Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.9 no.50 México Nov./Dez. 2018
https://doi.org/10.29298/rmcf.v9i50.235
Articles
Litter decomposition rate of Tamaulipan thorn scrub and an introduced plant species
1Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. México.
Litter decomposition is a key process for nutrients cycling in the ecosystems. Knowledge of the decomposition rate of vegetation litter of an area can helps to a better understanding of soil fertility and to propose sound management practices that favor the availability of nutrients for plants. The objectives of this study were i) to evaluate initial decomposition rate of litter produced in a Tamaulipan thorn shrub area and in a eucalyptus plantation (Eucalyptus camaldulensis) which is an introduced species in the region, and ii) to estimate correlation values between degradation weight loss by the decomposition process and climatic factors (rainfall and temperature). Thirty-two litterbags (1 mm mesh eye) containing litter of shrub and eucalyptus were place on the field. Remaining mass evaluations were made monthly for 6 month, removing four bags each month. Decomposition rate was higher for the thorn shrub (35.11 %) than for the eucalyptus litter (24.91 %). The decomposition constant (k year‒1), was higher for the Tamaulipan thorn shrub with k=0.934 than for eucalyptus (0.479). Decomposition litter mass remaining showed a negative correlation with temperature and evaporation, both for MET and eucalyptus, and only eucalyptus had a negative correlation to rainfall. The deposition of nutrients to the soil, as a result of litter decomposition would be slower in eucalyptus plantations than in MET areas, so the availability of nutrients may be limited at the time that rainfall pulses, which activate plants growth, occur and silvicultural practices must be established accordingly.
Key words: Eucalyptus; soil fertility; lost mass; remaining mass; k values; climatic variables
La descomposición de la hojarasca es un proceso clave en el reciclado de nutrientes en los ecosistemas. Conocer su velocidad de descomposición ayudará a una mejor comprensión de la fertilidad del suelo y a proponer acciones de manejo que favorezcan la disponibilidad de nutrientes. Los objetivos del presente estudio fueron: i) evaluar la descomposición temprana de la hojarasca en un área de Matorral Espinoso Tamaulipeco (MET) y una plantación de eucaliptos (Eucalyptus camaldulensis), especie introducida en la región; y ii) determinar la correlación entre la pérdida de peso de la hojarasca y la precipitación, la temperatura y la evaporación. Para su evaluación se colocaron 64 bolsas de tul (ojo de malla de 1 mm) con hojarasca de MET y eucalipto. Las evaluaciones de masa remanente se hicieron mensualmente, durante seis meses. La descomposición fue mayor en MET (35.11 %) que en eucalipto (24.91 %). La constante de descomposición (k año‒1) fue mayor para MET (k=0.934) que para eucalipto (k=0.479). La masa remanente de la descomposición de la hojarasca mostró correlación negativa con la temperatura y la evaporación, tanto en el sitio de MET como el de eucalipto y solo este tuvo correlación negativa con la precipitación. La deposición de nutrientes al suelo, producto de la descomposición de hojarasca, será más lenta en plantaciones de eucalipto que en áreas de MET, por lo que la disponibilidad de nutrientes pudiera limitarse cuando se presentan los pulsos de precipitación que activan el crecimiento vegetal y las prácticas silvícolas deberán realizarse de acuerdo a ello.
Palabras clave: Eucalipto; fertilidad del suelo; masa perdida; masa remanente; valor de k; variables climáticas
Introduction
Leaf litter is the main source of nutrients in forest soils, and it amounts to 80 % of the total nutrients that return to them (Santa-Regina and Tarrazona, 2001). The deposition of the leaves provides a cover to the soil and favors the edaphic environment. As the leaves decay, they become an important source of organic matter and activate the biogeochemical cycle of the elements (Gleissman, 2002).
In any ecosystem, leaf litter is the most importance source for the recycling of elements such as carbon, nitrogen, phosphorus, and others (Martín et al., 1996; Santa-Regina and Gallardo, 1989; Triska et al., 1984). Besides, it acts as an isolating layer protecting the soil from extreme temperature and humidity changes, it reduces erosion, and it favors water infiltration (Aerts, 1997).
The decomposition rate of leaves and the factors that control it contribute to the understanding of soil fertility and to the proposal of suitable management actions. Some of these factors are the climate, the chemical components, the hardness and thickness of the leaf litter, the decomposing fauna, the microorganisms, and those characteristics of the soil that are most related to the activity of decomposing agents, such as airing and organic matter content.
Thus, the temperature and moisture influence the mineralization of the organic matter. Precipitation plays a significant role, particularly during the first stages of litter decomposition, due to the leaching of labile substances (Salamanca et al., 2003), and, indirectly, in the activation of microorganisms (Brandt et al., 2007).
According to certain authors, the temperature accounts, to a larger or lesser extent, for the decomposition process of litter, as lower temperatures reduce the activity of the decomposer organisms (Trofymow et al., 2002); changes in temperature may affect the composition of the active flora and, consequently, the decomposition processes (Bertsch, 1995).
In their study on leaf litter decomposition in stands located at different altitudes, Shanks and Olson (1961) cite a decrease by an average of 2 % for each centigrade degree of descent in the mean temperature.
The chemical composition of leaf litter also determines its decomposition rate. Basically, two fractions can be distinguished: the soluble fraction, which represents the most labile components of leaf litter, such as sugars and proteins, which decay rapidly during the first stages of decomposition, due to the rapid growth of the microorganisms, favored, in temperate ecosystems, by a high concentration of labile carbon and nitrogen (Swift et al., 1979). The recalcitrant fraction, which has a lower decomposition rate, is made up of waxes, lipids, lignins, lignified carbohydrates, and phenols (Binkley, 1986).
The lignocelluloses index is an indicator of the resistance of leaf litter to microbial attack (Melillo et al., 1982). The lignin content and the lignin index have been inversely related to the decomposition rate of the leaf litter in the south of the Sonoran Desert (Martínez-Yrízar et al., 2007). Eucalyptus particularly has been described as a leading choice for the development of sustainable lignocellulosic biofuels (Healy et al., 2015). The lignin and cellulose contents vary between eucalyptus species, but, in general, Prinsen (2010) cites lignin contents of 26.91 % and cellulose contents of 48.07 % in the woody part of this plant.
Given the rapid growth and the adaptability of various eucalyptus taxa to the climate of northeastern Mexico and their potential use in the production as firewood and in the production of charcoal (Foroughbakhch et al., 2017), there is a possibility that these plantations may gain momentum in the region and displace the native taxa of the Tamaulipan thorn scrub (TTS).
Several studies have shown that the invader vegetal taxa have a variable impact on leaf litter decomposition rates (Reinhart and VandeVoort, 2006; Fargen et al., 2015). Furey et al. (2014) document lower leaf litter decomposition rates in monospecific plots of exotic species than in native plots, and they point out that the leaf litter of the natural mix of native species exhibited a better quality than that obtained from plots with exotic species.
Conversely, according to Jaeger et al. (2013), the leaf litter of Cinchona pubescens Vahl (an invader) decomposes more rapidly than that of native varieties, due to the higher content of N, P, and K in C. pubescens green leaves. This seeming contradiction is probably due to the quality of the leaf litter in terms of the concentration of certain elements, which determines the higher or lower decomposition rate.
There are many studies on the dynamics of the early stages of leaf litter decomposition (Kang et al., 2009); however, most have been carried out in forest ecosystems, and the shrubs are insufficiently represented in them (Zhang et al., 2008).
The Tamaulipan thorn scrub covers a surface area of 200 000 km2 in northeastern Mexico and in southern Texas (Peñaloza and Reid, 1989; López-Hernández et al., 2013); it is a valuable ecosystem, with important species for forest and silvopastoral production (Molina-Guerra et al., 2013), with a considerable ecosystemic complexity (Villarreal and Alanís, 2015) and a great floristic diversity (Muller, 1939).
Although the TTS covers a vast surface area, and despite its ecological and economic importance, studies on the dynamics of the decomposition of its leaf litter are extremely scarce.
Their short-term usefulness for the prediction and development of maximum limit of decomposition is debatable (Berg and Ekbohm, 1991; Harmon et al., 2009); however, they have been proven to be helpful for characterizing the initial stages of the decomposition process, and they allow reliable predictions of the decomposition rates of the litter of most ecosystems (Prescott, 2010).
The objectives of the present study were: to compare the decomposition rate of the litter in its early stages in a Tamaulipan thorn scrub (TTS) site consisting of native species, and in a plantation of Eucalyptus camaldulensis Dehnh., an introduced species, and to determine the correlation between the loss of weight resulting from the decomposition process of the leaf litter and the precipitation, temperature, and evaporation.
The first hypothesis was that eucalyptus leaves have a slower initial disintegration process than the Tamaulipan thorn scrub due to the physical and chemical characteristics of each type of leaf litter, since the climate and soil factors are identical for both types. The second hypothesis was that, in the study area, temperature has a higher correlation with leaf litter decomposition than rainfall.
Materials and Methods
Description of the study area
The study area is located within the campus of the Facultad de Ciencias Forestales de la Universidad Autónoma de Nuevo León (School of Forest Sciences of the Autonomous University of Nuevo León), UANL, at 24°47´N and 99°32´W), in the physiographic province of the Planicie Costera del Golfo Norte (Coastal Plain of the Northern Gulf region), close to the Eastern Sierra Madre.
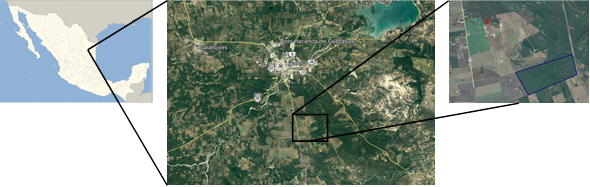
Red = Eucalyptus plantation; Blue = Tamaulipan thorn scrub.
Figure 1 Satellite image of the Linares campus of UANL, on which the study sites have been delimited.
The topography of the selected sites is flat or has a very slight slope, of 2º to 5º, according to O. K. Leontiev and G. I. Richagov (Lugo, 1989); their altitude is 350 m. The soils are deep, dark vertisols (FAO, 1988) of alluvial/colluvial origin, with a high content of clay, a moderately alkaline pH (6.5 -7.3), and a high content of calcium carbonates (Woerner, 1991).
According to Köppen’s classification, modified by García (1973), the climate of the area is semiwarm-subhumid, with two very irregular summer rainy seasons -March to June and September to October-, very warm summers, and occasionally severe frosts in winter. The mean annual precipitation is 805 mm, of which approximately 600 mm occur during the two summer periods mentioned above, with the highest precipitation in September. The rest of the rainfalls -around 20 %- occur in the autumn and winter, and the annual evapotranspiration, estimated using Thornthwaite’s method, reaches 1 150 mm (Návar et al., 1994).
The mean annual temperature is 22.6 °C, with a wide variation through the year; the maximum monthly average (39.8 °C) is registered in July, and the minimum monthly average (3.7 °C), in January (Camacho station, 1971-2000 data) (SMN, 2012).
Study sites
The study sites were located in an area originally occupied by the Tamulipan thorn scrub, which consists of a dense, widely diverse vegetation of shrubs and trees characterized by a broad interval of taxonomic groups, with differences in the habits of growth, leaf length, and phenology (López-Hernández et al., 2013). Associations of high or middle-sized shrubs with high trees prevail: thorny species with compound leaves, among which Acacia berlandieri Benth., Cordia boissieri A.DC., Karwinskia humboldtiana (Schult.) Zucc., Bumelia celastrina Kunth, Acacia rigidula Benth., Acacia farnesiana (L.) Willd., Cercidium macrum I. M. Johnst., Havardia pallens Benth., and other Prosopis species stand out for their abundance and coverage (Estrada and Marroquín, 1988).
The study sites were established in the TTS and in a eucalyptus plantation. The former has remained unused at least during the last thirty years, and it has a surface area of 10 hectares. The Eucalyptus camaldulensis plantation dates back to 1988 and covers a surface of 1.528 hectares.
The data for mean monthly temperature, rainfall and evaporation for the months of the experiment (Table 1) were obtained from the weather station of the School of Forest Sciences (FCF). The ombrothermic diagram constructed with the data of monthly rainfall and temperature in the year 2011-2012 (Figure 2), when the experiment was carried out, allowed the identification of a period of hydric deficit from November 2011 to April 2012.
Table 1 Climatological data for the dates of the leaf litter decomposition experiment (November 2011 - June 2012).
| Sampling month | Mean monthly precipitation (mm) |
Mean monthly temperature (oC) |
Mean monthly evaporation (mm) |
|---|---|---|---|
| November | 5.0 | 19.4 | 95.8 |
| December | 3.5 | 16.6 | 92.35 |
| January | 32.0 | 14.5 | 91.16 |
| February | 3.0 | 19.1 | 118.98 |
| March | 10.2 | 20.1 | 151.15 |
| April | 19.6 | 23.2 | 162.87 |
| May | 90.9 | 25.1 | 174.56 |
Field and laboratory work
The litter was collected in October, 2011, using five mesh baskets, with an approximate surface of 0.25 m2, at specific points in the TTS site (Figure 3) and in the eucalyptus plantation. A mix was made with the litter collected in each site and placed in tulle bags of 8 ×10 cm with a 1 mm mesh, each filled with up to 10 g of litter from the TTS and 6 g of the plantation litter. The bagged mixes were then collected in the study site (November, 2011) and distributed at random in four points at random (replications) in each site. The number of bags per point was eight, adding up to a total of 32 per site. The difference in the initial weight of the leaf litter in each bag was due to the greater availability of litter in the TTS at the start of the experiment.
Five additional bags of litter from each site (TTS and eucalyptus plantation) were dried in a (Riossa HCF-102-D) digital oven in order to establish the conversion of fresh weight to dry weight utilized to determine the loss of mass and the remaining mass on the different sampling dates.
Every month, from January to June, 2012, a minimum of four litter bags (one per point) were collected and taken to the laboratory, where they were cleaned and washed in order to remove any accumulation of earth that might alter their weight, and they were subsequently dried at 60 °C in a (Riossa HCF-102-D) digital oven, until a constant weight was obtained. The weight loss was utilized as an indicator of the decomposition of the litter.
Statistical analyses
The values of the remaining mass of litter (n=4) per site for each month were compared by means of a single-factor variance analysis, followed by a Tukey test (α=0.05) in order to determine the months with significant changes in weight loss. The assumptions of normality (Shapiro-Wilk) and homoscedasticity (Levene’s test) were previously corroborated.
The percentage of remaining mass of litter was calculated using the following equation:
Where:
w 0 = Dry weight of the sample in the tulle bag before being placed in the field
w t = Dry weight of the same content between the time when the litter bags were placed in the field until the time when they were collected
The value of the composition constant (decomposition rate) k (k year-1) was calculated using SigmaPlot 12.5 (Systat Software Inc.,) for the entire duration of the experiment (January - June, 2012). Estimations were carried out for each repeat (n=4), in order to statistically compare the value of k between the TTS and the plantation. k was estimated using a negative exponential model (Olson, 1963) based on the percentage values of remaining mass and the time:
Where:
X 0 = Percentage of the dry weight of the leaf litter at the starting time (year)
X t = Percentage of the dry weight of the leaf litter at time t (year)
k = Decomposition constant (year-1)
Pearson’s correlation analyses were carried out to determine whether there was a correlation between the climate variables (temperature, rainfall, and evaporation) and the loss litter mass.
The k (year-1) of both sites was compared using a t test for independent samples. The k year-1 values were transformed to log10 in order to meet the assumptions of normality and homoscedasticity. The statistical analyses were carried out with the SPSS software, version 18 (SPSS Inc).
Results and Discussion
The average values (n=4) of remaining mass of litter ranged between 61.01 % and 70.78 % for the vegetation of the TTS, and between 75.09 % and 90.79 % for the eucalyptus plantation (Table 2).
Table 2 Values (%) of the remaining mass of litter for each study site and assessment period, expressed as a fraction of a year, from the time when the leaf litter bags were placed in the field (time 0).
| Site | Repeat | Time (year) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.17 | 0.25 | 0.33 | 0.42 | 0.5 | ||
| TTS | 1 | 100 | 63.58 | 71.64 | 72.09 | 60.9 | 55.07 |
| 2 | 100 | 70.60 | 75.82 | 72.09 | 67.01 | 68.21 | |
| 3 | 100 | 88.81 | 71.79 | 72.99 | 70.90 | 70.15 | |
| 4 | 100 | 60.15 | 51.49 | 55.37 | 45.22 | 66.12 | |
| Average | 100a | 70.78 ±6.39b | 67.69 ±5.48b | 68.13 ±4.26b | 61.01 ±5.65b | 64.89 ±3.37b | |
| Eucalyptus | 1 | 100 | 92.22 | 94.81 | 94.81 | 83.70 | 78.52 |
| 2 | 100 | 86.30 | 85.19 | 82.96 | 83.33 | 78.89 | |
| 3 | 100 | 99.26 | 96.30 | 94.07 | 86.30 | 70.74 | |
| 4 | 100 | 85.37 | 79.26 | 87.04 | 88.52 | 72.22 | |
| Average | 100a | 90.79 ±3.21ab | 88.89 ±4.05ab | 89.72 ±2.85ab | 85.46 ±1.21bc | 75.09 ±2.11c | |
The average ± standard error (n=4) is shown. Different letters indicate significant differences (Tukey, P<0.05) between sampling dates at each site.
The decomposition percentage was higher in the TTS (35.11 %) than at the eucalyptus site (24.91 %) (Figura 4). The latter percentage differs from the findings of García-Arrese (1997), who compared the decomposition rate of the leaf litter of Quercus robur L., Eucalyptus globulus Labill., Pinus pinaster Ait. and Pinus radiata D. in forests of Santiago Compostela, Spain; according to this author, the eucalyptus litter had lost approximately 32 % of its mass after six months. Farfán-Valencia and Urrego (2007) registered losses of 37 % of leaf litter weight after six months in Eucalyptus grandis W. Hill ex Maiden plantations in agroforest systems in Colombia. Although neither of these studies worked with the same Eucalyptus species, we must take into account that the areas where they were carried out have a mean precipitation of approximately 2 000 mm, which is much higher than that of the present study, and lower mean temperatures.
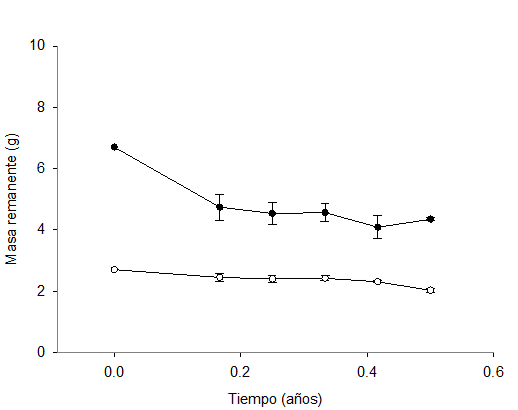
Tiempo (años) = Time (years); Masa remanente = Remaining mass
The vertical bars represent the standard error (n=4).
Figure 4 Remaining mass (grams) of eucalyptus (○) and TTS (●) leaf litter in the six months of the experiment.
The loss of mass in eucalyptus species seems to be favored by rainfall, as observed in the positive correlation between mass loss and precipitation obtained in this study.
The lower decomposition rate of E. camaldulensis, compared to that of the TTS is probably due to the lignin and cellulose contents. Values of 33.34 % cellulose and 24.10 % lignin are observed in E. globulus leaves (Kiffer et al., 2018); while certain species of Acacia (A. dealbata Link), had 17.4 % holocellulose and 19.10 % lignin (Abubacker and Prince, 2013). Likewise, according to the literature, the decomposition rates of leaf litter are positively associated with N or P concentrations (Aerts, 1996).
In a previous study carried out in the Linares campus of UANL, N concentrations of 1.03 % were determined in the TTS litter (López et al., 2015). Woods and Raison (1983) register values of 0.40 to 0.71 % of N for E. delegatensis R. T. Baker, E. pauciflora Sieb. ex Spreng and E. dives Schau. It is likely that the higher N content in the TTS litter may also influence its high decomposition rate.
Marmolejo et al. (2013) report an average maximum percentage of litter decomposition of 25.8 % after 10 months in four TTS sites. This percentage is below the decomposition rate of 34.48 % at six months determined by this study. If the rate were to be calculated for a period of 10 months using the generated equation, the value would be 41.78 %. In a study of Prosopis velutina Woot. carried out in Arizona, 32 weeks after the beginning of the decomposition process, 100 % of remaining mass (0 % degradation) was obtained with treatments on dry soil, while the percentage for treatments on moist soil (with 2% and 12% of water in the porous space of the soil, respectively) was 57 %; therefore, the authors consider that soil moisture controls the decomposition process, especially when rainfall events are small and occur in discrete pulses (Lee et al., 2014).
Mass losses of 20 to 47 % have been observed in Prosopis glandulosa Torr. for a similar climate period to that of the present study in Las Cruces, New Mexico ((Shaefer et al., 1985).
The degradation rate of litter in the TTS was also much higher than the annual losses documented for the litter of Mediterranean shrub species, which ranged between 15 % and 19 % (Schlesinger and Hasey, 1981). This is probably due to the fact that the litter collected and put into the bags consisted mostly of leaves, while in other cases it may have contained a higher proportion of branches. These have a higher concentration of lignin, a group of complex aromatic polymers present in the cell walls of the plant, which are resistant to enzyme degradation and serve as a barrier to the access to carbon labile compounds by microbial organisms (Austin and Ballaré, 2010) during the first stages of decomposition (O’Connell and Sankaran, 1997); therefore, the decomposition of the leaves is quicker.
The results of the variance analysis showed that the remaining mass of litter differed between sampling dates (P=0.001 for TTS, and P=0.0001 for eucalyptus) (Table 2); the largest losses were registered on the fourth and fifth dates, both of which exhibited the same value (P=0.135). In the litter from the TTS, the largest mass loss occurred already on the first date since the time when the bags were placed in the field, with a 25.7 % reduction from November to January (Figure 4).
A rapid mass reduction phase during the decomposition process was observed in Acacia mangium Willd. in a region of India with a warm-humid climate. This initial rapid decomposition phase occurred at five months, with 60 % of mass loss; however, the following three months were considered as a slow mass loss phase, with only 30 % (Kunhamu et al., 2009).
The decomposition constant (k year‒1) was higher for the TTS (P=0.004), with a value of 0.934 (r2=0.668), compared to eucalyptus (k= 0.479; r2=0.722) (Figure 5). There are no records of the decomposition rate k for Eucalyptus camaldulensis in the literature, unlike other eucalyptus species, for which monthly or daily data are available (Farfan-Valencia and Urrego, 2007; Goya et al., 2008). This makes it difficult to compare it with k year-1 estimations. However, in a subalpine forest ecosystem in Australia, the decomposition rate of E. delegatensis (R. T. Baker), E. pauciflora (Sieb. ex Spreng) and E. dives (Schau.) was studied during 29 months, and the decomposition rate (k day‒1) was estimated in 0.68, 0.53 and 0.47, respectively (Woods and Raison, 1983). The authors consider that higher moisture conditions in the southern exposure for E. pauciflora and E. dives in relation to the northern exposure of E. delegatensis may partly account for the differences observed between their decomposition rates.
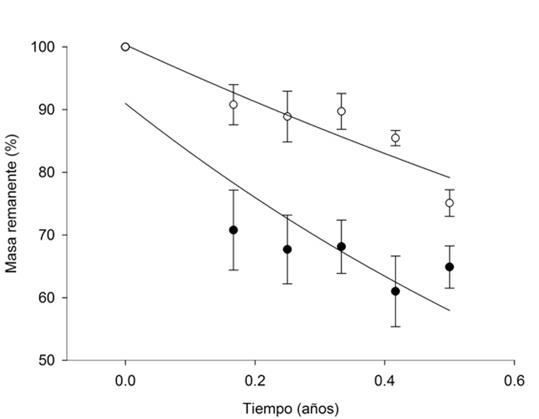
Tiempo (años) = Time (years); Masa remanente = Remaining mass
The generated equations are: Y=100.35 e-0.479x for eucalyptus litter and Y=91.00 e-0.934x for thornscrub litter. The vertical bars represent the standard error (n=4).
Figure 5 Remaining mass as percentage of eucalyptus (○) and TTS (●) litter decomposition on the various sampling dates.
There is little information on the decomposition constant (k) for the TTS. In a previous study carried out in the same area as the present one, k values of 0.50 (year‒1) are indicated for a well preserved thornscrub (Marmolejo et al., 2013); these values differ notably from those estimated in the present work.
The litter decomposition rates in general are widely variable, due to the differences in geographical location, climate conditions (Salamanca et al., 2003) and quality of the litter (Swift et al., 1979; Binkley, 1986).
Likewise, in ecosystems with a larger variety of climates, the fall of litter is not constant throughout the year, and the species have various rates, as in the case of the Tamaulipan thorn scrub. This probably explains the differences registered in the decomposition rates mentioned by Marmolejo et al. (2013) for the same vegetation type.
In their analysis of the decomposition rate of the litter of woody shrub species in a Mediterranean ecosystem, Rodríguez-Pleguezuelo et al. (2009) cite a value of 1.01 for Retama sphaerocarpa (L.) Boiss; this value is slightly higher than that obtained by the present study for the TTS as a whole (0.934). In both areas, the mean annual temperature is similar, of 20.8 °C and 22.6 °C, although the rainfall is much lower in the Mediterranean area (449 mm). Fioretto et al. (2003) estimate a litter decomposition rate (k) of 0.71 for Myrtus communis (L.), and of 0.31 for Cistus incanus (L.), which indicates that not only the climate but also the chemical composition plays a major role (Berg et al., 1995; Fioretto et al., 1998).
Annual estimates have been carried out in certain shrub species of the Chihuahuan Desert; for example, k=0.654 for Prosopis glandulosa Torr., and k=0.777 for a mix of annual plants (Schaefer et al., 1985).
According to Cuevas and Medina (1988) and Landsberg and Gowers (1997), the litter decomposition rates tend to be higher in rainy tropical forests than in dry ones, and, at the same time, they are higher in the tropics than elsewhere. Nevertheless, due to the large variations resulting from all the involved factors, a significant overlap in the decomposition rates may be detected between different ecosystems or soil uses. In this study, the remaining mass exhibited a negative correlation with the registered ambient temperature and evaporation both for the TTS (r=-0.474, P=0.019; r=-0.571, P=0.004) and for the eucalyptus plantation (r=-0.693, P=0.0001; r=-0.697, P=0.0001), and only the eucalyptus litter had a correlation with the rainfall (r=-0.709, P=0.0001). This may be due to the fact that the study region has a rather low level of rainfall within the semiarid-subhumid interval, with 164.2 mm accumulated during the six sampling months. For this reason, the litter decomposition seems to be more affected by the temperature, whose average during the six months was 20.6 °C.
Conclusions
The percentage of leaf litter mass loss in the Tamaulipan thorn scrub (35.11 %) was 10 % higher than at the eucalyptus plantation (24.91 %) six months after the bags were placed in the field. Likewise, the decomposition constant (k year-1) was higher for the TTS, with a value of 0.934, than for the eucalyptus plantation (0.479). Therefore, we accept the first hypothesis, i.e. that the leaf litter of eucalyptus has a slower initial disintegration process than that of the Tamaulipan thorn scrub. The lower decomposition rate of the eucalyptus litter with respect to that of TTS implies that also the deposition of nutrients to the soil will be slower. In semiarid regions the rainfall pulses are a key factor for the growth of the vegetation, and a slow decomposition may limit the availability of nutrients when rainfalls occur. This has implications on the management, the forestry practices, and the possibilities of regeneration of the species to be established.
The percentage of remaining mass of the litter of both the TTS and the eucalyptus plantation has a negative correlation with the registered ambient temperature and evaporation, while only the eucalyptus litter has a negative correlation with the rainfall.
Acknowledgments
The authors wish to express our gratitude to the Facultad de Ciencias Forestales de la Universidad Autónoma de Nuevo León, (School of Forest Sciences of the Autonomous University of Nuevo León) for the logistic facilities provided for this research, as well as to Jonathan Bautista for his contribution in the in-field data collection.
REFERENCES
Abubacker, M. N. and M. Prince. 2013. Decomposition of lignin and holocellulose of Acacia dealbata Link (Mimosoideae) leaves, twigs and barks by fungal isolates from virgin forest ecosystem of Doddabetta Belt of Nilgiris. Biosciences Biotechnology Research Asia 10(2):719-726. DOI: http://dx.doi.org/10.13005/bbra/1187. [ Links ]
Aerts, R. 1996. Nutrient resorption from senescing leaves of perennials: are there general patterns? Journal of Ecology 84(4):597-608. [ Links ]
Aerts, R. 1997. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79: 439-449. DOI: http://www.jstor.org/stable/3546886. [ Links ]
Austin, A. T. and C. L. Ballaré. 2010. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proceedings of the National Academy of Sciences of the United States of America (PNAS) 107(10):4618-4622. https://doi.org/10.1073/pnas.0909396107. [ Links ]
Berg, B. and G. Ekbohm. 1991. Litter mass-loss rates and decomposition patterns in some needle and leaf litter types. Long-term decomposition in a Scots pine forest. VII. Canadian Journal of Botany 69(7): 1449-1456. DOI: https://doi.org/10.1139/b91-187. [ Links ]
Berg, B., M. B. Johansson, R. Calvo de Anta, A. Escudero, A. Gärdenäs, R. Laskowski, M. Madeira, E. Mälkönen, C. McClaugherty, V. Meentmeyer and A. Virzo De Santo. 1995. The chemical composition of newly shed needle litter of Scots pine and some other pine species in a climatic transect. X Long-term decomposition in a Scots pine forest. Canadian Journal of Botany 73:1423-1435. DOI: https://doi.org/10.1139/b95-155. [ Links ]
Bertsch, H. F. 1995. La Fertilidad de los suelos y su manejo. San José, Costa Rica. 157 p. [ Links ]
Binkley, D. 1986. Forest Nutrition Management. Ed. Wiley. New York, NY, USA. 290 p. [ Links ]
Brandt, L., J. Y. King and D. Milchunas. 2007. Effects of ultraviolet radiation on litter decomposition depend on precipitation and litter chemistry in a shortgrass steppe ecosystem. Global Change Biology 13:2193-2205. DOI: 10.1111/j.1365-2486.2007.01428.x. [ Links ]
Cuevas, E. and E. Medina. 1988. Nutrient dynamics within Amazonian forest II. Fine root growth, nutrient availability and leaf litter decomposition. Oecologia 76(2):222-235. https://doi.org/10.1007/BF00379956. [ Links ]
Estrada, E. y F. Marroquín. 1988. Leguminosas de Nuevo León: Sinopsis de las especies de Linares. Reporte Científico Núm. 9. Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. Linares, N. L., México. 39 p. [ Links ]
Organización de las Naciones Unidad para la Alimentación (FAO). 1988. World Reference Base for Soil Resources. World Soil Resources Report Num. 84. Ed. ISSS-ISRIC-FAO. Rome, Italy. 88 p. [ Links ]
Farfán-Valencia, F. y J. B. Urrego. 2007. Descomposición de la hojarasca y liberación de nutrientes de Coffea arabica, Cordia alliodora, Pinus oocarpa y Eucalyptus grandis, en sistemas agroforestales con café. Cenicafé 58(1):20-39. http://biblioteca.cenicafe.org/bitstream/10778/205/1/arc058(01)020-039.pdf . (1 de marzo de 2017). [ Links ]
Fargen, C., S. M. Emery and M. M. Carreiro. 2015. Influence of Lonicera maackii invasion on leaf litter decomposition and macroinvertebrate communities in an urban stream. Natural Areas Journal 35(3):392-403. https://doi.org/10.3375/043.035.0303. [ Links ]
Fioretto, A., A. Musacchio, G. Andolfi and A. Virzo De Santo. 1998. Decomposition dynamics of litters of various pine species in a Corsican pine forest. Soil Biology and Biochemistry 30(6):721-727. DOI: https://doi.org/10.1016/S0038-0717(97)00182-X. [ Links ]
Fioretto, A., S. Papa and A. Fuggi. 2003. Litter-fall and litter decomposition in a low Mediterranean shrubland. Biology and Fertility of Soils 39(1): 37-44. DOI: https://doi.org/10.1007/s00374-003-0675-5. [ Links ]
Foroughbakhch, R., A. Carrillo-Parra, J. L. Hernández-Piñero y M. A. Guzmán-Lucio. 2017. Crecimiento y producción de una plantación subtropical de eucalipto en un suelo degradado del Noreste de México. Madera y Bosque 23(3):71-85. DOI: https://doi.org/10.21829/myb.2017.2331130. [ Links ]
Furey, C., P. A. Tecco, N. Perez-Harguindeguy, M. A. Giorgis and M. Grossi. 2014. The importance of native and exotic plant identity and dominance on decomposition patterns in mountain woodlands of central Argentina. Acta Oecologica 54: 13-20. http://dx.doi.org/10.1016/j.actao.2012.12.005. [ Links ]
García, E. 1973. Modificaciones al sistema de clasificación climática de Köppen (para adaptarlo a las condiciones de la República Mexicana). Ed. Universidad Autónoma de México, Instituto de Geografía. México D. F., México. 246 p. [ Links ]
García-Arrese, A. M. 1997. Influencia de la composición química de la hojarasca en su descomposición en sistemas forestales. In: II Congreso Forestal Español. 14 a 18 de junio. Lourizán, Pontevedra, España. pp. 313-318. [ Links ]
Goya, J. F., J. L. Frangi, C. A. Pérez and F. Dalla T. 2008. Decomposition and nutrient release from leaf litter in Eucalyptus grandis plantations on three different soils in Entre Ríos, Argentina. Bosque 29(3): 217-226. [ Links ]
Gliessman, S. R. 2002. El concepto de agroecosistemas. In: Gliessman, S. R. (comp.). Agroecología: Procesos ecológicos en agricultura sostenible. Turrialba, Costa Rica, CATIE. pp. 17-24. [ Links ]
Harmon, M. E., W. L. Silver, B. Fasth, H. Chen, I. C. Burke, W. J. Parton, S. C. Hart, W. S. Currie and Long-term Intersite Decomposition Experiment Team (LIDET). 2009. Long-term patterns of mass loss during the decomposition of leaf and fine root litter: an intersite comparison. Global Change Biology 15(5): 1320-1338. https://doi.org/10.1111/j.1365-2486.2008.01837.x. [ Links ]
Healy, A. L., D. J. Lee, A. Furtado, B. A. Simmons and R. J. Henry. 2015. Efficient Eucalypt Cell Wall Deconstruction and Conversion for Sustainable Lignocellulosic Biofuels. Frontiers in Bioengineering and Biotechnology 3: 190. https://doi.org/10.3389/fbioe.2015.00190. [ Links ]
Jaeger, H., M. J. Alencastro, M. Kaupenjohann and I. Kowarik. 2013. Ecosystem changes in Galapagos highlands by the invasive tree Cinchona pubescens. Plant and Soil 371:629-640. DOI: https://doi.org/10.1007/s11104-013-1719-8. [ Links ]
Kang, H. Z., B. Berg, C. Liu and C. J. Westman. 2009. Variation in mass-loss rate of foliar litter in relation to climate and litter quality in Eurasian forests: Differences among functional groups of litter. Silva Fennica 43(4):549-575. [ Links ]
Kiffer, W. P. Jr, F. Mendes, C. G. Casotti, L. C. Costa and M. S. Moretti. 2018. Exotic Eucalyptus leaves are preferred over tougher native species but affect the growth and survival of shredders in an Atlantic Forest stream (Brazil). PLoS ONE 13(1): e0190743. https://doi.org/10.1371/journal.pone.0190743. [ Links ]
Kunhamu, T. K., B. M. Kumar and S. Viswanath. 2009. Does thinning affect litterfall, litter decomposition, and associated nutrient release in Acacia mangium stands of Kerala in peninsular India? Canadian Journal of Forest Research 39:792-801. https://doi.org/10.1139/X09-008. [ Links ]
Landsberg, J. and S. Gowers. 1997. Application of Physiological Ecology to Forest Management. Ed. Academic Press. San Diego, CA USA. 354 p. [ Links ]
Lee, H., J. Fitzgerald, D. B. Hewins, R. L. McCulley, S. R. Archer, T. Rahn and H. L. Throop. 2014. Soil moisture and soil-litter mixing effects on surface litter decomposition: A controlled environment assessment. Soil Biology and Biochemistry 72:123-132. DOI: http://dx.doi.org/10.1016/j.soilbio.2014.01.027. [ Links ]
López-Hernández, J. M., H. González-Rodríguez, R. G. Ramírez-Lozano, I. Cantú-Silva, M. V. Gómez-Meza, M. Pando-Moreno y A. E. Estrada-Castillón. 2013. Producción de hojarasca y retorno potencial de nutrientes en tres sitios del estado de Nuevo León, México. Polibotánica 35:41-64. http://www.scielo.org.mx/scielo.php?script= (17-marzo-2017). [ Links ]
López H., J. M., H. González R., R. G. Ramírez L., J. I. del Valle A., I. Cantú S., M. Pando M., A. E. Estrada C. y M. V. Gómez M. 2015. Producción de hojarasca y depósito potencial de nutrientes de las hojas en el Matorral Espinoso Tamaulipeco. Revista Mexicana de Ciencias Forestales. 6(30): 74-89. [ Links ]
Lugo, J. H. 1989. Diccionario geomorfológico. Universidad Autónoma de México. México, D. F., México. 478 p. [ Links ]
Marmolejo M., J. G., C. M. Cantú A. y M. A. Gutiérrez S. 2013. Degradación de la hojarasca en sitios con vegetación primaria y secundaria del matorral espinoso tamaulipeco. Revista Mexicana de Ciencias Forestales 4(17):174-181. [ Links ]
Martín, A., I. Santa Regina y J. F. Gallardo. 1996. Eficiencia, re-translocación y balance de nutrientes en bosques de Quercus pyrenaica bajo diferentes pluviometrías en la Sierra de Gata (Centro Oeste Español). Ecología 10:79-93. [ Links ]
Martínez-Yrízar, A., S. Núñez and A. Búrquez. 2007. Leaf litter decomposition in a southern Sonoran Desert ecosystem, northwestern Mexico: Effects of habitat and litter quality. Acta Oecologica 32:291-300. DOI: 10.1016/j.actao.2007.05.010. [ Links ]
Melillo, J. M., J. D. Aber and J. F. Muratore. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63(3):621-626. https://doi.org/10.2307/1936780. [ Links ]
Molina-Guerra, V. M., M. Pando-Moreno, E. Alanís-Rodríguez, P. A. Canizales-Velázquez, H. González-Rodríguez y J. Jiménez-Pérez. 2013. Composición y diversidad vegetal de dos sistemas de pastoreo en el matorral espinoso tamaulipeco del Noreste de México. Revista Mexicana de Ciencias Pecuarias 4(2):361-371. [ Links ]
Muller, C. H. 1939. Relations of the vegetation and climatics types in Nuevo León, México. The American Midland Naturalist 21:687-729. [ Links ]
Návar, J., T. Cavazos y P. A. Domínguez. 1994. Los balances hidrológicos mensuales con tres probabilidades de precipitación en el estado de Nuevo León. In: Pota S., C., J. A. Ramírez F., M. M. Rangel y I. Navarro-L. (eds.). Actas de la Facultad de Ciencias de la Tierra UANL 8. Linares, N.L., México. pp. 71-82. [ Links ]
O’Connell, A. M. and K. V. Sankaran. 1997. Organic matter accretion, decomposition and mineralization. In: Nambiar, E. K. S. and A. G. Brown (eds.). Management of soil, nutrient and water in tropical plantation forests. Australian Center for International Agricultural Research. Australian Centre for International Agricultural Research. Canberra, Australia. pp. 443-473. [ Links ]
Olson, J. S. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44(2):322-331. http://www.jstor.org/stable/1932179?seq=1&cid=pdf-reference#references_tab_contents . (28 de febrero de 2017). [ Links ]
Peñaloza R. y N. Reid. 1989. Pasado, presente y futuro del uso de la tierra en el matorral tamaulipeco del noreste de México. In: Simposio Agroforestal en México: sistemas y métodos de uso múltiple del suelo. Tomo II. Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. 14-16 de noviembre. Linares, N.L., México. pp. 663-692. [ Links ]
Prescott, C. E. 2010. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133-149. DOI: https://doi.org/10.1007/s10533-010-9439-0. [ Links ]
Prinsen, P. 2010. Composición química de diversos materiales lignocelulósicos de interés industrial y análisis estructural de sus ligninas. Trabajo de fin de Máster. Departamento de Biotecnología Vegetal, Instituto de Recursos Naturales y Agrobiología de Sevilla. Consejo Superior de Investigaciones Científicas. Sevilla, España. 82 p. [ Links ]
Reinhart, K. O. and R. VandeVoort. 2006. Biodiversity research: effect of native and exotic leaf litter on macroinvertebrate communities and decomposition in a western Montana stream. Diversity and Distributions 12:776-781. [ Links ]
Rodríguez-Pleguezuelo, C. R., V. H. Durán, J. L. Muriel, F. J. Martín and D. Franco. 2009. Litter decomposition and nitrogen release in a sloping Mediterranean subtropical agroecosystem on the coast of Granada (SE, Spain): Effects of floristic and topographic alteration on the slope. Agriculture, Ecosystems and Environment 134(1):79-88. DOI: https://doi.org/10.1016/j.agee.2009.05.019. [ Links ]
Salamanca, E. F., N. Kaneko and S. Katagiri. 2003. Rainfall manipulation effects on litter decomposition and the microbial biomass of the forest floor. Applied Soil Ecology 22:271-281. https://doi.org/10.1016/S0929-1393(02)00153-1. [ Links ]
Santa-Regina, I. y J. F. Gallardo. 1989. Ciclos biogeoquímicos en bosques de la Sierra de Bejar (Provincia de Salamanca). Options Méditerranéens-Série Séminaires 3:147-149. [ Links ]
Santa-Regina, I. and T. Tarrazona. 2001. Nutrient cycling in a natural beech forest and adjacent planted pine in northern Spain. Forestry 74:11-28. https://doi.org/10.1093/forestry/74.1.11. [ Links ]
Schaefer, D., Y. F. Steinberger and W. G. Whitford. 1985. The failure of nitrogen and lignin control of decomposition in a North American Desert. Oecologia 65 (3):382-386. DOI: https://doi.org/10.1007/BF00378913. [ Links ]
Schlesinger, W. H. and M. M. Hasey. 1981. Decomposition of chaparral shrub foliage: losses of organic and inorganic constituents from deciduous and evergreen leaves. Ecology . 62(3):762-774. https://doi.org/10.2307/1937744. [ Links ]
Shanks, R. E. and J. S. Olson. 1961. First year breakdown of leaf litter in southern Appalachian forests. Science 134:194-195. DOI: 10.1126/science.134.3473.194. [ Links ]
Sistema Meteorológico Nacional (SMN). 2012. Datos para estación Camacho, municipio Linares, estado de Nuevo León. http://smn.cna.gob.mx/es/informacion-climatologica-ver-estado?estado=nl (13 de junio de 2017). [ Links ]
Swift, M. J, O. W. Heal and J. M. Anderson. 1979. Decomposition in terrestrial ecosystems. Blackwell Scientific. Oxford, UK. 372 p. [ Links ]
Triska, F. J., J. R. Sedell, K. Cromack Jr., S. V. Gregory and F. M. McCorison. 1984. Nitrogen budget for a small coniferous forest stream. Ecological Monographs 54:119-140. https://doi.org/10.2307/1942458. [ Links ]
Trofymow, J. A., T. Moore, B. Titus, C. Prescott, T. Morrison, M. Siltanen, S. Smith, J. Fyles, R. Wein, C. Camire, L. Duschene, L. Kozak, M. Kranabetter and S. Visser. 2002. Rates of litter decomposition over 6 years in Canadian forests: influence of litter quality and climate. Canadian Journal of Forest Research 32(5):789-804. DOI: http://doi.org/10.1139/x01-117. [ Links ]
Villarreal González, J. G. y G. J. Alanís Flores. 2015. Impacto del marrano alzado y el jabalí europeo en hábitats del matorral espinoso tamaulipeco en el noreste de México. CIENCIA U.A.N.L. 18(72):23-29. [ Links ]
Woerner, M. 1991. Métodos químicos para el análisis de suelos calizos de zonas áridas y semiáridas. Facultad de Ciencias Forestales, UANL. Linares, N.L., México. 105 p. [ Links ]
Woods, P. V. and R. J.Raison. 1983. Decomposition of litter in sub-alpine forests of Eucalyptus delegatensis, E. pauciflora and E. dives. Australian Journal of Ecology 8:287-299. https://doi.org/10.1111/j.1442-9993.1983.tb01326.x. [ Links ]
Zhang, D., D. Hui, Y. Luo and G. Zhou. 2008. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. Journal of Plant Ecology 1(2):85-93. DOI: https://doi.org/10.1093/jpe/rtn002. [ Links ]
Received: March 13, 2018; Accepted: October 05, 2018











 texto em
texto em 




