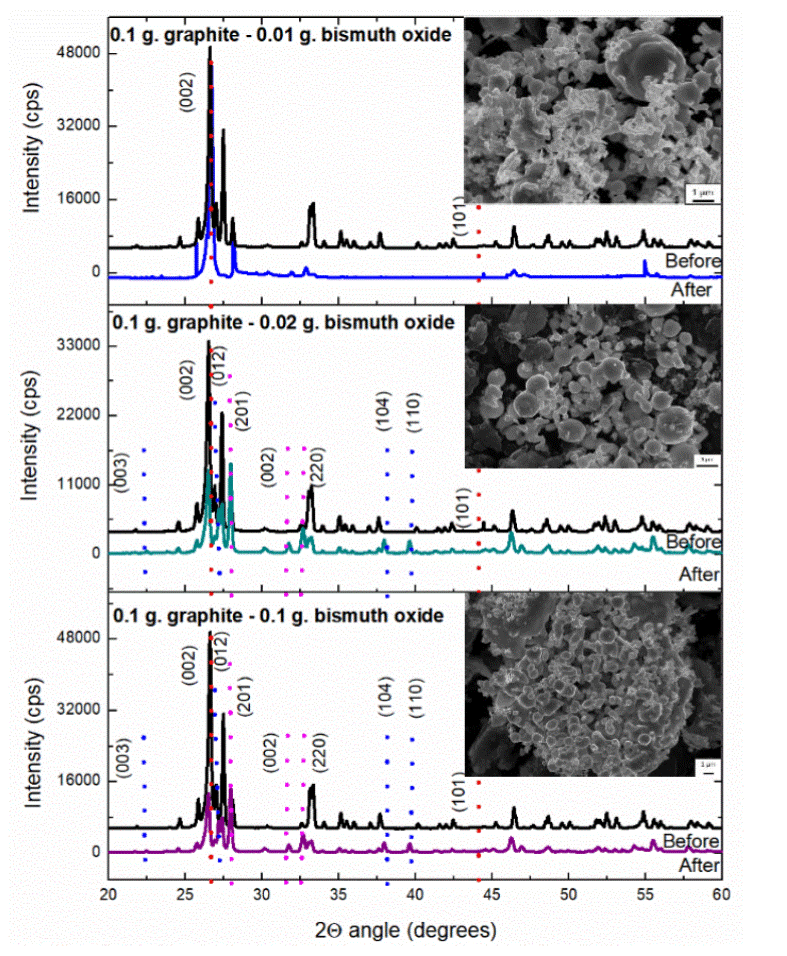
Figure 6 XRD patterns of the a sample (black line) before, (color line) after microwave irradiation during 1 min at 1200 W, of a sample with a) 0.1 g graphite - 0.01 g bismuth oxide, b) 0.1 g graphite - 0.02 g bismuth oxide, and c) 0.1 g graphite - 0.1 g bismuth oxide. Along with SEM image of the irradiated sample.
The capacitance and resistance of the materials in this experimental section show an increasing of the capacitance in all the samples and the values are observed in table 5, the behavior demonstrated an increasing in the capacitance values when the ratio between carbon and bismuth oxide in the sample is observed, this is due to the property of energy absorbance which results in an increase of temperature, the capacitance increase in a larger proportion of 209.8% when a ratio of the reactants is 1:10, where a complete transformation of α-Bi2O3 to ß-Bi2O3 is observed and no other products are obtained, 43.63% for the sample with reactants ratio 1:5 and finally, 27.65% for the sample with equal weight ratio of the reactants; in the last two samples a transformation of α-Bi2O3 to ß-Bi2O3 is observed, however, a reduction to metallic bismuth is achieved, which reduces the capacitance of the composite as is known.
Table 5 The resistance and capacitance of the samples with different ratio of reactants, irradiated during 1 min.
| Reactants ratio (Bi2O3:C) | Resistance (MΩ) | Capacitance (pF) | Percent capacitance increase (%) | ||
| Before MW | After MW | Before MW | Before MW | ||
| 1:10 | 7.48 | 1.44 | 22.8 | 70.65 | 209.8 |
| 1:5 | 6.18 | 3.34 | 29.38 | 42.2 | 43.63 |
| 1:1 | 4.74 | 2.44 | 44.66 | 57.01 | 27.65 |
In the next group of experiments the quantity of reactants and the ratio between them were analyzed as in the previous section, however, in this case the time of microwave's irradiation was changed at 2.5 min. First, the composition of the sample was 0.1 g of graphite and 0.01 g of α-Bi2O3; in the second sample was increased the quantity of bismuth oxide to 0.02 g and constant weight of 0.1 g of graphite; finally, in the third experiment the quantity of reactants is the same, weight of 0.1 g each one. XRD shows the initial sample with the mixture of reactants, where is demonstrated the presence of hexagonal graphite and monoclinic bismuth oxide (PDF charts 89-7213 and 27-0053, respectively) as is observed in Figure 7, black lines. After microwave irradiation, the XRD results of the first two samples in Figure 7-a and -b, show the change of phase of bismuth oxide from a to β and Figure 7c shows the bismuth oxide change and the reduction of the Bi5+ to Bi0. The morphology of the structures obtained with 0.1 g of graphite and 0.01 g of Bi2O3 show melted structures, as in the micrograph of the Figure 7a; forthe sample with 0.1 g of graphite and 0.02 g of Bi2O3, the Figure 7b shows big structures of ß-Bi2O3 with size from 10 to 40 μm and irregular shape; finally, the micrograph of the Figure 7c show the sample with the same weight composition of reactants, where hemispherical ß-Bi2O3 is observed (as the XRD result demonstrated), the size of bismuth oxide particles are approximately 1 micrometer average.
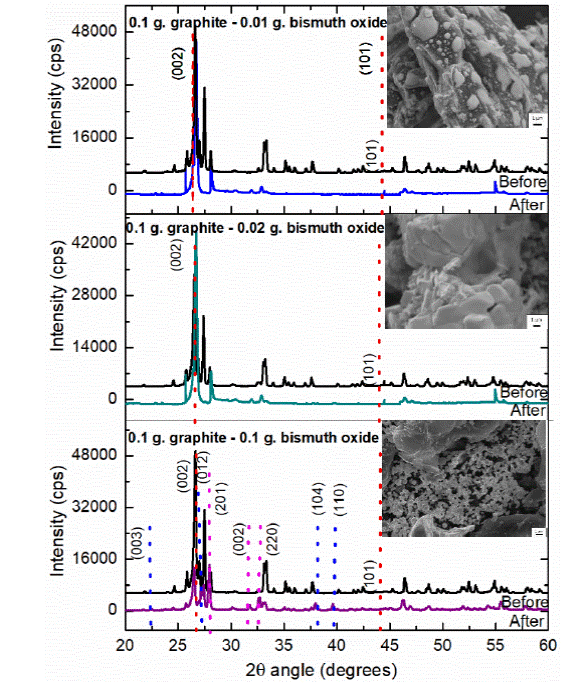
Figure 7 XRD patterns of samples before (black-line) and after (color-line) microwave irradiation at 1200 W during 2.5 min; along with SEM image of the corresponding irradiated sample. For a) 0.1 g graphite / 0.01 g bismuth oxide sample, b) 0.1 g graphite / 0.02 g bismuth oxide sample, and c) 0.1 g graphite / 0.1 g bismuth oxide sample.
Bismuth oxide - aluminum oxide - carbon composites were tested as capacitors, giving the results of Table 6, were the better results are obtained at 1:10 ratio of reactants with a percent of increasing of 88.28%, the results are similar to the sample with ratio 1:5 with 80.87% of capacitance increasing, and finally, the capacitance decrease to 23.82% in the sample with reactants ratio of 1:1 due to during the phase transformation of bismuth oxide, the quantity of photons of microwave is enough to heat the carbon and create metallic bismuth, which is not a good material to be used as capacitor.
Table 6 The resistance and capacitance of the samples with different ratio of reactants, irradiated during 2.5 min.
| Reactants ratio (Bi2O3:C) | Resistance (MΩ) | Capacitance (pF) | Percent capacitance increase (%) | ||
| Before MW | After MW | Before MW | Before MW | ||
| 1:10 | 7.52 | 1.56 | 24.83 | 65.58 | 164.11 |
| 1:5 | 4.32 | 2.88 | 31.79 | 57.5 | 80.87 |
| 1:1 | 3.62 | 1.43 | 49.83 | 61.7 | 23.82 |
In the samples where the change of phase was from alpha bismuth oxide to beta bismuth oxide, the process of phase transformation is due to a rearregement from a monoclinic crystalline system to one with tetragonal crystalline system, the symmetry in all the samples was increased, as well as its capacitance.
Conclusions
The method employed let to obtain easily ß-Bi2O3 and metallic bismuth from α-Bi2O3 blend with graphite. The transformation of crystalline structure from a stable phase (α-Bi2O3 monoclinic) to a metastable one (ß-Bi2O3 tetragonal) was obtained in all the samples irradiated at least 30 s in the microwave oven for all the weight rates. The best rate in weight in all samples to obtain the change to ß-Bi2O3 was 0.1 g for each reactant. The time of blending before irradiation is important due to the distribution of the two reactants, 1 min is enough to get the transformation of crystalline phase, thus, equiaxial structures of 500 nm to 1 μm was obtained at 1 min and 2.5 min of blending and 30 s of irradiation. The effect of the microwave power level in the phase transformation was clear, no change was obtained at 600 and 960 W with a time of synthesis of 30 s and weight compositions of both reactants of 0.1 g, therefore, at these conditions, is necessary to use 1200 W of microwave's power level to get the phase transformation to tetragonal phase of bismuth oxide and metallic bismuth. The obtention of metallic bismuth is adjudge to the temperatures reached during the synthesis by the graphite layers. 1:10 ratio of reactants shows the best results independently of the time of microwaves irradiation due an exclusive α-Bi2O3 to ß-Bi2O3 transformation.











 nova página do texto(beta)
nova página do texto(beta)




