Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Intervención (México DF)
versão impressa ISSN 2007-249X
Intervención (Méx. DF) vol.14 no.28 México Jul./Dez. 2023 Epub 11-Jun-2024
https://doi.org/10.30763/intervencion.288.v2n28.67.2023
Research articles
Design and Evaluation of Biosensor Prototypes as a System to Detect Microbiological Risks for Organic Collections in Storage
*Universidad Militar Nueva Granada (UMNG), Colombia. est.lizeth.russy@unimilitar.edu.co
**Universidad Militar Nueva Granada (UMNG), Colombia. maria.patino@unimilitar.edu.co
This RESEARCH addressed the implementation of biosensor prototypes to detect microbiological risks in environments with organic collections. During the first phase, different combinations of supports, culture mediums, and pH indicators were tested in order to design the prototypes in the laboratory, while the second phase saw the prototypes tested in collection storage environments. The best prototypes were chosen during the experimental phase, taking into account the criteria of colony forming-unit (CFU), amount of CFU in the biosensors, and the diversity of isolated fungi. During the second phase, it was possible to conclude that environmental conditions are determining factors for the prototypes’ functionality.
Keywords: air; storage; biosensors; collections; organic materials; microorganisms
La presente INVESTIGACIÓN aborda la implementación de prototipos de biosensores para la detección de riesgo microbiológico en ambientes con colecciones de tipo orgánico. En su primera fase se ensayaron las diferentes combinaciones de soportes, medios de cultivo e indicadores de pH para el diseño de los prototipos en el laboratorio, y en la segunda, se evaluaron los prototipos de colecciones en ambientes de almacenamiento. Durante la fase experimental se escogieron los mejores prototipos teniendo en cuenta los criterios de: tiempo de unidades formadoras de colonias (UFC), cantidad de UFC en los biosensores y diversidad de hongos filamentosos aislados. En la segunda fase se logró concluir que las condiciones ambientales son determinantes para el funcionamiento de los prototipos.
Palabras clave: aire; almacenamiento; biosensores; colecciones; materiales orgánicos; microorganismos
Introduction
Microorganisms play an important role in the biodeterioration of cultural objects, as a result of the processes of growth and development and also of metabolic activities which will eventually cause physicochemical and mechanical alterations to their structure, with negative consequences for their preservation (Gacto & Gacto, 2011, pp. 108-110). Specimens made with organic material of protein or cellulose nature have been reported in collections of documents, archaeological textiles, bone or mummified remains, wood and furs, among others (González, Acevedo, Cases & Valenzuela 2016, pp. 176-179); these substrata are potential niches for agents of biodeterioration to develop under favorable environmental conditions (Nitiu et al., 2015, p. 428).
The process of biodeterioration in organic collections is usually detected at an advanced stage (Rojas, 2019, p. 21) and the necessary interventions by those responsible for their handling and storage to halt colonization and microbiological effects can involve other types of risks-some of an aesthetic nature-to the materials (Merritt, 2007, p. 2) or the modification of ultrastructural, chemical, and genetic components, which are usually a source of information for various studies (Lasprilla et al., 2014, pp. 25-26).
In Latin America, authors such as Borrego, Herrera, Paneque, and Quitral have confirmed the presence of airborne bacteria and fungi in libraries, archives, and museums and have linked this to the deterioration of collections as well as to the staff’s health (Borrego, Herrara & Paneque, 2021, p. 10; Quitral, 2020, p. 86). Due to the importance of identifying the air quality in locations that host collections, Villalba (2015, p. 23) documented biocontamination by means of impact studies to establish an index of environmental microbiological contamination (ICMA).
The microorganisms reported include genera of fungi such as Aspergillus, Cladosporium, Penicillium, Curvularia, Alternaria, Fusarium, Chaetomium, Phoma, Trichoderma, Mycelia sterilia, Mucor, and Chrysonilia (Borrego et al., 2010, p. 125; Villalba, 2015, p. 27), as well as bacteria, predominantly of the genera Micrococcus, Staphylococcus, and Bacillus (Skóra et al., 2015, p. 395). Many of these microorganisms have been reported to cause biodeterioration in various heritage materials as a result of their ability to produce hydrolytic enzymes, acids, and pigments that alter their properties (Borrego et al., 2010, p. 129).
Hence, a strategy for the early detection of the risk of microbial colonization in stored cultural objects is required, which must include parameters of temperature (degrees Celsius) and relative humidity (RH) through the analysis of bioaerosols revealing air quality in the storage environment, as suggested by Valentín et al. (2017, pp. 102-107). However, museums rarely implement such practices (Valentín, 2015, p. 345) due to the cost involved in hiring specialists and acquiring laboratory equipment.
Therefore, research into the biosensors developed-the most relevant of which is that by Nieves Valentín in 2015-in hand with the implementation of biosensors as alarm systems to detect the growth of microorganisms in display cases containing mummified human remains. Those biosensors were based on a support with high hygroscopicity composed of protein or cellulose materials, similar to those in the collections, along with a culture medium to which a marker could be added that changes color when microorganisms grow under favorable conditions of temperature and relative humidity (Valentín, 2015, p. 346).
The biosensors are placed in display cases, cupboards, and pieces of furniture or storage spaces along with the collection pieces and are linked to an apparatus that registers temperature and relative humidity to correlate the environmental conditions with the growth of microorganisms on the biosensor (Valentín, 2015, p. 344), which is influenced by micro-condensation within the display cases that hydrates the culture medium. The exposure time of biosen sors before the medium becomes denaturalized can be of up to three months (Valentín, 2015, p. 347). The alert system to rectify environmental conditions in the display cases, before biodeterioration of the objects in the collection occurs, is the presence of microorganisms growing on the biosensors.
Bearing in mind that Colombia has no prior research into biosensors, the aim of this research was to determine which characteristics, composition, and environmental conditions were required for the biosensor prototype to function as a system to detect microbiological risks and what types of microorganisms they detect in the storage environment of organic collections.
Methodology
Phase I. Design and Evaluation of Prototypes in the Laboratory
The features of the biosensor prototypes were selected in accordance with annotations made by Valentín (2015). The size of the prototype support was 4 x 4 cm, and three types of material support were chosen: linen, crepe paper, and goose feathers based on the bibliography consulted and the ease of obtaining them (Valentín, 2015, p. 348; Urkullu, 2001, p. 58; Assis et al., 2020, p. 5983).
We chose three types of culture medium: Sabouraud broth, which is widely used to recover filamentous fungi due to its composition of peptones and glucose (Guinea, Peláez, Alcalá & Bouza 2005, pp. 333-334); DG18 broth (dicloran-chloramphenicol glycerol), with a low availability of water (Aw), that favors the growth of xerophilous microorganisms, such as certain filamentous fungi reported in museum and library environments (Manrique, Patiño & Gutiérrez 2012, pp. 5-6), and lastly, nutrient broth as a medium used to grow microorganisms with low nutritional requirements, including fungi and bacteria.
Another characteristic of the biosensor prototype design was a pH indicator; four were proposed with the criteria of turning from neutral to slightly alkaline in a range of 7-8 or slightly acidic in a 6-6.5 pH range (Martín & Villegas, 2021, p. 111). The chosen indicators were bromothymol blue, bromocresol purple, litmus blue, and methyl red. An experiment in test tubes was conducted for all the indicators in order to identify the most appropriate. Each test tube had 3 ml of culture medium with one of the four indicators, to which were added 0.5 ml of a solution of Penicillium sp. conidia containing 4.8 x 10.5 (10^5-105) conidia/ml. The test tubes were incubated for a week at 25° C. The characteristics of the previously selected biosensors were combined, as seen in Figure 1.
Figure 1 Experimental design to evaluate the characteristics of supports, culture mediums, and pH indicators of different biosensor prototypes
| Support | Culture medium | pH Indicator | Repetitions | Control |
|---|---|---|---|---|
| Linen | Sabouraud broth | With indicator | 3 | 1 |
| Linen | Sabouraud broth | Without indicator | 3 | 1 |
| Linen | DG18 medium | With indicator | 3 | 1 |
| Linen | DG18 medium | Without indicator | 3 | 1 |
| Linen | Nutrient culture | With indicator | 3 | 1 |
| Linen | Nutrient culture | Without indicator | 3 | 1 |
| Paper | Sabouraud broth | With indicator | 3 | 1 |
| Paper | Sabouraud broth | Without indicator | 3 | 1 |
| Paper | DG18 medium | With indicator | 3 | 1 |
| Paper | DG18 medium | Without indicator | 3 | 1 |
| Paper | Nutrient culture | With indicator | 3 | 1 |
| Paper | Nutrient culture | Without indicator | 3 | 1 |
| Feathers | Sabouraud broth | With indicator | 3 | 1 |
| Feathers | Sabouraud broth | Without indicator | 3 | 1 |
| Feathers | DG18 medium | With indicator | 3 | 1 |
| Feathers | DG18 medium | Without indicator | 3 | 1 |
| Feathers | Nutrient culture | With indicator | 3 | 1 |
| Feathers | Nutrient culture | Without indicator | 3 | 1 |
(Table: María C. Patiño Ramírez, August 31, 2021).
Prepared in triplicate, in addition to a control, the linen, paper, and feather supports were sterilized in the autoclave, along with culture mediums with and without indicators, and the Petri dishes (121° C, 17 psi). They were subsequently oven dried at 70° C for 40 minutes, until the liquid culture medium had dehydrated (Figure 2).
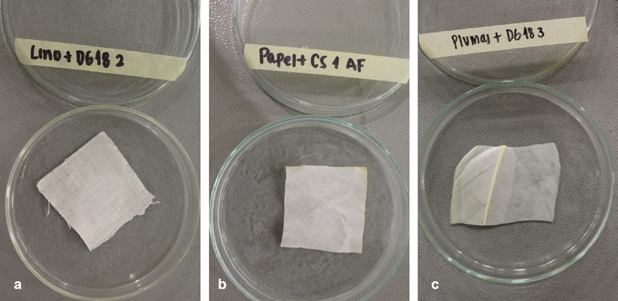
(Photograph: Lizeth P. Russy-Velandia, September 10, 2021)
Figure 2 Design of the biosensor prototypes in Petri dishes a) Biosensor Prototype LINEN:DG18, b) Biosensor Prototype PAPER:CS, c) Biosensor Prototype FEATHER:DG18
We prepared a total of 108 biosensor prototypes for use in the Archivo Central de la Universidad Militar Nueva Granada (UMNG) in the Cajica, Cundinamarca campus. They were all placed in the center of the storage area, approximately one meter above ground, except for the control; the Petri dishes containing biosensors were exposed to the environment for one hour, so the microorganisms suspended in the air could be sedimented onto the supports. After the period of exposure, the Petri dishes were closed and taken back to the laboratory where they were separated into two sets of 54 prototypes each to begin the tests under different conditions of temperature and relative humidity.
The first set was exposed in a curing chamber in the UMNG Materials Laboratory under constant conditions of 90% HR and 23° C for a period of four weeks, with weekly follow-up to photographically register and quantify the colony forming units (CFU) in the prototypes. The second was placed on a table in the UMNG Multiple Laboratory I at ambient temperature and relative humidity. The monthly average of these environmental variables was obtained by placing the prototypes close to each other in an Onset HOBO Datalogger; the same photographic follow-up was carried out over a period of four weeks.
Following this, a qualitative and quantitative description was made of the colonies that grew on the prototypes in each set. In the case of filamentous fungi, morphotypes and even genus were identified by means of tinction with lactophenol blue, observation under the 40X optic microscope, and follow-up of taxonomic codes (Barnett & Hunter, 1998, pp. 6-197; Franco et al., 2012, pp. 137,138, 184 and 434-437). The prototypes from both sets that did not present CFU growth after four weeks were rehydrated with a saline solution of 0.9% p/v (Valentín, 2015, p. 348) and incubated at 25° C to identify whether there were spores or conidia which had been unable to develop in the conditions they were under.
The best biosensor prototypes were selected based on three criteria: 1) time of appearance of the CFU of filamentous fungi over the course of four weeks, registering the first time colony growth had been observed on the biosensors with the formula:
CFUH being the number of colonies counted on the biosensor; 2) total CFU of filamentous fungi; and 3) diversity of filamentous fungi in relation to the different number of isolated morphologies for each support. The frequency of appearance (Fa) was calculated over the total number of isolated filamentous fungi in each prototype with the formula:
where N° CFUMorphotypes is the number of times a certain morphotype appeared on the same support during the identification process, and the Total CFUHS is the type of CFU that grew on each support. The results were analyzed by means of descriptive statistics to compare those parameters in the evaluated prototypes.
Phase II. Evaluation of the Prototypes in Environments of Collection Storage
The prototypes that showed the least time for the appearance of CFU in Phase I, a significant number of accumulated CFU, and a greater diversity of morphologies were prepared once again and, along with a control, placed in triplicate next to a Datalogger in the storage areas of selected collections in the cities of Bogota, Mede llin, and the municipality of Cajica, Colombia. The biosensors were deployed over several weeks, with a weekly photographic registry to monitor the growth of CFU and filamentous fungi. The prototypes that showed no growth of CFU after four weeks were taken back to the laboratory, rehydrated with a saline solution of 0.9% p/v (Valentín, 2015, p. 348), and incubated at 25° C for two weeks. The results obtained were correlated with the variables of relative humidity and temperature (RH and T°) in each of the storage areas to analyze the prototypes’ performance.
Results and discussion
Phase I. Design and Evaluation of the Prototypes in the Laboratory
Selection of pH indicator
Throughout the tests with pH indicators, bromothymol blue presented a change from neutral pH to acid in all the culture mediums with Penicillium sp. activity; this indicator displayed a more noticeable change of color compared to all the other indicators in the nutrient broth; therefore, it was chosen as the pH indicator that would serve to observe color changes on the supports resulting from organic acids produced by metabolizing sugars contained in the culture medium (Arora, 2013, p. 3).
Selection of the Best Biosensor Prototypes
Selection was made exclusively with the data collected in the test of the first set of 54 prototypes conducted in the curing chambers (90% RH-23° C) since the test with the second set of prototypes exposed to ambient relative humidity and temperature on the laboratory table did not present CFU growth until after they had been rehydrated with saline solution at the end of the fourth week. Following incubation, there was growth of Penicillium sp., Cladosporium sp., Epicoccum sp., Paecilomyces sp., and Fusarium sp., a result that is linked to the environmental conditions, which did not exceed 70% RH or 23° C. The average monthly conditions in the laboratory were 55.5% RH and 18.95° C, hence natural rehydration of the culture mediums was not obtained and, therefore, no colonies of filamentous fungi were observed.
Time of Appearance and Quantity of CFU/cm2 of Filamentous Fungi
As mentioned earlier, these criteria were only evaluated for the first set of prototypes that had microorganism growth. Through photographic follow-up over four weeks, we obtained an average of growth replicas of CFU /16 cm2; that is to say, for the total area of supports (4 x 4 cm2) exposed to 90% RH and 23° C, we calculated the standard deviation (Figure 3), which allowed us to see how scattered our data was around the average value of the three tests (Lee et al., 2015, pp. 221-222).
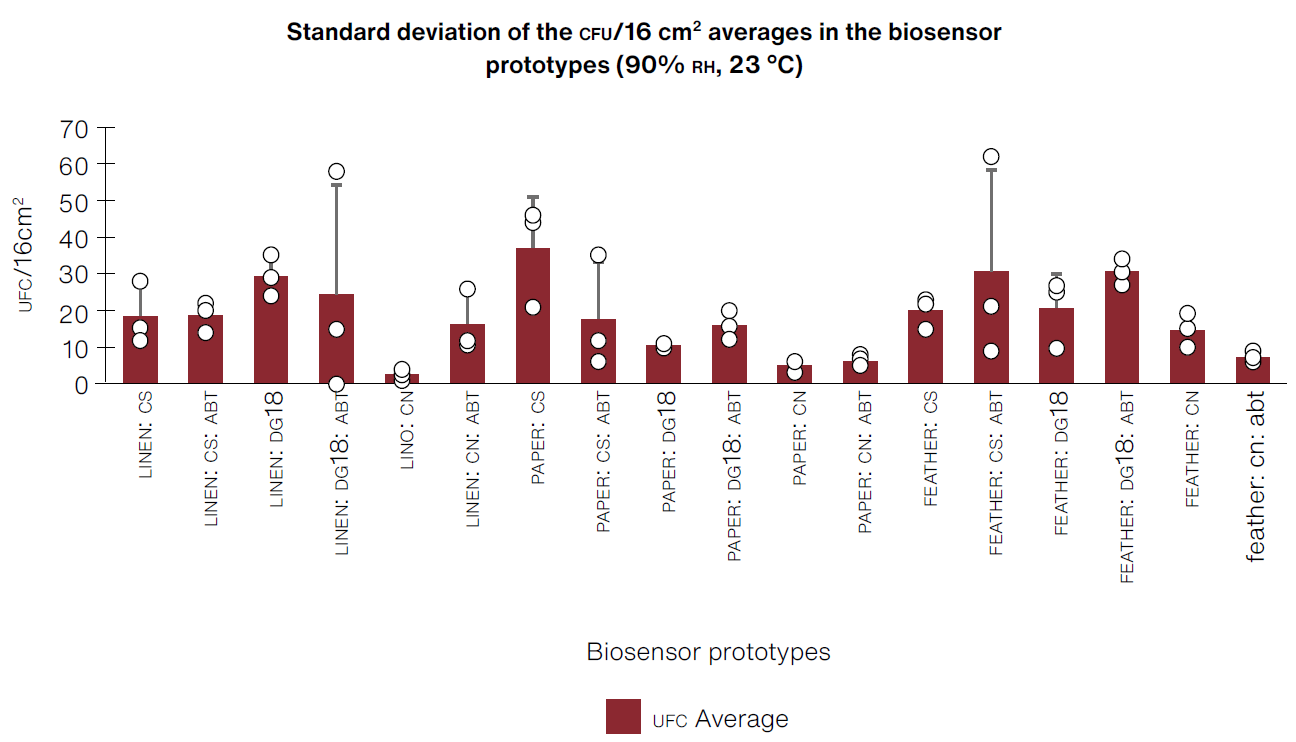
*CS:Sabouraud broth, CN:nutrient broth, DG18:dichloran-glycerol chloramphenicol broth, ABT:bromothymol blue
(Table: Lizeth P. Russy-Velandia, April 15, 2022)
Figure 3 Standard deviations obtained from the average of CFU/16 cm2 repetitions, with each biosensor prototype under conditions of 90% RH-23° C in the curing chamber.
High values for standard deviation in certain prototypes indicate that, in most cases, the data extends to a wider range with regard to the median (Barde & Barde, 2012, p. 113), which could be linked to the positioning of prototypes on shelves during the test in the curing chamber and its interaction with the surrounding water vapor.
Keeping in mind the criteria of time of appearance of CFU, quantity of CFU /cm2 of filamentous fungi so as to select the best biosensors, we identified that prototypes made of PAPER:CS, FEATHER:CS, and FEATHER:CS:ABT had the shortest time for CFU appearance (as of the first week) and showed the highest accumulated quantity of CFU/cm2 of filamentous fungi by the end of the fourth week (Figure 4).
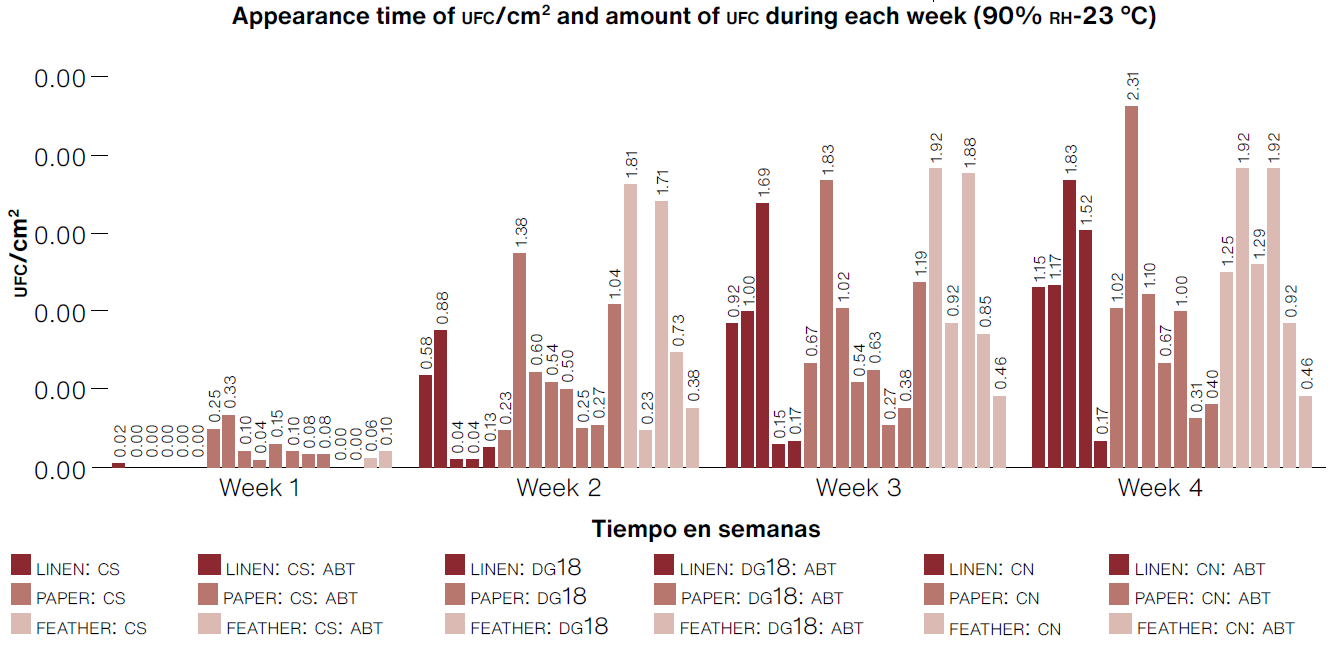
*CS:Sabouraud broth, CN:nutrient broth, DG18: dichloran-glycerol chloramphenicol broth, ABT: bromothymol blue
(Table: Lizeth P. Russy-Velandia, May 18, 2022)
Figure 4 Number of CFU/cm2 for each biosensor prototype over the four-week test period under conditions of 90% RH-23° C in the curing machine.
In the case of prototypes made with a linen support, we observed a growth of 0.02 CFU/cm2 during the first week in the Sabouraud culture medium; however, by the end of the test, there was greater isolation of filamentous fungi in the DG18 medium, indicating that although it takes a week longer to start detecting the microorganism, it is possible to isolate xerophile microorganisms that tend to grow with low water requirements. Based on the analysis presented above, the best biosensors were PAPER:CS, FEATHER:CS, QQ total of 18 morphotypes with the most frequent being Cladosporium sp. morphotype No. 1 with 22.2% (Figure 5).
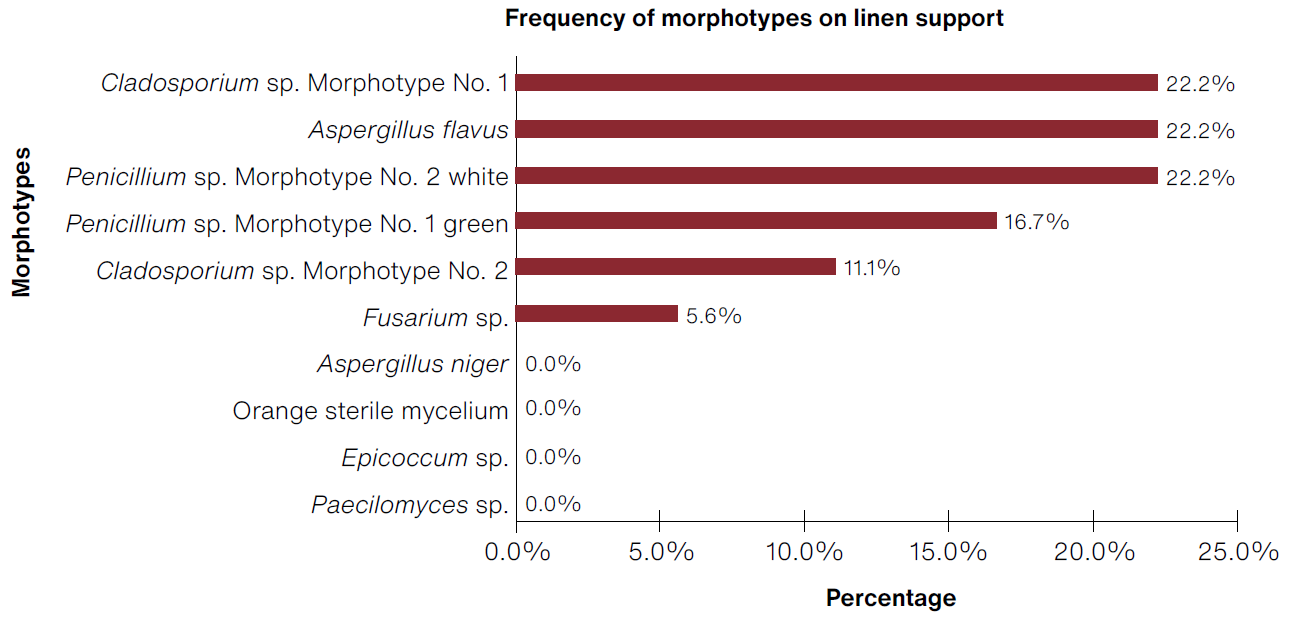
(Table: Lizeth P. Russy-Velandia, May 30, 2022)
Figure 5 Percentage of frequency of morphotypes identified on the linen support.
The prototypes with the greatest diversity of morphotypes on a linen support were the prototypes LINEN:CS and LINEN:CS:ABT, with four morphotypes each, and LINEN:DG18 and LINEN:DG18:ABT, presenting three and four morphotypes (Figure 6). Based on the selection criteria, it was determined that the prototype with the greatest detection capacity for the linen support was LINEN:DG18, with a cumulative average of 1.83 CFU/cm2. Although the appearance of filamentous fungi was slowin the first week, by the end of exposure in optimal conditions it produced the highest number of colony growth (Figure 7).
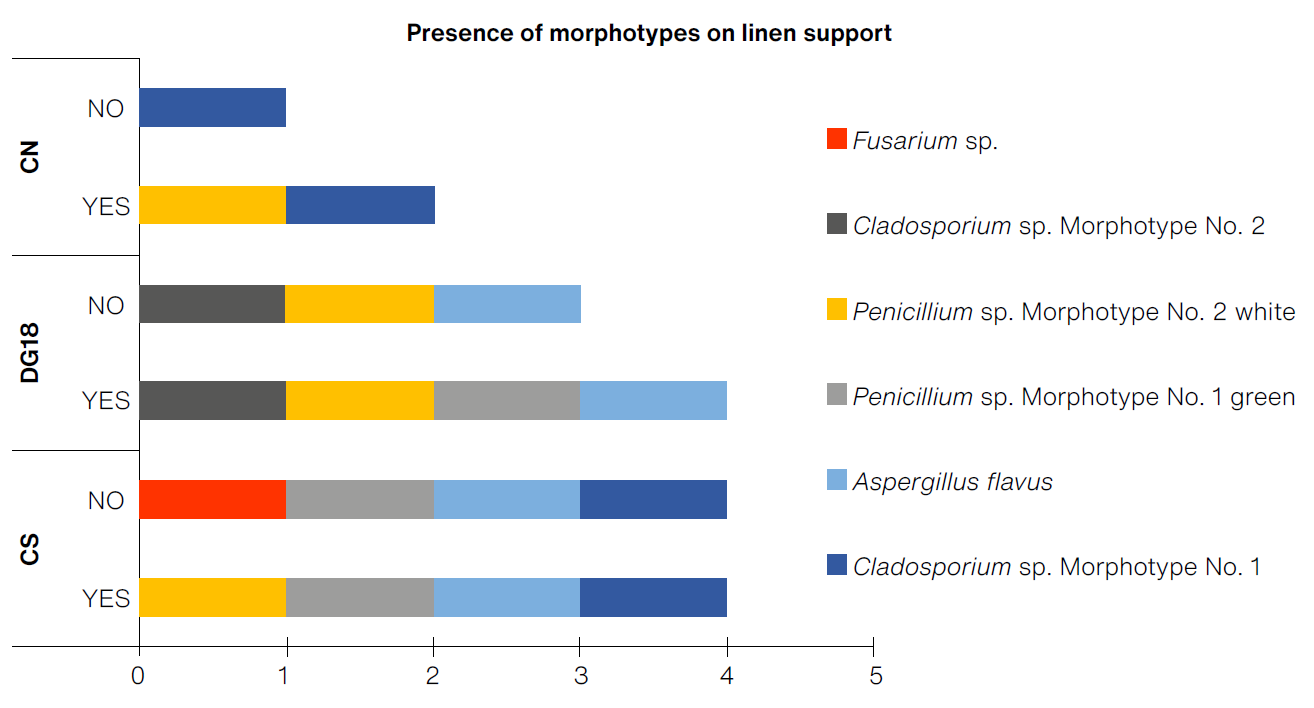
(Table: Lizeth P. Russy-Velandia, June 21, 2022)
Figure 6 Presence of morphotypes on the linen support according to the three culture mediums, with and without indicator.
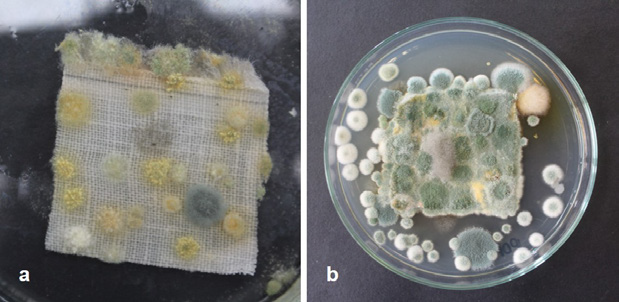
(Photograph: Lizeth P. Russy-Velandia, June 2, 2022)
Figure 7 a) Biosensor Prototype LINEN:DG18, b) Support of the same prototype sown in culture medium dicloran-glycerol chloramphenicol: the growth of Penicillium sp. morphotype No. 2 can be observed.
Regarding biosensors made of paper, a total of 22 morphotypes were obtained, with the most frequent being Cladosporium morphotype No. 1 (27.3%) and Penicillium sp. morphotype No. 1 green (18.2%) (Figure 8).
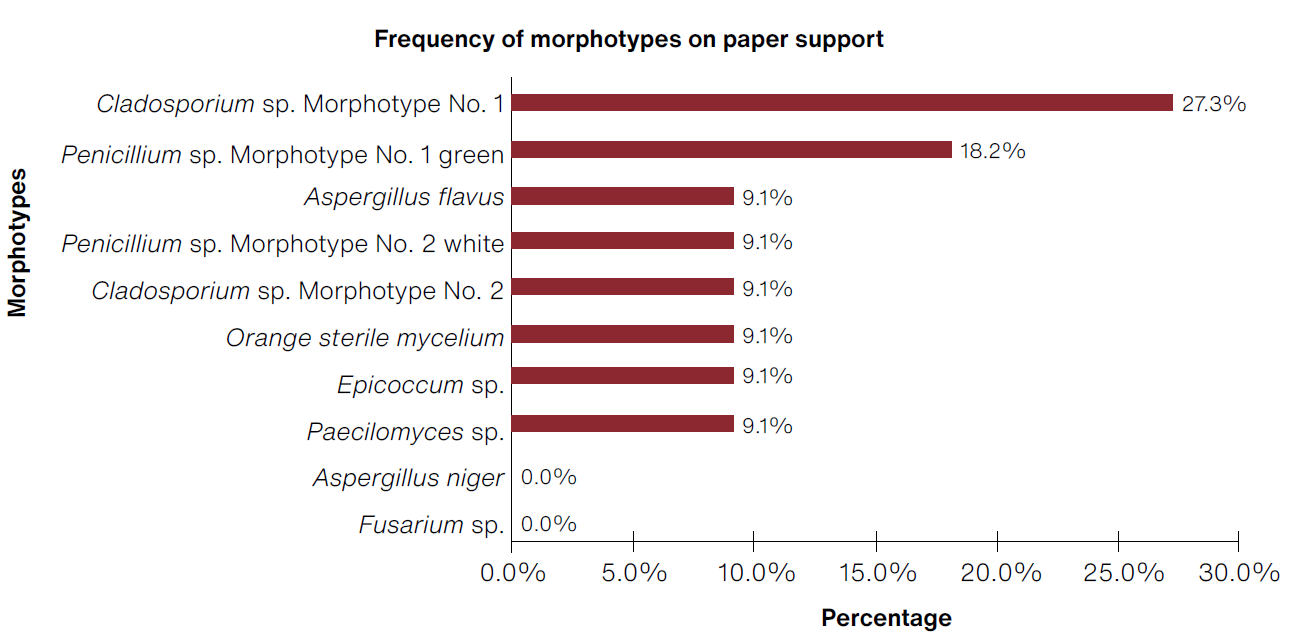
(Table: Lizeth P. Russy-Velandia, June 23, 2022)
Figure 8 Percentage of frequency of morphotypes identified on the paper support.
The greatest diversity of morphotypes were found on prototypes made of crepe paper (PAPER:CS, with six morphotypes) (Figure 9). Based on the selection criteria, it was determined that among the crepe paper supports, the prototype with the greatest detection capacity was PAPER:CS, with a cumulative of 2.31 CFU/cm2 and a brief time before the appearance of CFU, even during the first week, showing a greater cumulative growth of fungi colonies when compared to other prototypes at the end of the test.
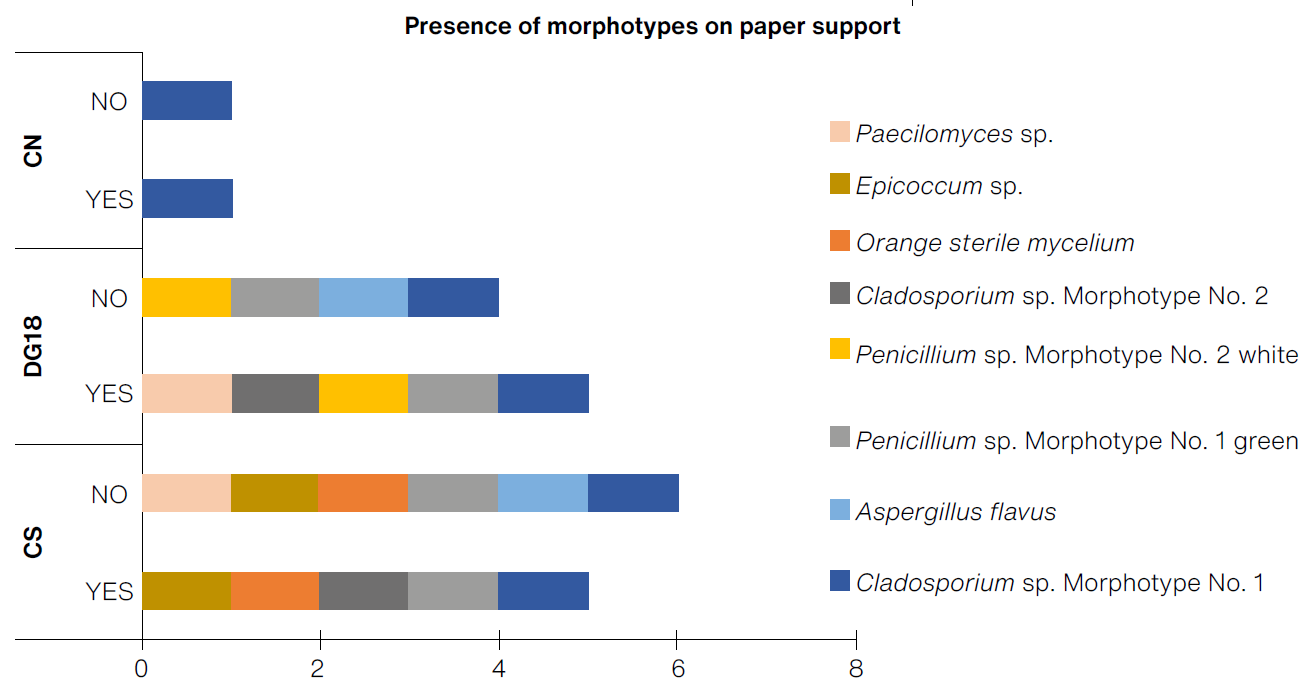
(Table: Lizeth P. Russy-Velandia, June 23, 2022)
Figure 9 Presence of morphotypes on the paper support, according to the three culture mediums, with and without indicator.
The prototypes made of goose feathers (Figure 10) presented the following frequency over a total of 21 morphotypes: 23.8% Cladosporium morphotype No. 1, 23.8% Aspergillus flavus, and 19.0% Aspergillus niger.
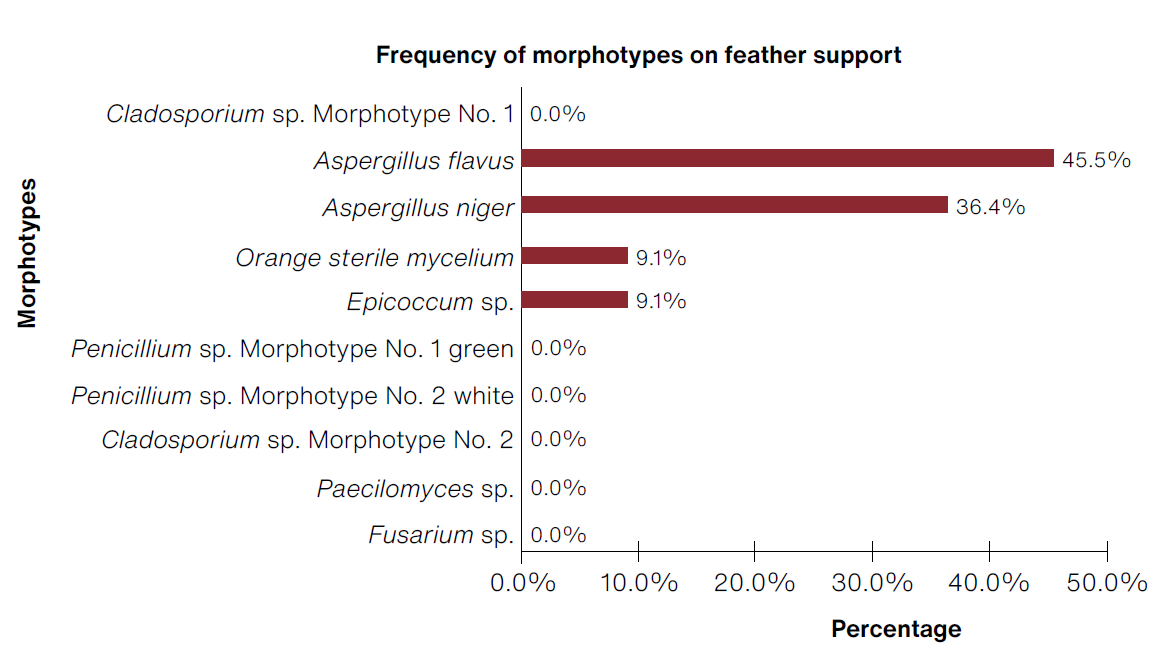
(Table: Lizeth P. Russy-Velandia, May 30, 2022)
Figure 10 Percentage of frequency of morphotypes identified on the feather support.
The greatest diversity of morphotypes were found on prototypes FEATHER:CS and FEATHER:CS:ABT, with five morphotypes each (Figure 11). Based on the selection criteria, it was determined that the goose feather support prototype with the best detection capacity was FEATHER:CS, with a cumulative of 1.25 CFU/cm2 and rapid detection of colonies of filamentous fungi as of the first week of the test.
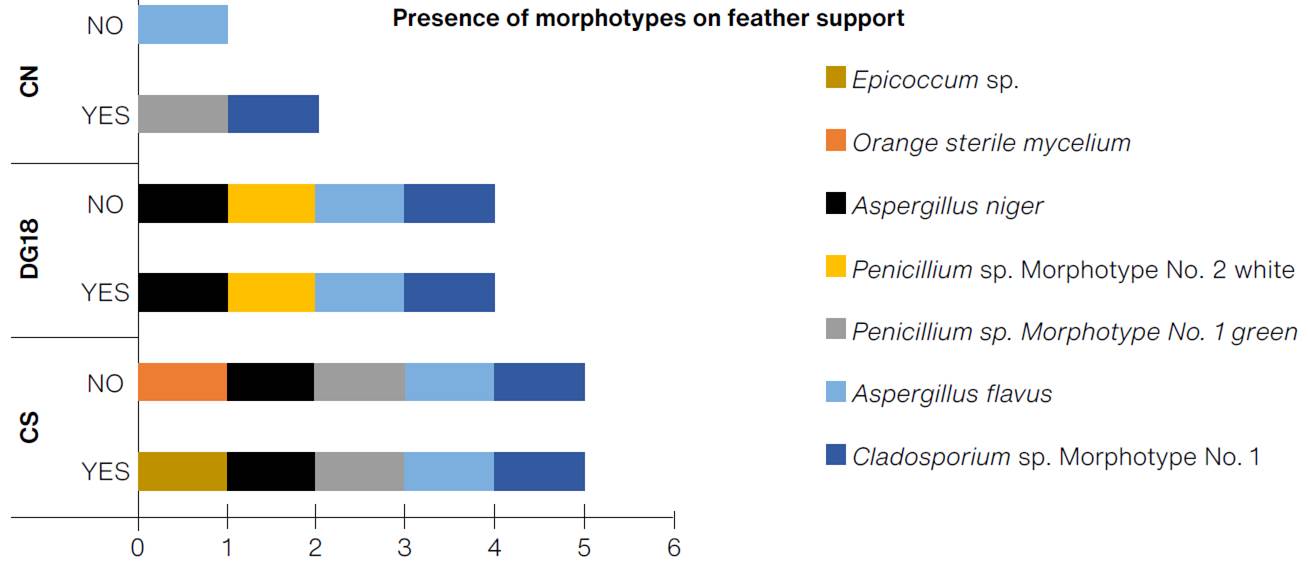
(Table: Lizeth P. Russy-Velandia, June 25, 2022)
Figure 11 Presence of morphotypes on the feather support, according to the three culture mediums, with and without indicator.
During the course of the tests, it was concluded that the pH indicator was not crucial for making biosensors since the results obtained were very similar with or without it and did not affect the growth of CFU/cm2, while the changes of color with the bromoethanol blue indicator were imperceptible.
As for the results obtained in the curing chamber, the biosensor prototypes made with nutrient culture isolated eight morphotypes of bacteria. Given that these were not visible to the naked eye, the growth of bacteria could only be observed once the supports were transferred and planted in Petri dishes with the same culture medium used to create the biosensors and incubated in the laboratory at 37° C for a week; hence they were not taken into consideration for the study.
Phase II. Evaluation of Prototypes in Environments of Collection Storage
The same biosensor prototypes were set out in two different Colombian cities in order to compare them. Bogota has an ambient temperature range between 14.5 and 22.5° C and a percentual relative humidity between 77 and 83 points (Natarajan, Rodríguez & Vellei., 2015, p. 244), while Medellin presents a range between 23.3 and 27.2° C and 62.6% RH (Restrepo-Betancur, Peña-Serna & Martínez-González, 2019, p. 315). Figure 12 summarizes the locations of the biosensors, the type of collection stored, as well as the average monthly temperature and relative humidity.
Figure 12 Location and collections of an organic nature in storage, where the best types of biosensor prototypes were placed as a system to detect microbiological risk, along with a record of the monthly average of temperatures and relative humidity
| Location of exposure | Biosensor Prototypes | Organic Collection | T° | %RH |
|---|---|---|---|---|
|
Museo Colonial (Bogota) |
Linen with DG18 broth | Collection of Colonial furniture: tapestries of various types, vegetable fibers and animal fur; also includes textiles with silver, gold, or copper thread as well as hand-painted textiles | 17.8 | 52.9 |
|
Universidad Militar Nueva Granada (Cajica) |
Paper with Sabouraud broth | Central archive: Documents, accounting ledgers, and registries in cardboard boxes | 19.9 | 59.2 |
| Feather with Sabouraud broth | José Ricardo Cure Hakim Zoological Collection: mammal furs and birds in storage | 17.7 | 62.0 | |
|
Museo Universitario Universidad de Antioquia (Medellín) |
Linen with DG18 broth | Anthropological collection: vegetable fiber textiles and archaeological textiles. | 22.7 | 57.3 |
| Paper with Sabouraud broth | Historical Collection: paintings, graphic works, photographs, texts and books in cardboard boxes | Not reported | Not reported | |
| Feather with Sabouraud broth | Natural Science Collection: taxidermied animals, skeletons, study skins, among other specimens | 19.5 | 58.7 |
(Table: Lizeth P. Russy-Velandia, July 11, 2022).
The prototype placed in the Colonial Museum of Bogota did not show any detection of CFU or filamentous fungi. The same occurred with the prototypes exposed in the UMNG, a result that is linked to the temperature and relative humidity registered in the three storage environments (Figure 12): on the one hand, in every collection, it does not go above 20° C, and although average monthly relative humidity can reach 62% in the José Ricardo Cure Hakim Zoological Collection, no microorganism growth was detected there either. In the city of Medellin, the registered average monthly temperature range was between 22.7 and 19.5° C, with a relative humidity of 58.7-57.3% (Figure 12); that is to say, quantities under 60%, which prevented the rehydration of the culture medium in those environmental conditions.
Since it did not display growth of CFU of filamentous fungi in any of the storage environments located in the different cities, the prototypes were taken to the laboratory to be rehydrated and incubated for a week at 25° C; thus, we proved that the prototypes had sedimented spores which did not grow until they were placed in optimal temperature and humidity conditions. Fungi of the genera Cladosporium, Penicillium, and Aspergillus grew, which are typically reported as biological contaminants in indoor sites (Khan & Karuppayil, 2012, pp. 406-414; Cepeda et al., 2019, pp. 42-44). The microorganisms found in those environments and recovered from the biosensors match the results obtained by Valentín (2015, p. 349), who reported contamination by environmental micro organisms corresponding to Cladosporium sp. and Penicillium chrysogenum (2017, p. 349) as well as by Borrego, Herrera, and Paneque and Penicillium in storage environments (2021, pp. 5-6), who reported the predominance of Aspergillus, Cladosporium.
The reported optimal conditions for the growth of microorganisms are between 25 and 30° C and 70 to 75% RH (Mallo et al., 2017, p. 621); however, none of the storage environments in this study reached an average monthly temperature over 25° C, and the highest percentage of relative humidity was 62 points. When environmental conditions are not favorable, the microorganisms’ capacity to begin reproductive processes diminishes (Mallo et al., 2017, p. 621). These results concur with those obtained in Phase I of the tests.
Conclusions
The environmental conditions of the positive control in Phase I (90% RH, 23° C) were determining factors for the development of filamentous fungi on our biosensor prototypes; the results obtained in the field phase demonstrate that the use of such biosensors is not viable in open storage environments with environmental conditions of relative humidity ranging between 52.9 and 62.0% RH and temperature ranging 17.7-19.9° C. The results of this study corroborate what was reported by Valentín, who stated that, as a rule, one does not visually detect the development of microorganisms on biosensors in museum display cases with reasonably good environmental conditions for a period of 30 to 60 days (Valentín et al., 2017, p. 104).
Moreover, placing biosensors in open spaces could promote the dehydration of the supports, which is why Valentín proposed that this strategy for preventing biodeterioration be applied in casings and containers (Valentín, 2015, p. 347). Among the microorganisms recovered from the biosensors, the most prominent are the fungi Cladosporium sp. and Penicillum., sp, as well as certain potentially dangerous species such as Aspergillus flavus, microorganisms that are frequently signaled as bio-contaminants in indoor spaces.
Thanks
We are especially grateful to Doctor Nieves Valentín for reviewing this document and for her feedback during the development of the research described.
REFERENCES
Arora, M. (2013). Cell culture media: a review. Mater Methods, 3(175),1-29. doi: https://doi.org/10.13070/mm.en.3.175 [ Links ]
Assis, T., Pawlak, J., Pal, L., Jameel, H., Reisinger, L. W., Kavalew, D., Campbell, C., Pawlowska, L. y Gonzalez, R. W. (2020). Comparison between uncreped and creped handsheets on tissue paper properties using a creping simulator unit. Cellulose, 27, 5981-5999. [ Links ]
Barde, M. P. y Barde, P. J. (2012). What to use to express the variability of data: Standard deviation or standard error of mean? Perspectives in Clinical Research, 3(3), 113-116. doi: https:// doi.org/10.4103/2229-3485.100662 [ Links ]
Barnett, L. y Hunter, B. (1998). Illustrated genera of imperfect fungi. The American Phytopathological Society Press. [ Links ]
Borrego, S. F., Herrera, O. y Paneque, I. (2021). Calidad micológica ambiental en archivos cubanos y su impacto en la salud del personal. Anales de la Academia de Ciencias de Cuba, 11(3), 1-17. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2304-01062021000300023 [ Links ]
Borrego, S. F., Perdomo, I., Guiamet, P. y Gómez de Saravia, S. (2010). Estudio de la concentración microbiana en el aire de depósitos del Archivo Nacional de Cuba. Augmdomus, 1, 118-137. https://revistas.unlp.edu.ar/domus/article/view/97/117 [ Links ]
Cepeda, R., Luque, L., Ramírez, D., Franco, P. y Fabra, M. (2019). Monitoreo de hongos ambientales en laboratorios y reservas patrimoniales bioarqueológicas. Boletín Micológico, 34(2), 33-49. doi: https://doi.org/10.22370/bolmicol.2019.34.2.1909 [ Links ]
Franco, A. E., Cepero, M. C., Cárdenas, M. E., Estupiñán, N. y Restrepo, S. (2012). Biología de hongos. Universidad de los Andes. [ Links ]
Gacto, M. y Gacto, M. (2011). Los microorganismos y el arte. Anales de Biología, 33, 107-115. https://www.um.es/analesdebiologia/numeros/33/PDF/33_2011_13.pdf [ Links ]
González, C., Acevedo, N., Cases, B. y Valenzuela, G. (2016). Tejidos para la muerte: análisis textil y egiptológico de vendajes funerarios del Museo Nacional de Historia Natural. Universum, 31(1), 173-189. doi: http://dx.doi.org/10.4067/S0718-23762016000100011 [ Links ]
Guinea J., Peláez, T., Alcalá, L. y Bouza, E. (2005). Evaluation of Czapeck agar and Sabouraud dextrose agar for the culture of airborne Aspergillus conidia. Diagnostic Microbiology and Infectious Disease, 53(4), 333-334. doi: https://doi.org/10.1016/j.diagmicrobio.2005.07.002 [ Links ]
Khan, H. A, A. y Karuppayil, S. M. (2012). Fungal pollution of indoor environments and its management. Saudi Journal of Biological Sciences, 19(4), 405-426. doi: https://doi.org/10.1016/j.sjbs.2012.06.002 [ Links ]
Lasprilla Rosero, L., Forero Lizarazo, L. M., Bernal Gómez, C. M., Alba Álvaro, W. R., Torres Landínez, A. Y., López Lagos, M. I., Vega Acosta, N. L., Rivera Zavala, J. C., Buitrago-Hernández, S. M., Suárez Díaz, H. A., Ortegón Meneses, L. A. y Tamayo Díaz, E. R. (2014). Identificación de agentes contaminantes de la colección del herbario de la Universidad Pedagógica y Tecnológica de Colombia, Tunja (Boyacá-Colombia). Conexión Agropecuaria JDC, 4(2), 25-44. https://revista.jdc.edu.co/index.php/conexagro/article/view/207 [ Links ]
Lee, D., In, J. y Lee, S. (2015). Standard deviation and standard error of the mean. Korean Journal of Anesthesiology, 68(3), 220-223. doi: https://doi.org/10.4097/kjae.2015.68.3.220 [ Links ]
Mallo, A. C., Nitiu, D. S., Eliades, L. A. y Saparrat, M. C. N. (2017). Fungal Degradation of Cellulosic Materials used as Support for Cultural Heritage. International Journal of Conservation Science, 8(4), 619-632. https://ri.conicet.gov.ar/bitstream/handle/11336/71511/CONICET_Digital_Nro.3193845c-d6ba-4b2c-ab1f-e1903321f181_A.pdf?sequence=2&isAllowed=y [ Links ]
Manrique, A., Patiño, M. C. y Gutiérrez, A. (2012). Estudio del microbiodeterioro del fondo documental Anselmo Pineda de la Biblioteca Nacional de Colombia. Conservamos. Guía técnica de preservación en bibliotecas, 5(5), 3-41. [ Links ]
Martín, D. y Villegas, M. A. (2021). Implementación de sensores de pH para valorar la conservación preventiva en un taller de restauración de pintura. Revista PH. Instituto Andaluz del Patrimonio Histórico, (102), 98-116. doi: https://doi.org/10.33349/2021.102.4608 [ Links ]
Merritt, J. (Agosto de 2007). Mold: prevention of microorganism growth in museum collections. Conserve O Gram, 3(4), 1-5. https://www.nps.gov/museum/publications/conserveogram/03-04.pdf [ Links ]
Natarajan, S., Rodriguez, J. y Vellei, M. (2015). A field study of indoor thermal comfort in the subtropical highland climate of Bogota, Colombia. Journal of Building Engineering, 4, 237-246. doi: https://doi.org/10.1016/j.jobe.2015.10.003 [ Links ]
Nitiu, D., Mallo, A., Elíades, L., Saparrat, M. y Vázquez, H. (2015). Monitoreo de la carga fúngica ambiental y de otros bioaerosoles en un depósito de restos momificados del NOA del Museo de la Plata (Argentina): un estudio de caso. Boletín de la Sociedad Argentina de Botánica, 50(4), 427-436. doi: https://doi.org/10.31055/1851.2372.v50.n4.12906 [ Links ]
Quitral, Y. A. (2020). Contaminación biológica en bibliotecas, reflexiones sobre una emergencia silenciosa. Revista Eletronica da ABDF, 4(número especial), 86-101. https://revista.abdf.org.br/abdf/article/view/126/130. [ Links ]
Restrepo-Betancur, L. F., Peña-Serna, C. y Martínez-González, M. F. (2019). Climate change in the city of Medellin-Colombia, throughout fifty years (1960-2010). DYNA, 86(209), 312-318. https://www.redalyc.org/journal/496/49662418037/49662418037.pdf [ Links ]
Rojas, L. P. (2019). Diagnóstico de biodeterioro de la colección de anatomía de la Universidad Militar Nueva Granada y evaluación de un método para su control [trabajo de grado]. Pontificia Universidad Javeriana. Colombia. Repositorio Institucional. https://repository.javeriana.edu.co/bitstream/handle/10554/43143/Trabajo%20de%20grado%20%20-%20Lina%20Rojas.pdf?sequence=5&isAllowed=y [ Links ]
Skóra, J., Gutarowska, B., Pielech-Przybylska, K., Stepien, L., Piwrezak, K., Piotrowska, M. y Pietrowski P. (2015). Assessment of microbiological contamination in the work environments of Museums, archives and libraries. Aerobiología, 31, 389-401. doi. https://doi.org/10.1007/s10453-015-9372-8. [ Links ]
Urkullu, T. (2001). Investigación del comportamiento de algunos textiles utilizados como soporte de pintura como fuente de documentación a procesos de restauración [tesis de Doctorado]. Universidad Complutense de Madrid. [ Links ]
Valentín, N. (2015). Biosensores como sistemas de alarma para detectar riesgos de biodeterioro en restos momificados. Estudios preliminares. Boletín del Museo Arqueológico Nacional, 33, 344-354. http://www.man.es/man/estudio/publicaciones/boletin-info/2010-2019/2015-33-18-valentin-info.html [ Links ]
Valentín, N., Sánchez, B., Durán, D., Muro, C., Herráez, Ma. I., Vilanova, O., Montero, J., Manrique, A. y Gaztañaga, A. (2017). Desarrollo de tecnologías para la detección precoz de contaminantes biológicos. Aplicaciones a vitrinas de aire y anoxia. En Ciencia y arte VI (pp. 101-119). Ministerio de Educación, Cultura y Deporte. https://www.libreria.culturaydeporte.gob.es/libro/la-ciencia-y-el-arte-vi-ciencias-experimentales-y-conservacion-del-patrimonio_1495/ [ Links ]
Villalba, L. S. (2015). Caso de estudio: modelo preliminar para evaluar biocontaminación en depósitos de archivo: parámetro de calidad de aire. Conservamos. Guía técnica de la preservación en bibliotecas 9(9), 22-30. https://www.academia.edu/20447081/Caso_de_estudio_modelo_preliminar_para_evaluar_biocontaminaci%C3%B3n_en_dep%C3%B3sitos_de_archivo_par%C3%A1metro_de_calidad_de_aire [ Links ]
Received: February 13, 2023; Accepted: August 18, 2023; Published: February 16, 2024











 texto em
texto em 



