Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista ALCONPAT
versão On-line ISSN 2007-6835
Rev. ALCONPAT vol.6 no.1 Mérida Jan./Abr. 2016
Applied research articles
Mechanical and chemical behavior of calcium sulfoaluminate cements obtained from industrial waste
1 Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Av. Industria Metalúrgica 1062, Parque industrial Saltillo-Ramos Arizpe, Ramos Arizpe, Coah., México CP. 25900.
A calcium sulfoaluminate clinker was synthesized calcining a mixture of fly ash, fluorogypsum, aluminum slag, and calcium carbonate at 1250 ºC. The clinker was mixed with 15, 20, or 25% e.p. of CaSO4·½H2O. The pastes were prepared with a water/cement ratio of 0.5. Compression resistance of cements cured in potable water and corrosive mediums at 40 ºC was evaluated. The cements cured in potable water developed compressive strengths of 38-39 MPa; those immersed in corrosive mediums showed a decrease in this property after the chemical attack. Ettringite was the main product of hydration. The degradation of the cements by chemical attack was due to a decalcification and dealumination of the pastes.
Keywords: calcium sulfoaluminate; ettringite; compressive strength; chemical attack
Se sintetizó un clínker de sulfoaluminato de calcio calcinando una mezcla de ceniza volante, fluoryeso, escoria de aluminio y carbonato de calcio a 1250 °C. El clinker fue mezclado con 15, 20 o 25 % e.p. de CaSO4·½H2O. Las pastas se prepararon con relaciones agua/cemento de 0.5. Se evaluó la resistencia a la compresión de cementos curados en agua potable y en medios corrosivos a 40 °C. Los cementos curados en agua potable desarrollaron resistencias a la compresión de 38-39 MPa, los inmersos en medios corrosivos presentaron una disminución en esta propiedad después del ataque químico. La etringita fue el principal producto de hidratación. La degradación de los cementos por ataque químico es debida a una descalcificación y dealuminación de las pastas.
Palabras clave: sulfoaluminato de calcio; etringita; resistencia a la compresión; ataque químico
Foi produzido um clínquer de sulfoaluminato de cálcio a partir da calcinação a 1250oC de uma mistura de cinza volante, escória de alumínio, carbonato de cálcio e gesso de flúor. Esse clínquer foi misturado com 15%, 20% e 25% e.p. de CaSO4·½H2O. As pastas foram preparadas com relação água/cimento igual a 0,5. Foi avaliada a resistência à compressão das pastas curadas em água potável e em meios corrosivos a 40oC. As pastas curadas em água alcançaram resistências à compressão de 38-39 MPa, enquanto as pastas imersas em meios corrosivos apresentaram uma redução da resistência frente ao ataque químico. A etringita foi o principal produto da hidratação desses cimentos. A degradação dessas pastas de cimento por ataque químico ocorreu devido a uma descalcificação e dealuminização dos produtos hidratados.
Palavras chave: sulfoaluminato de cálcio; etringita; resistência à compressão; ataque químico
1. INTRODUCTION
Cement is a material utilized in the construction of cities and houses, with a growing demand dependent upon demographic growth. The use of concretes constituted with appropriate materials, conveniently provided and well-consolidated, ensures the durability of the constructions. The most frequently used binding material in construction is Portland cement; however, in its production processes large quantities of fossil fuels and, in a parallel manner in its decarbonation process of raw materials large quantities of CO2, are emitted into the atmosphere, contributing around 7% of the global CO2 emissions (Roy et al., 1999; Gartner et al., 2004). There are a variety of alternative and viable materials (industrial wastes) that can be used for the substitution of Portland cement. Their use is based on the importance of minimizing CO2 emissions, as well as increasing interest in the production of cementive materials that develop good mechanical properties and good stability in corrosive environments.
An alternative to partially or completely substitute the use of Portland cement is the manufacture of calcium sulfoaluminate (CSA) cement which presents a crystalline structure that consists of a tridimentional arrangement of AlO4 tetrahedrons interlinked with Ca+2 and SO4
-2 ions located in the existent intervals (Sharp J.H. et al., 1999). This type of cement has low CO2 emissions, forming at a temperature of approximately 1250 ºC, in addition to developing good mechanical properties (compression resistance) (Older, 2005; Zhou et al., 2006; Garcia-Maté et al., 2015; Hargis et al., 2014). On the other hand, there is a large quantity of wastes such as slag, gypsum, and fly ash among others, that show considerable quantities of Al2O3, CaO, and CaSO4 in their chemical composition, these being the main components for the production of CSA clinkers. An important advantage derived from the manufacture of this clinker from industrial waste is the decrease in air, soil, and visual contaminations that these create (stored outdoors) and the decrease in CO2 emissions to the environment. Li et al. reported the synthesis of CSA utilizing high alumina fly ash, bauxite, and limestone as the starting materials (Li et al., 2007), where the main stages of synthesis were CSA and tricalcium silicate (C3S). A sulfoaluminate-belita was synthesized at 1150 ºC from fly ash, powders, and muds collected from industrial processes. As a product of synthesis, the clinker obtained showed stages such as gehlenite (formed by the decomposition of belita at temperatures higher than 1100 ºC), CSA, and belita (Li et al., 2001). During the synthesis of CSA utilizing industrial wastes, the formation of gehlenite is obtained as a secundary product of synthesis, this stage being responsible for the decreased formation of CSA (Arjunan et al., 1999). The synthesis of a sulfoaluminate-ferrite through the use of lime, gypsum, red mud, and bauxite at 1250 ºC showed stages such as C4(A3F)3
2. EXPERIMENTAL PROCEDURE
The materials utilized were fly ash (fa), aluminum slag (as), and fluorogypsum (fg). The latter two were subject to a grinding process until a particle size less than 106 µm (#140 ASTM mesh) was obtained. Subsequently, the chemical composition of each material was analyzed through X-ray fluorescence (XRF), the results of which are shown in Table 1.
Table 1. Chemical composition in oxides of the raw material.
| Oxides | Aluminum Slag as (e.p. %) | Fly Ash fa (e.p.%) | Fluorogypsum fg (e.p.%) |
| Na 2 O | 2.496 | - | - |
| MgO | 5.000 | 1.377 | - |
| Al 2 O 3 | 63.19 | 24.81 | - |
| SiO 2 | 11.69 | 59.49 | 0.103 |
| SO 3 | 0.833 | - | 56.33 |
| Cl 2 | 4.636 | - | - |
| K 2 O | 2.203 | 1.716 | - |
| CaO | 7.263 | 4.806 | 43.24 |
| TiO 2 | 0.983 | 1.663 | - |
| MnO | 0.503 | - | - |
| Fe 2 O 3 | 1.203 | 6.126 | - |
Based on the chemical composition, the wastes were mixed in the required proportions in order to obtain CSA in accordance with the following reaction: as + fa + fg + CaCO3 = CSA (80% e.p.) + Ca2SiO4 (20% e.p.). The proportions of each starting material were adjusted with CaCO3 (97% purity). The starting materials were homogenized in plastic containers with acetone and alumina balls for 4 hours; they were subsequently dried at 80 ºC for 12 hours. Pills 2 cm in diameter were made through uniaxial pressing at 45 MPa, which were then subject to thermal treatment at 1250 ºC for 4 hours. The formation of the CSA was corraborated through X-ray diffraction. The clinker obtained was grounded until obtaining a specific superficial area of approximately 3800 cm2/g (ASTM C-204), and was subsequently mixed with 15, 20, or 25% e.p. calcium sulfate (CaSO4·½H2O) in order to prepare the CSA cement. The pastes were prepared with a water/cement ration of 0.5 in accordance with the procedure indicated in the Mexican standard NMX-C-085-ONNCC-2002 (NMX-C-085). The nomenclature of the systems was the following: 515, 520, and 525, where the first number indicates the water/cement ration and the latter two indicate the calcium sulfate content. The mixtures were emptied into Nyalcero molds and vibrated for 60 seconds in order to eliminate porosity. The already filled molds were covered with plastic and placed in isothermic chambers at 40 ºC for 24 hours. Subsequently, the cubes were removed from the molds and placed in containers with water (potable water) in order to begin humid curing at the aforementioned temperature for 1, 3, 7, 14, or 28 days; after each curing period, the compressive strength was evaluated. For the effect of comparison, Ordinary Portland cement (OPC commercial) samples were elaborated as control references. These samples were elaborated and cured under the same conditions as the CSA-based cements.
For chemical durability tests, samples were cured for 7 days in potable water which were subsequently submerged in corrosive mediums for 14, 28, or 42 days for the chemical resistance evaluating by means of measuring their compressive strength. The samples were submerged in 2 liters of H2SO4 0.5 N, MgCl2 06 N, and Na2SO4 0.04 N solution (elaborated with deionized water) at 40 ºC in order to simulate aggressive conditions. The compressive strength measurements were done in an automated hydraulic press (Controls model 50-C7024) with a 250 kN capacity, using a loading speed of 350 N/s. The tests were done in accordance with the procedure described in the standard ASTM C109/C109-M95 (ASTM-C109). In order to identify the stages present resulting from the hydration reactions and the chemical attack, samples with 28 (potable water) and 42 (corrosive medium) days of immersion were analyized through X-ray diffraction (XRD). Fragments were selected, preferably from the surface of each one of the cubes tested in a chemical attack, and were submerged in methanol and dried for 48 hours in an oven at 40 ºC. Samples were analyzed before and after the chemical attack through Scanning Electron Microscopy (SEM).
3. RESULTS
Figure 1 shows the diffraction pattern of the synthesized clinker at a temperature of 1250 ºC. The reflections corresponding to the CSA were primarily observed. Secondary stages such as gehlenite (Ca4Al2SiO7), calcium aluminate (CaAl2O4), mayenite (Ca12Al14O33), and pleochroite (Ca20Al26Mg3Si3O68) were formed. Belite (Ca2SiO4) was obtained in a lesser percentage than what was intended due to the thermodynamic stability of secondary stages formed at 1250 ºC. On the other hand, gehlenite and spinel are considered non-cementing stages due to their small or lack of reactivity in the presence of water, but these could act as reinforcement stages.
Figure 2 shows the compressive strength results of systems 515, 520, and 525 cured from 1 to 28 days at 40 ºC. In system 515, a gradual development of the compressive strength was observed from day one and up to 14 days; at 28 days the compressive strength decreased, favored by the delayed ettringite formation. System 520 showed a decrease in compressive strength at 7 days with a slight increase at 14 and 28 days; however, a low strength developed ending with 24.25 MPa. In system 525, an increase in the compressive strength was observed in function with time (from 1 to 14 days); at subsequent times it remained at the same strength of 38.9 MPa at 28 days. It is possible that ettringite formed almost in full during the first days of curing, and the subsequent increase is due to the growth of this stage within the pores weak zones (micro-cracks). The presence of gypsum and CSA residue in the days following the start of the curing process indicates that the hydration reactions shall continue with the passage of time. This system showed the best compressive strength values. Based on the aforementioned, a greater quantity of gypsum increases the mechanical resistance due to the formation of the largest quantity of ettringite from the first days of curing. The compressive strength results obtained were found within the value established in the standard NMX-C061-ONNCCE-2001 (20-40 MPa at 28 days of curing). The experimentally obtained value for the OPC cement was 38 MPa.
Figures 3 and 4 show the XRD patterns of systems 515 and 525 at 1, 14, and 28 days of curing at 40 ºC. In system 515 (Figure 3), during the first day reflections were observed corresponding to ettringite and these reflections increased in intensity at 14 and 28 days of curing. In system 525 (Figure 4), during the first day reflections were observed corresponding to the CSA and gypsum, these latter reflections were observed up to 14 days later. The reflections corresponding to ettringite were visible from day one and increased in intensity at 14 and 28 days. The gradual increase in intensity of the reflections of the hydration products indicated the delayed ettringite formation. This was not enough to affect the mechanical properties given that the increase of this stage possibly took place within the present pores. For both systems, reflections corresponding to the spinel and gehlenite were observed without apparent changes in the intensity of the reflections in function to the time of curing, due to these two stages being inert in the presence of water.
Figure 5 shows the micrographs of fracture surfaces of systems 515 and 525 at 1, 14, and 28 days of curing at 40 ºC. In system 515 during the first day, a dense microstructure is seen with the presence of some cracks distributed in the matrix. At 14 days, a microstructure is observed with a denser and more compact matrix where the cracks decreased in quantity explaining the increase in compressive strength that was presented in the curing time. At 28 days of curing, some weak zones were observed generated by the growth of hydrates giving rise to a decrease in the compressive strength of the material.
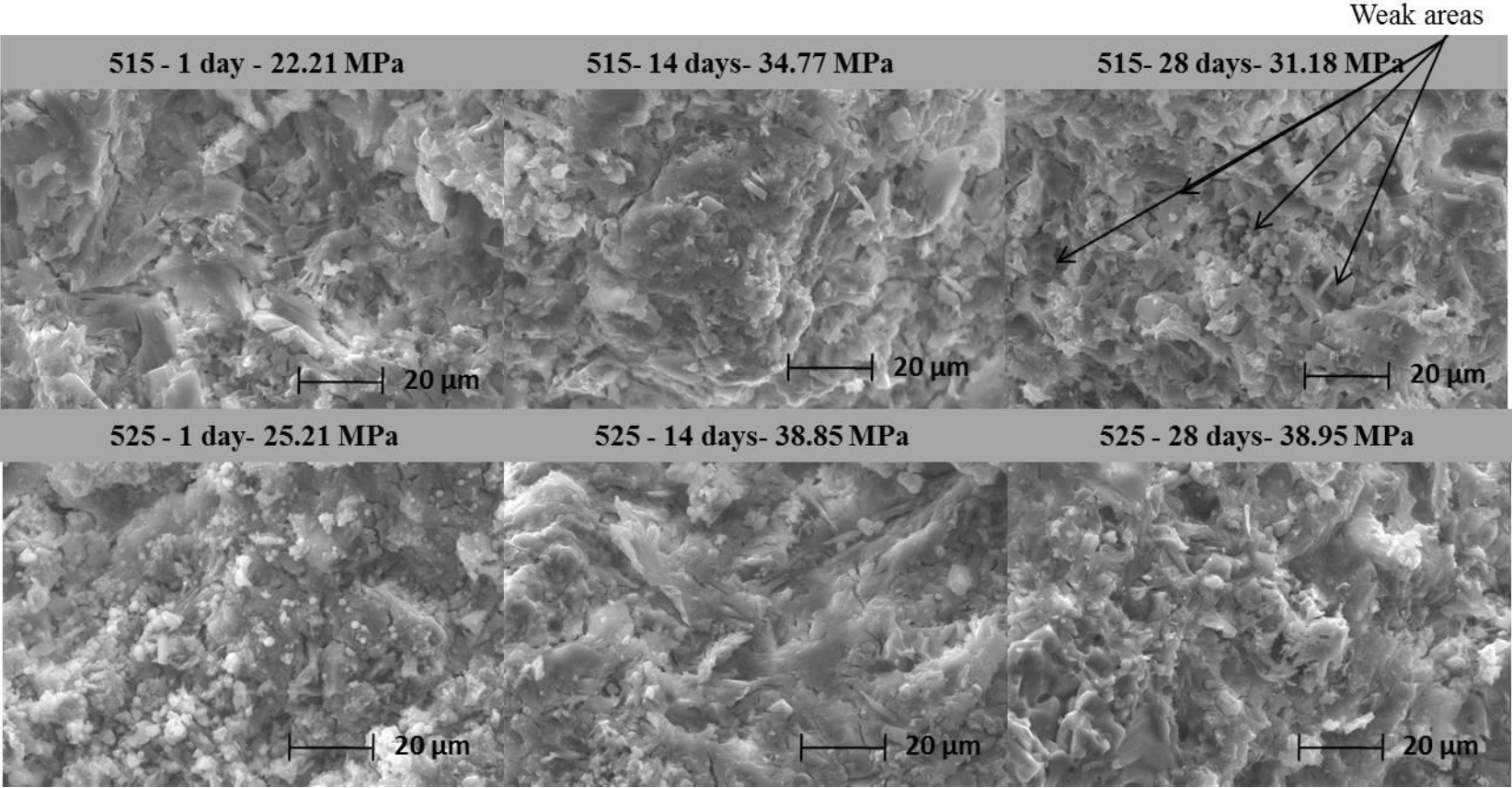
Figure 5. Micrographs of fracture surfaces of samples of systems 515 and 525 at 1, 14, and 28 days of curing at 40 ºC.
In the surface microstructures of system 525 on day one, white nodes were observed immersed in a dense ettringite matrix. These white nodes are associated with the formation of gypsum. At 14 days of curing, greater densification of the matrix was presented similar to the microstructure observed at 28 days, corroborating the similarity in mechanical properties at 14 and 28 days of curing. System 525 developed the best compressive strength, showing greater stability in function to time by not showing decreases in resistance. Due to the aforementioned, the stability of this system was evaluated immersed in aggressive solutions. Samples of this cement were cured for 7 days in potable water (38.95) and subsequently immersed in corrosive solutions.
Figure 6 shows the compressive strength results obtained from the samples after being immersed in corrosive mediums for 7, 14, 28, and 42 days at 40 ºC. The samples immersed in a solution of NaSO4 0.04 N developed the greatest compressive strength at 42 days of curing (34.63 MPa). The samples immersed in solutions of H2SO4 and MgCl2 developed similar compressive strength at 42 days with values of 28.10 and 29.2 MPa, respectively. A decrease in compressive strength of 28.6 and 25.83% was observed in samples immersed in H2SO4 and MgCl2 solutions, respectively. These variations could be attributed to the interaction of the samples with the Cl-, Mg+2, SO4 -2, and Na+ ions present in the corrosive solutions. The samples immersed in potable water did not show a decrease in compressive strength at any time during the curing (1-28 days).
The results obtained from the XRD characterization of the samples immersed in aggressive solutions at 42 days of curing are shown in Figure 7. High intensity reflections were observed corresponding to gypsum in samples immersed in a H2SO4 solution, indicating a degradation of the ettringite caused by the diffusion of SO4 -2 ions inside of the microstructure, causing a dissolution of the material. For samples immersed in MgCl2 and Na2SO4, a pattern was observed similar to the one described above, except that the reflections corresponding to gypsum had low intensity indicating a moderate attack on the material.
Some damage could be seen during a visual analysis, where a softening of the surface was observed which was greater in the samples immersed in the acid solution, indicating a greater aggressiveness by this solution.
Figure 8 shows a micrograph of a sample immersed in a H2SO4 0.5 N solution at 40 ºC. The region of chemical attack was observed from the surface with a depth of approximately 129 µm, visually observing a grayer shade than the rest of the sample. The EDS analysis indicated a migration of Al+3 ions caused by the attack of the acid solution. A 22 µm thick crack formed due to a change in volume, caused by the migration of calcium ions and sulfates to the exterior, increasing the attack given that the new surfaces were exposed to the corrosive solution. Under the crack a dense and compact matrix was observed, suggesting that it is a zone where the corrosive medium did not penetrate.
Figure 9 shows the microstructure of a sample immersed in a MgCl2 0.6 N solution for 42 days at 40 ºC. The depth of the chemical attack was approximately 380 µm, being greater than the one observed in the cement attack with a H2SO4 solution. In the area above the crack, an interface with a high Mg content was observed due to a slow diffusion of Mg+2 ions in the sample, indicating an ionic exchange with calcium ions. The non-corroded area showed a compact microstructure.
Figure 10 shows the microstructure of a sample immersed in a Na2SO4 0.04 N solution for 42 days at 40 ºC. An attack depth of 46.7 µm with a granular appearance was observed; these are possibly gypsum nodes, the product of a decalcification of the material. Alumina particles inside the matrix were observed, indicating a migration towards the exterior of calcium and sulfate ions. The cement immersed in this solution showed a greater resistance to the attack and its compressive strength was 34.63 MPa at 42 days of immersion; this is possibly due to the Na+ ions having displaced the Ca+2 ions, forming part of the crystalline network.
4. CONCLUSIONS
A calcium sulfoaluminate clinker was obtained from the synthesis of a mixture of aluminum slag, fly ash, and fluorogypsum. The clinker showed in its composition stages such as calcium sulfoaluminate, gehlenite, calcium aluminate, mayenite, and belite.
The cements elaborated with calcium sulfoaluminate synthesized from industrial wastes developed compressive strengths of 38.95 MPa, similar to those developed by the Ordinary Portland pastes cured under the same conditions.
The pastes immersed in a (Na2SO4) solution showed a high resistance to attack by sulfates, developing a compressive strength of 34.63 MPa after 42 days of immersion.
The degradation of pastes by the attack of H2SO4 and MgCl2 occurs by dealumination and decalcification processes. The samples immersed in H2SO4 and MgCl2 developed a compressive strength of 28.10 and 29.2 MPa, respectively, after 42 days of curing.
5. Referencias
Arjunan, P., Silsbee, M. R., Roy, D. M. (1999) Sulfoaluminate-belite cement from low-calcium fly ash and sulfur-rich and other industrial by-products. Cement and Concrete Research, Vol. 29: pp. 1305-1311. [ Links ]
ASTM C109/C109-M95, (1995), Standard test method for compressive strength of hydraulic cement mortars (using 2-in. or [50 mm] cube specimens), Vol 04.01 Cement, Lime, gypsum. [ Links ]
ASTM C-204, (1995), Fineness of Hydraulic Cement by Air Permeability Apparatus, Annual, Book of ASTM Standars. Section 4. Construction. Volume 04.01. Cement, Lime, Gypsum. [ Links ]
Burciaga-Diaz, O., Escalate-García J. I. (2012) "Strength and durability in acid media of alkali silicate activated metakaolin geopolymers ”, Journal of the American Ceramic Society, Vol 97, 7: pp.2307-2313 [ Links ]
Gallardo M., Almanza J. M., Cortés D. A., Escobedo J. C., Escalante-García J. I. (2014)"Synthesis and mechanical properties of a calcium sulphoaluminate cement made of industrial wastes ", Materiales de Construcción, Vol 64, 315, e023: pp. 1-8. [ Links ]
García-Maté M., De la Torre A., Leon-Reina L., Losilla E., Aranda M. A. G., Santacruz I. (2015), "Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement", Cement and Concrete Composites, Vol 55: pp.53-61. [ Links ]
Gartner, E. (2004), “Industrially interesting approaches to “low-CO 2 ” cements ", Cement and Concrete Research, Vol. 34, 9: pp. 1489-1498. [ Links ]
Hargis C. W., Telesca A., Monteiro P. J. M., "Calcium sulfoaluminate (Ye'elimite) hydration in the presence of gypsum, calcite, and vaterite ", Cement and Concrete Research, Vol 65: pp.15-20. [ Links ]
Katsioti, M., Tsakiridis P. E., Agatzini-Leonardou, S., Oustadakis, P. (2005), "Examination of the jarosite-alunite precipitate addition in the raw meal for the production of Portland and sulfoaluminate-based cement s ", International Journal of Mineral Processing, Vol. 76: pp. 217 - 224. [ Links ]
Li, H., Agrawal, D. K., Cheng, J., Silsbee, M. R. (2001), "Microwave sintering of sulphoaluminate cement with utility wastes ", Cement and Concrete Research, Vol. 31: pp 1257- 1261. [ Links ]
Li, J., Ma, H., Zhao, H. (2007), "Preparation of sulphoaluminate-alite composite mineralogical phase cement from high alumina fly ash ", Key Engineering Materials, Vol. 334-335: pp. 421-424. [ Links ]
Martin, J. J., Márques G., Alejandre F. J., Hernandez M. E. (2008),"Durability of API class cement pastes exposed to aqueous solutions containing choride, sulphate and magnesium ion ", Materiales de construcción, Vol 58,292: pp. 1701-1707. [ Links ]
Mehta, P. K. (1967) “Expansion characteristics of calcium sulfoaluminate hydrates ”, Journal of the American Ceramic Society, Vol. 50, 4: pp. 204-208. [ Links ]
Moore, A., Taylor, H. F. W. (1968), “Crystal structure of ettringite ", Nature, Vol. 218: pp. 1048 - 1049. [ Links ]
NMX-C-061-ONNCCE-2001. (2001), Industria de la construcción-cemento-determinación de la resistencia a la compresión de cementantes hidráulicos, Organismo Nacional de Normalización y Certificación para la Construcción y Edificación, México DF. [ Links ]
NMX-C-085-ONNCCE-2002 (2002), Industria de la construcción-Cementos hidráulicos-Método estándar para el mezclado de pastas y morteros de cementantes hidráulicos, Organismo Nacional de Normalización y Certificación para la Construcción y Edificación, México DF. [ Links ]
Older, I. (2005), "Cements containing calcium sulfoaluminate”, Special Inorganic Cements, Modern Concrete Technology 8, Taylor and Francis Group: pp. 63-81. [ Links ]
Roy, D. M. (1999), "Alkali-activated cements opportunities and challenges ", Cement and Concrete Research, Vol. 29, 2: pp 249-254. [ Links ]
Sersale, R., Frigiones, G., Bonavita, L. (1998), "Acid depositions and concrete attack: main influences", Cement and Concrete Research, Vol. 28 pp. 19-24. [ Links ]
Sharp J. H., Lawrence C. D., Yang R. (1999) “Calcium sulfoaluminate cements-low-energy cements, special cements or what?”, Advances in Cement Research, Vol 11, 1: pp 3 -13 [ Links ]
Singh, M., Kapur, P. C., Pradip. (2008),"Preparation of calcium sulphoaluminate cement using fertiliser plant wastes ", Journal of Hazardous Materials, Vol. 157: pp. 106-113. [ Links ]
Singh, M., Upadhayay, S. N., Prasad, P. M. (1997), "Preparation of iron rich cements using red mud ”, Cement and Concrete Research, Vol. 27, 7: pp. 1037-1046. [ Links ]
Taylor, H. F. W., Famy, C., Scrivener, K. L. (2001), "Delayed ettringite formation ", Cement and Concrete Research, Vol. 31, 5: pp. 683-693. [ Links ]
Zhou, Q., Milestone, N. B., Hayes, M. (2006) "An alternative to Portland Cement for waste encapsulation-The calcium sulfoaluminate cement system", Journal of Hazardous Materials, Vol. 136, 1: pp. 120-129. [ Links ]
Zhou, Q., Milestone, N. B., Hayes, M. (2006) "An alternative to Portland Cement for waste encapsulation-The calcium sulfoaluminate cement system", Journal of Hazardous Materials, Vol. 136, 1: pp. 120-129. [ Links ]
Received: August 29, 2015; Accepted: December 10, 2015











 texto em
texto em 











