Introduction
The postharvest life of horticultural products is frequently limited by rot due to fungal development (da Silva et al., 2019). A specific case is given by the affectation of fruits such as apple (Heinmaa et al., 2019) and citrus (Gandarilla-Pacheco et al., 2020) by Penicillium spp., which may limit consumption and shelf life. The use of plant extracts can reduce microbial development (León-Fernández et al., 2019). Among available alternatives, essential oils can be used to address such problems (Antonioli et al., 2020; Perczak et al., 2020). In particular, the use of a wide spectrum of essential oil as that of thyme (Thymus vulgaris) may constitute an option against several fungal species (Reyes-Jurado et al., 2019).
Essential oils (Eo) are liquid mixtures of volatile organic compounds, belonging mainly to the terpenes group. They have lipophilic character, strong aromatic properties, and their biosynthesis occurs as part of the secondary metabolism of some groups of plants, mainly through the mevalonic acid pathway (Rehman et al., 2016). Terpenoids and phenylpropanoids are the main constituents of Eo, which provide them with a characteristic aroma and different biological properties (da Silva et al., 2020). Also, they act as antimicrobial (Danzi et al., 2020), and insecticidal agents (Benelli & Pavela, 2018). On this basis, Eo can be applied in food preservation (Fellenberg et al., 2020), in alternative medicine, and in the therapeutic area (Rivera et al., 2015).
In order to favor an adequate inhibitory effect, direct contact of the plant extract with the vegetable to be protected has been frequently recommended (Rusin et al., 2021). In this regard, the essential oils have been used as part of biopolymeric coatings (Akhter et al., 2019) to reduce microbial development (Naeem et al., 2018). However, these materials must often include a lipid component to reduce permeability to water vapor (Galus & Kadzińska, 2015), which limits the action of the Eo due to a dissolution effect. According to Peretto et al. (2014), the controlled release of an Eo in the headspace of the system to be protected can cause greater inhibitory effect, which suggests that an encapsulated handling should be done.
Encapsulation consists of trapping one substance by another of polymeric nature (Zuidam & Shimoni, 2010). Among the advantages of encapsulation are the controlled release of such substance, the stability of active ingredients, increase of bioavailability, protection of the loaded ingredients from degradation, masking off-flavors, including bitterness and astringency of some ingredients, increasing of flavor and food safety, and improvement of stability against oxidation (Barroso et al., 2021).
The essential oils are volatile at ambient conditions and can be degraded by oxygen. Therefore, it is necessary to increase the stability, the activity, and the volatilization rate, which can be attended through an encapsulation technique (Mutlu-Ingok et al., 2020). The Ionic gelation by drip extrusion is a simple and efficient encapsulation method. The technique has low cost and low requirement of sophisticated equipment (Aceval et al., 2019), which give it high potential to be part of a technology transfer strategy aimed at the use of essential oils. Ionic gelation is based on the use of anionic polymers such as sodium alginate which, when combined with calcium ions, induce the formation of a gel, which finally creates a capsule (Bušić et al., 2018; Caporaso & Formisano, 2016) that traps the solution components. The controlled release of an encapsulated component constitutes one of the main goals of this practice and depends on the structure of capsules (Gorbunova et al., 2018), including porosity (Bušić et al., 2018), which may depend on the gelation time (González et al., 2015). In this context, the work aimed at controlling the release kinetics of thyme essential oil through ionic gelation encapsulation to assess the potential of inhibiting the development of Penicillium spp.
Material y Methods
Chemical reagents
Calcium chloride (CaCl2), ethanol (EtOH96), glutaraldehyde, sodium alginate (C6H7O6Na; AlgNa), and tween 80 were acquired with Reactivos Química Meyer S.A de C.V. Sodium cacodylate buffer ((CH3)2AsO2Na) was acquired with Sigma-Aldrich Química S.A. de C.V.
Ionic gelation encapsulation
Thyme (Thymus vulgaris L.) essential oil (Eo) provided by Laitz S.A. de C.V. (Mexico) was used. Eo was mixed with tween 80 at 1:1 ratio. Homogenization was applied with an Ultra-Turrax equipment (T25 basic, IKA Labortechnik, Staufen, Germany) operated at 13,500 rpm during 4 min. Solutions of AlgNa were prepared at 0.5 and 1.0 % and solutions of CaCl2 at 0.8 and 2.5 %. The Eo-tween mixture was combined with each of the AlgNa solutions to form emulsions with 2000 mL m-3 Eo. The Eo-AlgNa emulsions were dropped into the CaCl2 solutions, where residence was allowed for 10, 20, 40, and 60 min. At the end of each time, the formed capsules were washed twice by immersion in distilled water for 10 s to remove superficial calcium and they were stored in distilled water at 4 °C. Based on the combination of AlgNa/CaCl2 conditions, treatments 0.5/0.8, 0.5/2.5, 1.0/0.8, and 1.0/2.5 were formed with different gelation times. Capsules were evaluated in terms of diameter (
Essential oil release kinetics
A methodology was developed to evaluate essential oil concentration in emulsions. Solutions of Eo were prepared in ethyl alcohol 96° (EtOH96) in the range of 100 to 2000 mL m-3 and absorbance at 275 nm was read with an UV-vis spectrophotometer (DR 500, HACH, Germany). A linear regression was applied and Equation (2) was obtained with a determination coefficient of 0.9993, where
Capsules were prepared as described above. From each treatment, 33 samples of capsules weighing between 1 and 3 g were formed and placed at 25 °C. At intervals of 12 h, until completing 120 h, capsule samples were removed from each treatment to evaluate Eo content. Each sample was grounded in the Ultra Turrax equipment for 5 min at 13,500 rpm in 40 mL EtOH96. The system was allowed to settle, the liquid absorbance was measured at 275 nm, the concentration of Eo was quantified with Equation (2), and the volume of Eo (
Data were fitted to Equation (5) using the software Sigma Plot (SPSS Inc., 2000) and the resulting model was derived with respect to time (Equation 6), where
A container of volume
Container hermetic conditions were accepted, which allowed the output flow to be considered null
The right side of Equation (8) describes the variation of the essential oil volume inside the recipient (Equation 9) and, after the division of both sides by the container volume
The essential oil that was incorporated into the headspace came from capsules placed in the container. Thus, the product of essential oil concentration change of capsules and the mass of capsules
The substitution of Equation (11) into Equation (10) resulted in Equation (12) and the consideration of Equation (6) in Equation (12) resulted in Equation (13), which constituted the transient mass balance of the essential oil in the container headspace over time, where
Equation (13) was solved with the Fourth Order Runge-Kutta method (Burden & Faires, 2010) and results were fitted to models with the form of Equation (14) to facilitate the analysis, where
Encapsulation efficiency
AlgNa/CaCl2 treatments corresponding to 0.5/0.8 and 1.0/0.8 were selected to evaluate the encapsulation efficiency. Samples of capsules of 1 g were crushed in the Ultra-Turrax equipment for 5 min at 13,500 rpm and mixed with 40 mL EtOH96. After settling, absorbance was measured at 275 nm in the liquid with an UV-vis spectrophotometer (DR 500, HACH, Germany) and the theoretical concentration of essential oil (
Microscopic structure
The structure of capsules was observed using a Jeol Scanning Electron Microscope (JMS-035; Jeol Ltd., Akishima, Japan). Capsules were fixed with 3 % glutaraldehyde in 0.1 M cacodylate buffer for 24 h. The liquid was withdrawn and two additional washes with cacodylate buffer were carried out. Then, gradual dehydration was carried out with sequential washes in ethanol between 30 and 100 %. Drying at critical point was performed, a gold coating was applied, and samples were observed in the scanning electron microscope at 15,000x.
Evaluation of fungal inhibition
Orange fruits with fungal development were obtained from a local market. The contaminating fungus was sampled, incubated in Petri dishes with potato-dextrose-agar (PDA) culture medium, and observed with an optical microscope to identify the species that were present. The fungus Penicillium digitatum was selected as a working model. Samples of such fungus were placed in immersion in 1 L of sterilized distilled water and successive dilutions were made up to 10-5. PDA culture media were prepared in Petri dishes and these were inoculated at the center with the microbial dilution with bacteriological loop. The plates were individually placed in closed 1-L containers. The AlgNa/CaCl2 treatment corresponding to 1.0/0.8 with immersion of 10 min was used to conduct fungal inhibition tests. Samples of 1.0, 1.5, or 2.0 g were placed in felt bags inside the containers, together with the Petri dishes. A Control treatment was established with Petri dishes not exposed to the essential oil. Fungal growth was observed on the plates for five days.
Data analysis
Behaviors of the essential oil encapsulation and release kinetics were evaluated considering three variation factors: the concentration of sodium alginate, with two levels (0.5 and 1.0 %), the concentration of calcium chloride, with two levels (0.8 and 2.5 %), and the immersion time in the solutions, with four levels (10, 20, 40, and 60 min). Data were subjected to analysis of variance, complemented with mean comparison routines applied with the Tukey's test, performed with a significance level of 0.5. All evaluations were carried out in triplicate.
Results and Discussion
Physical characteristics of capsules
Capsules of thyme essential oil were formed through ionic gelation with AlgNa and CaCl2. Chains of D-mannuronic and L-guluronic acids interacted with calcium ions and formed a gel with a reticular structure (Ching et al., 2017). When the AlgNa-Eo emulsion fell into the CaCl2 solution, there was instantaneous gelation at the interface, which allowed the encapsulation of the Eo. Over time, the calcium ions diffused into each droplet and interacted with the AlgNa (Lee & Mooney, 2012). Gelation occurred by a cooperative union of calcium ions with residues of guluronic acid involving their dimerization. With the addition of calcium, the alignment of two opposite chains of guluronic acid with a hydrophilic cavity was induced and favored the union with calcium ions using oxygen atoms of the carboxylic groups, resulting in egg-box structures (Ching et al., 2017). Scanning electron microscope observation showed several layers of alginate from the outside to the inside, which was not very evident with low gelation times, but it was very noticeable with longer times (Figure 1). Consistent with the report of Bušić et al. (2018), high porosity was found.
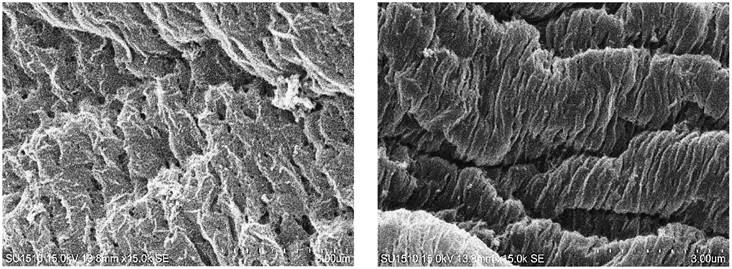
Figure 1 Structure of capsules obtained with ionic gelation from sodium alginate and calcium chloride solutions, with residence times in the calcium solution of 10 (left) and 20 min (right).
The capsules had a diameter in the range of 2.16 to 2.81 mm, similarly to that obtained by Aceval et al. (2019). Likewise, they had thickness between 0.20 and 0.46 mm and both dimensions were affected by the composition of the gelation system and by the residence time in the CaCl2 solution (Figure 2). The increase in AlgNa favored larger encapsulation diameters. Due to the polymeric nature of such compound, the increase in concentration caused higher viscosity, which caused a larger droplet size at the outlet of the dosing elements. Likewise, the capsule diameter tended to increase with higher concentration of CaCl2, but the contrast was significant only with residence times greater than 10 min.
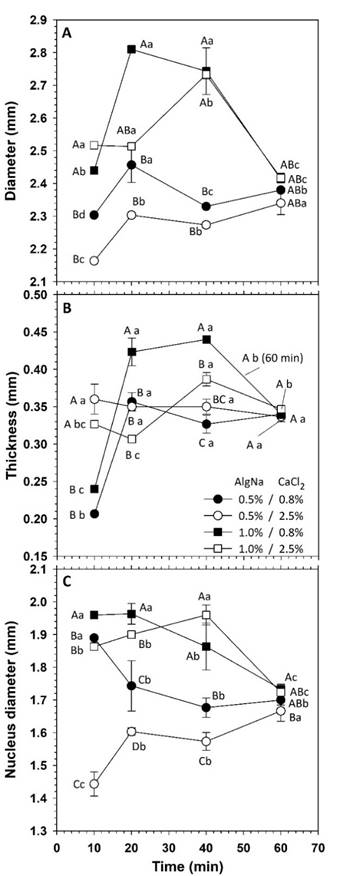
Figure 2 Dimensions of capsules prepared through ionic gelation. Equal capital letters indicate non-significant difference between means at the same time. Equal lowercase letters indicate non-significant difference between means within a treatment. Error bars indicate standard deviations.
Although gelation occurs instantaneously, the calcium ions diffuse inwards as time passed, causing a larger capsule size (Ching et al., 2017). However, the time in the calcium solution affected the diameter of capsules in different manner. With 0.5 % AlgNa, the diameter remained in the range of 2.16 to 2.45 mm and, although with immersion of 20 min values were higher, the contrast was considered moderate.
With 1.0 % of AlgNa, the residence time caused increase in diameter with the transition from 10 to 20-40 min of immersion, but a reduction in size was observed with 60 min. With long residence times, the capsules reached a homogeneous size, independently of the composition, with an average value of 2.39 (± 0.04) mm at 60 min and, although the contrast with respect to 10-min immersion was significant (p ≤ 0.05), the difference only varied within 2.55-5.67 %.
In the case of thickness, encapsulation with 2.5 % CaCl2 caused values of 0.34 (± 0.03) mm, with no significant difference between different times, which suggested that, from the first contact of AlgNa with calcium ions, the gel membrane properties were not affected by additional residence times. On the other hand, with 0.8 % CaCl2, the thickness varied within 0.20-0.24 mm with 10 min immersion and between 0.35 and 0.44 mm with times of 20-40 min.
The procedure used corresponded to a direct gelation and the thickness of the membrane capsule is proportional to the reaction time in the calcium bath (Caporaso & Formisano, 2016), so the development of the capsule must be stopped by removing the spheres from the solution. Although this coincided with the systems with 0.8 % CaCl2, it was not observed with 2.5 % concentration. However, similarly to the diameter, with a time of 60 min, the thickness tended to a homogeneous value of 0.34 (± 0.01) mm in all systems, without significant difference between them (Figure 2B).
The time of 60 min was the longest period evaluated at work. As time passes, the porosity of the capsular membrane decreases due to a greater polymer crosslinking with calcium ions, which might have caused contraction and even matrix dehydration due to the expulsion of water.
The behavior with 0.8 % CaCl2 suggested that, depending on composition, it is feasible to control the diameter and the thickness of capsules. Based on this result, mixtures with that concentration of CaCl2 were selected to evaluate the behavior of the encapsulation systems. However, as the capsule structure is not rigid and changes with the interaction between ions, a more stable state is reached as time advances, ceasing the dependency to the system composition.
On the other hand, the amount of trapped oil is determined by the dimensions of the capsule core. The lowest
Table 1 Encapsulation efficiency through ionic gelation based on sodium alginate at 0.5 and 1.0 % and CaCl2 at 0.8 % with different residence times in the calcium solution.
| SystemAlgNa/CaCl2 | t (min) | η (%) | SystemAlgNa/CaCl2 | t (min) | η (%) |
|---|---|---|---|---|---|
| 0.5 %/0.8 % | 10 | 92.95b (2.16) | 1.0 %/0.8 % | 10 | 94.01ab (1.89) |
| 20 | 88.23d (1.64) | 20 | 90.13cd (2.08) | ||
| 40 | 90.71c (1.95) | 40 | 92.29cd (1.56) | ||
| 60 | 93.67ab (2.01) | 60 | 95.18a (1.85) | ||
| HSD | 2.12 |
The symbols t and η indicate the residence time in the calcium solution and the encapsulation efficiency, respectively. Equal letters indicate non-significant difference. HSD is honest significant difference (Tukey, 0.05). Values in parentheses correspond to standard deviations.
Systems based on the 0.8 % CaCl2 solution were evaluated in terms of encapsulation efficiency (
Kinetics of essential oil release
The release of the essential oil from the capsules had a logarithmic behavior and data were properly fitted to Equation (14), with determination coefficients higher than 0.99 in all cases. Thus, the increase in concentration in the headspace of containers occurred rapidly at the beginning and thereafter the rate of change gradually slowed down, so the presence of Eo tended to a constant value (Figure 3). This behavior was due to the release of Eo was carried out in a closed container and, over time, the concentration gradient between the interior of the capsules and the headspace was gradually reduced, thereby also reducing the release. However, if release develops in systems with higher dimensions such as a greenhouse, it shall be more important the evaluation of the concentration change as a function of the distance from the liberation point.
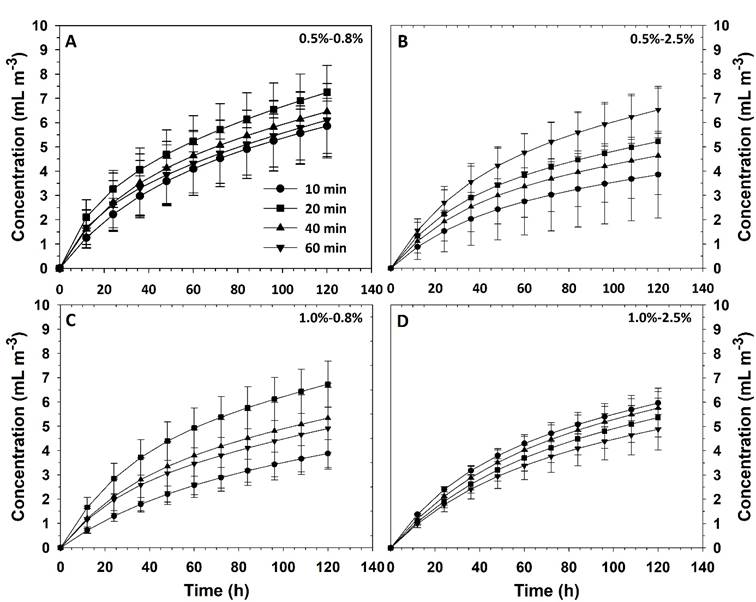
Figure 3 Thyme essential oil release kinetics affected by the composition of AlgNa-CaCl2 systems and the immersion time in the calcium solution.
The constant
Table 2 Time and kinetics constant average values for different essential oil concentrations, in a 1 L container, generated from the release from 1 g of capsules.
| Variation factor |
|
|
|
|
|
|---|---|---|---|---|---|
| Concentration of AlgNa | |||||
| 0.5 % | 6.59 a (0.52) | 5.4167a (0.4444) | 38.26a (8.3737) | 114.78a (25.12) | 254.19a (55.63) |
| 1.0 % | 6.44 a (0.45) | 4.8611b (0.3333) | 40.94a (9.20) | 122.82a (27.61) | 271.99a (61.14) |
| HSD | 0.99 | 0.4722 | 21.50 | 64.68 | 143.24 |
| Concentration of CaCl2 | |||||
| 0.8 % | 7.09 a (0.05) | 5.9167a (0.5278) | 34.01a (12.35) | 102.02b (37.05) | 225.93b (82.06) |
| 2.5 % | 5.98 b (0.40) | 4.6778b (0.1944) | 43.04a (1.93) | 129.12a (5.78) | 285.95a (52.80) |
| HSD | 0.99 | 0.5833 | 21.58 | 24.74 | 23.38 |
| Time | |||||
| 10 min | 5.89b (0.57) | 4.8333b (0.4167) | 41.81a (6.64) | 125.44a (19.94) | 277.80a (44.15) |
| 20 min | 6.62ab (1.01) | 5.1667a (0.4444) | 40.66a (5.91) | 112.11ab (63.59) | 248.27b (48.82) |
| 40 min | 6.41ab (0.51) | 5.1111a (0.7778) | 38.65a (6.58) | 115.94ab (19.74) | 256.76ab (43.71) |
| 60 min | 7.14a (0.58) | 5.0278a (0.6389) | 37.37a (21.20) | 121.97b (17.74) | 270.11ab (39.28) |
| HSD | 1.13 | 0.2500 | 23.09 | 22.28 | 27.30 |
t1/2 , t7/8 and t0.99 are required times to reach 50.0, 87.5, and 99.0 % of the maximum concentration in the container, respectively. Equal letters indicate non-significant difference. Values in parentheses are standard deviations. HSD is honest significant difference (Tukey, 0.05).
The constant
Although the constant
Average
These results indicated that, based on the concentration of AlgNa and CaCl2 solutions, it is feasible to control the release rate of Eo, but this can only be performed with immersion times between 10 and 20 min within the calcium solution, since different behaviors were not obtained with longer residence times. However, the higher release rate with 20 min was caused by a lower encapsulation efficiency (Table 1), where the superficial oil volatilized faster than that of the nuclear region. In this regard, since the superficial concentration of essential oil cannot be controlled, the 10-min residence time was considered a better alternative to attend the encapsulation of the essential oil.
Potential for fungal inhibition
The formulation AlgNa/CaCl2/t-1.0 %/0.8 %/10min was used to evaluate the potential of the capsules for fungal inhibition, due to the larger nuclear diameter and high encapsulation efficiency obtained. A searching process was carried out for the required quantity of capsules to inhibit the development of Penicillium digitatum, which had been inoculated on culture plates, through the release of essential oil in their headspace.
The growth of the fungus in the Control treatment, without essential oil exposure, varied from low to moderate on the first day of incubation, from moderate to high on the second, and it was high in all the cases after the third day (Table 3). In contrast, when the inoculated medium was exposed to the presence of 2.0 g of capsules, the released oil inhibited totally the fungus development. In order to find the capsules minimum inhibitory quantity (MIQ), the test was repeated with batches of 1.0 g of capsules and a low to moderate growth was observed in the first two days. A moderate growth was noted afterwards until the end of the five days evaluation period. These results indicated that the MIQ was between 1.0 and 2.0 g. In this sense, a third test with batches of 1.5 g of capsules showed that, with this quantity, a total inhibition of the fungus was also achieved. Although results indicated that the MIQ was between 1.0 and 1.5 g of capsules, the quantity used in the last test, that is, 1.5 g of capsules, was accepted as the minimum required to cause the inhibition of the fungus.
P. digitatum is an important factor that limits the shelf life of citrus fruits and it is known as green mold. Besides, other species of the genus Penicillium limit the consumption of fruits as apple due to the production of mycotoxins (Heinmaa et al., 2019). Talibi et al. (2014) pointed out that the use of essential oils, particularly those of cinnamon and thyme, have potential to control most rots of citrus in postharvest, including green mold, blue mold, and sour rot.
Table 3 Development of the fungus Penicillium digitatum inoculated in culture medium exposed to the release of thyme essential oil from capsules placed in the headspace of closed containers of 1 L.
| Day | Assay 1 | Assay 2 | Assay 3 | |||
|---|---|---|---|---|---|---|
| Control | Treat: 2.0 g | Control | Treat: 1.0 g | Control | Treat: 1.5 g | |
| 1 | Low | Null | Moderate | Low | Low | Null |
| 2 | Moderate | Null | High | Moderate | Moderate | Null |
| 3 | High | Null | High | Moderate | High | Null |
| 4 | High | Null | High | Moderate | High | Null |
| 5 | High | Null | High | Moderate | High | Null |
Control: handling without the presence of capsules. Treat: handling with 1.0, 1.5, and 2.0 g.
The proposed mechanism of action of essential oils against microorganisms includes the affectation of the cell membrane, causing its disruption and making it more permeable, altering ion transport processes, thus modifying the concentration of electrolytes like K+, Ca2+, and Na+. Also, it has been suggested the interaction with membrane proteins, which may cause the disruption of the functions of mitochondria, which conduces to an imbalance in ATP concentration and, eventually, the death of the cell (Mutlu-Ingok et al., 2020).
Talibi et al. (2014) pointed out that the use of essential oils in citrus fruits can be made difficult by possible problems of phytotoxicity or alteration of sensory attributes. In this regard, the results of the present work showed that a direct contact with the product is not required, since the essential oil is released in the headspace. This use based on the release of essential oil vapors in the headspace was consistent with the use of biopolymeric films, where Peretto et al. (2014) incorporated carvacrol and methyl cinnamate at 0.75 %, and these compounds were vaporized from there and achieved fungal control in strawberry fruits.
Also, Martínez-Romero et al. (2007) reported the high effectiveness of an essential oil through the use of carvacrol vapors at a concentration of 1000 mL m-3 in the headspace of grapefruits. Similarly, López-Gómez et al. (2021) released vapors of eugenol, bergamot, and grapefruit essential oil at a concentration of 100 mL m-3 in a modified atmosphere system that allowed the quality of mushroom slices to be maintained for 12 d.
The use of essential oils to reduce microbial growth is normally based on direct contact through biopolymeric coatings, but the results of this work showed that the use of vapors with controlled release can be done through encapsulation. In this regard, a release analysis based on Equation (13), with the parameters of the AlgNa/CaCl2/t-1.0/0.8/10min system showed that the concentration of Eo in the headspace that is required to cause inhibition was less than 100 mL m-3, which contrasted with the concentrations that must be used with biopolymeric coatings, fluctuating between 500 and 1000 mL m-3 (Valle-Ortiz et al., 2019), indicating that the release of essential oils from encapsulation systems constitutes a better strategy to control the fungus development.
Nowadays, the evidence for using essential oils to reduce losses caused by microbial development is growing. However, it is necessary to attend several aspects to improve the potential for using such substances in the conservation of horticultural products. In this regard, Mutlu-Ingok et al. (2020) pointed out the following aspects that may part of future researches for using vapors of essential oils: the quantity to be used should consider not only the minimum inhibitory concentration (MIQ) but also the maximum sensorially permitted concentration (MSC) to avoid the modification of the quality attributes of the product, for which designed mixtures of different essential oils may be applied, considering the synergistic effect between them. The determination of MIQ must also consider the control of both, the growth and mycotoxin production of fungi, the part of the plant to be protected, the geographical aspects, and the extraction method of the essential oil. Authors explained that it is also necessary to investigate the antifungal actions not only against monocultures, but also against polycultures. In addition, new strategies for improving the stability of essential oils are required. In relation to this, an encapsulation strategy as that showed in the present work can be adequate, together with the modeling of the kinetics release, which can allow determining the quantities to be used in a better form, once the MIQ and the MSC have been established.
Conclusions
Thyme essential oil (Eo) was encapsulated by ionic gelation with sodium alginate (AlgNa) and calcium chloride (CaCl2). The concentration of solutions and the residence time (











 nova página do texto(beta)
nova página do texto(beta)



