Plants are a natural source of secondary metabolites (SMs) widely used in the pharmaceutical, agrochemical, and cosmetic industries (Banerjee et al. 2022, Lee & Huyn 2023). These SMs are biosynthesized by different biochemical pathways which are susceptible to both genetic and environmental factors and plant ontogeny (Brown et al. 2003, Li et al. 2020); therefore, variations in the biosynthesis and accumulation of these metabolites may occur because of the spatial and temporal patterns that take place during plant development and/or in response to biotic and abiotic stresses (Gobbo-Neto & Lopes 2007, Pavarini et al. 2012, Yang et al. 2018). Alternatively, the change from vegetative to the reproductive stage can also modify the metabolic profile and trigger the production of a certain group of SMs in plants (Brown et al. 2003, Verma & Shukla 2015). As part of our continuing interest to establish the biosynthetic origin of the tri-nor-sesquiterpene urechitol A (Figure 1) and other campechane skeleton-related terpenoids, we have evaluated the influence of the flowering stage in the accumulation of urechitol A in transformed plants of P. andrieuxii.
Pentalinon andrieuxii (Müll. Arg.) b.f. Hansen & Wunderlin is an herbaceous Mexican plant belonging to the Apocynaceae family (Morales 2009); the roots of the plant are used by Mayan healers to treat cutaneous leishmaniosis (Chan-Bacab et al. 2003, Lezama‐Dávila et al. 2014) and its phytochemical knowledge includes production of secondary metabolites such as coumarins, sterols and terpenoids (Yam-Puc et al. 2009, Pan et al. 2012). The terpenoids reportedly produced by P. andrieuxii include betulinic acid, a triterpene with several biological properties such as antiparasitic, antiviral, and antileukemic properties (Lezama‐Dávila et al. 2014, Gupta et al. 2015, Meira et al. 2016, Varikuti et al. 2022), and urechitol A, a tri-nor-sesquiterpenoid with an unusual carbon skeleton of unknown biosynthetic origin (Yam-Puc et al. 2009). A recent investigation into the accumulation dynamics of terpenoids in wild plants of P. andrieuxii showed that while betulinic acid accumulates in plant leaves at all stages of plant development, with no apparent relationship to plant ontogeny, maximum production of urechitol A occurs in plants in the adult stage and is restricted to the root tissue (Hiebert-Giesbrecht et al. 2016). More recently, genetically transformed plants of P. andrieuxii, regenerated from hairy root cultures, kept under growth chamber-controlled conditions, showed higher contents of betulinic acid and urechitol A than those in the wild plants used as control (Hiebert-Giesbrecht et al. 2020). On the basis of the hypothesis that the flowering stage of P. andrieuxii has a positive influence on its production of terpenoids, the aim of this study was to compare the accumulation of the sesquiterpene urechitol A in plants of P. andrieuxii, both transformed and wild type, before flowering induction and at full bloom.
Materials and methods
Plant material. The transformed plants of P. andrieuxii used in this study were regenerated from A. rhizogenes (ATCC-15834)-transformed roots (Hiebert-Giesbrecht et al. 2020). The plants were kept for four years in a growth chamber with a 16/8 h (light/dark) photoperiod, a temperature of 24.5 ± 0.2 °C, and an illumination of 36.07 μM/m2/s; wild type plants of P. andrieuxii were propagated in vitro and kept under similar conditions.
Greenhouse acclimation and flowering induction. Plants of P. andrieuxii kept in the growth chamber were first transferred to greenhouse conditions for four weeks [temperature of 30 ± 2 °C and natural light (approximately 13 h of light and 11 h of darkness) with an average intensity of 398 μM / m2 /s]. Flowering was induced by exposing plants to natural ambient conditions, i.e. 38 to 42 °C and normal day/night lighting periods. Plants were monitored regularly for up to eight weeks (July to September 2019) and once flower buds developed, a sample of root tissue was collected, and the plants were returned to greenhouse conditions.
Sampling and roots extraction. Roots tissue samples of P. andrieuxii were collected before flowering induction (July 2019) and during full bloom (August 2019). For sampling, roots of each plant were collected, washed, frozen with liquid nitrogen, freeze-dried, ground, and stored. Extraction of the root tissue samples was carried out following the procedure reported by Hiebert-Giesbrecht et al. (2016) with some modifications. The samples (ca. 1 g) were extracted with 50 mL of methanol under sonication (three times, 30 min ea) conditions; plant material was removed by filtering through filter paper and the solvent was evaporated under reduced pressure at 35 ° C to produce the corresponding methanol crude extract, which was then sonicated with 50 mL of dichloromethane (CH2Cl2) (three times, 30 min ea) to give a dichloromethane-soluble fraction used for analyses.
Detection and quantification of urechitol A by gas chromatography-mass spectrometry. The presence and concentration of urechitol A in the root extracts were established by gas chromatography/mass spectrometry (GC-MS) analyses using an Agilent 7890B Gas Chromatograph coupled to an electron impact (70 eV) mass spectrometer (Agilent 5977A), scanning 50 to 600 amu; data was processed using the ChemStation software (Agilent Technologies), while separations were carried out following a method reported by Hiebert-Giesbrecht et al. (2020) with minor modifications, i.e., an injection volume of 3 μL, a 50:1 split, and nitrogen at 1.5 mL/min as carrier gas. The presence of urechitol A in the extracts was confirmed by comparing the GC-MS chromatographic profiles of the different samples with the chromatographic profile of an authentic sample of pure urechitol A, isolated from a root extract of P. andrieuxii following the procedure reported by Yam-Puc et al. (2009), used as reference standard. Quantification was carried out using a calibration curve (R 2 = 0.99) prepared with five dilutions (0.01 to 0.1 mg/mL) of the standard, injected in triplicate, following the method reported by Hiebert-Giesbrecht et al. (2016 and 2020). The increases of urechitol A content measured as fold increase in relation to the flowering stage was calculated using the equation “Content of urechitol A at flowering stage/Content of urechitol A before flowering stage” for each plant line.
Statistical analyses. Results are reported as means ± SD, statistical analysis is based on multiple comparison of two-way ANOVA, considering two factors: time (before and after flowering) and the plant lines genotype [no- transformed plant (wt) and transformed plants (2 -8)] (P < 0.0001), followed by Tukey’s test with (P ≤ 0.05). Statistical analyses were performed using software Graph pad Prisma version 10.0.2.
Results
Flowering induction. Both transformed and wild-type (control) plants of P. andrieuxii were successfully acclimatized to greenhouse conditions (natural light and room temperature, Figure 2A); while none of the plants flowered when kept under the previously described greenhouse conditions, all plants flowered when exposed to ambient natural light and temperature (above 32 °C, Figure 2B), with flower buds observed after three weeks of flowering induction (Figure 2C) and full bloom displaying on the sixth week after induction (Figure 2D, E, F).

Figure 2 A) Transformed plants of Pentalinon andrieuxxi in greenhouse conditions (natural light, 30 °C); B) Transformed plants of P. andrieuxii under floral induction conditions (natural light, 36 to 42 °C); C) Transformed plants with flower buds three weeks after floral induction; D) WT plant at full bloom; E) and F) Transformed plants in full bloom.
Influence of flowering on the production of urechitol A in P. andrieuxii. The evaluation of the influence of the flowering stage and the plant’s genotype on the production of urechitol A showed that while the flowering stage did strongly influence the production of the sesquiterpene in the roots of P. andrieuxii (89 % as the cause of content variation, F = 986.5, P < 0.0001), neither the plant’s genotype, nor the interaction of the two factors, appears to have a significant influence (3.9 %, F = 6.9, P = 0.0006 and 3.7 %, F = 5.97, P = 0.0014, respectively) in the production of urechitol A. The content of urechitol A increased from a minimum of 43 to a maximum of 91 times (0.29 - 0.55 mg/gDW -1, Figures 3 and 4) in the different plants, both transformed and wild type, at full bloom.
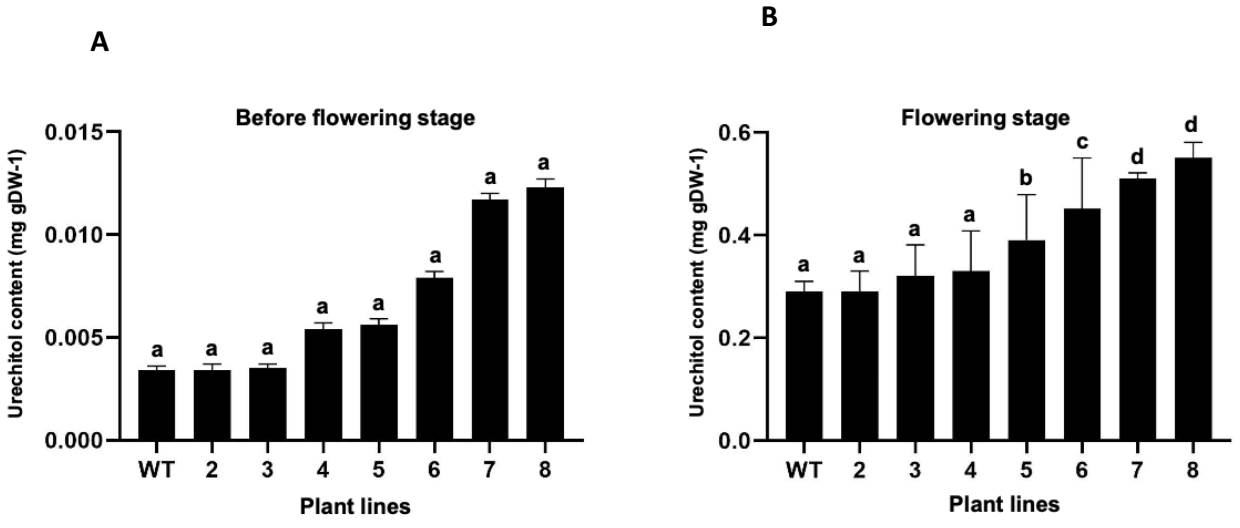
Figure 3 Urechitol A content in root extracts of plants of Pentalinon andrieuxii [wild type (wt) and transformed plants lines (2-8)]. A) Before flowering induction; B) At full bloom. Bars represent mean values ± standard deviation. Different letters indicate statistically significant differences (P ≤ 0.05).
Discussion
Flowering is a complex process, sensitive to environmental conditions such photoperiod, temperature, and their interaction (Amasino 2010, McClung et al. 2016). It has been reported that ambient temperature increases the rate of flowering in some species and many transition to flower in response to environmental cues such as daylength and temperature (Amasino 2010, Wigge 2013, Jagadish et al. 2016). The rise in temperature has a strong effect in plant development particularly in herbaceous species (Büntgen et al. 2022). In this investigation, plants of P. andrieuxii transitioned to flower when exposed to temperature above 32 °C; these initial results strongly indicated that the plant perceived higher temperatures as an important environmental signal to flower, reflecting its adaptation to a hot and tropical environment. These results also showed that P. andrieuxii can shift from vegetative to reproductive stage when exposed to natural photoperiod and ambient temperature conditions, defining ambient temperature as the physiological, non-stressful temperature range for a given species (Capovilla et al. 2015). These findings are also in agreement with reports in the literature describing temperature as strongly influencing plant flower transition (Wigge 2013, McClung et al. 2016) and with a report describing the strong inductive effect of temperature independent of photoperiodic cues (Balasubramanian et al. 2006). To the best of our knowledge, this is the first study about the influence of temperature in the flowering in P. andrieuxii; future studies will provide a deeper understanding about the importance of factors such as temperature and natural light in the flowering of this and other species, similarly adapted to the high ambient temperatures of the tropics.
Influence of flowering stage in the content of urechitol A in P. andrieuxii. Since it is well known that biosynthesis and storage of SMs are dependent of organ tissue and the developmental stage of the plant, a particular secondary metabolite pathway may not be activated during a particular stage of plant growth and development (Broun et al. 2006, Bartwal et al. 2013); accordingly, the wide range of changes that take place in all the plant organs during the flowering stage of plants, which can be specific for each species, will affect the general production of SMs (Cho et al. 2017, Isah 2019). There are a number of studies reporting on the positive effect of the flowering stage on the production of terpenoids, e.g., the production of guaianolides in roots of Cichorium intybus L. (Bogdanović et al. 2014), the nine-times increase in the production of artemisinin during the flowering of Artemisa annua (Ferreira et al. 1995, Mannan et al. 2011, Towler & Weathers 2015), and the increased content of terpenes such as α-pinene, β-caryophyllene, α-humulene, and cannabidiol during the flowering stage of Cannabis sativa (Tremlová et al. 2021).
A recent study on the spatial distribution of terpenoid production in wild plants of P. andrieuxii showed that while the triterpene betulinic acid was only found in the leaves and the tri-nor-sesquiterpene urechitol A only in the roots, the production of the former is constant during plant development, while the latter is only found in the roots of adult plants (Hiebert-Giesbrecht et al. 2016); these findings suggested that the production of urechitol A was associated with a particular stage of plant growth. This investigation showed that the flowering stage of P. andrieuxii has a positive influence on the terpenoid production and accumulation in the roots of the plant, with the contents of the tri-nor-sesquiterpenoid urechitol A increasing from a minimum of 43 to a maximum of 91 times during the flowering stage in all plants, both transformed and wild type. Interestingly, and while the content of urechitol A in all plant lines was within range of that previously reported in wild plants (Hiebert-Giesbrecht et al. 2016), the largest increases in urechitol A production were observed in plant lines with the lowest contents of urechitol A before flowering (i.e., wild type, and plant lines 2 and 3, with an increase of 85, 84, and 91 times, respectively) (Figure 3); alternatively, the plant lines with the highest contents of urechitol A before flowering showed the smallest increases (i.e., plant lines 7 and 8, with increases of 43 and 44 times). These results strongly suggest that a maximum production for the tri-nor-sesquiterpene is established by the plant, in response to its interaction with the surrounding environment.
It is well known that secondary metabolite production is under genetic control, involving a complex network of genes that encode transcription factors that, in turn, regulate the expression of other genes which encode enzymes that catalyze the formation of secondary metabolites, and that this gene expression is modulated throughout plant development as well as by environmental factors (Laitinen at al. 2005, Pasquali et al. 2006, Guitton et al. 2010); accordingly, it has been reported that high expression of the gene linalool synthase produces a high content of linalool and linalyl acetate in full bloom inflorescences of Lavandula angustifolia L (Guitton et al. 2010). These findings confirm the fact that the plant genome determines the type, the organ, and the time when a specific metabolite is to be produced, while the content may depend on developmental stage of the plant and environmental factors. In this context, molecular knowledge of P. andrieuxii will allow the identification of genes and enzymes involved in the biosynthetic pathway of urechitol A.
The intense metabolic activity that takes place during flowering promotes cellular division and differentiation demands metabolites such as sugars, organic acids, and amino acids for the formation of new cell structures and reproductive organs; these primary metabolites also produce precursors for the biosynthesis of secondary metabolites in all parts of the plant, including underground and non-photosynthetic tissues like roots (Broun et al. 2006, Huang et al. 2019). Roots play an important role in the storage of SMs and, for many species, it has been reported that the flowering phase affects positively the accumulation of SMs in roots, e.g., the highest yield of salidroside in roots of Rhodiola seminovii occurs during reproductive stage (Terletskaya et al. 2022). Roots may also be the biosynthetic site of SMs such as the tropane alkaloids hyoscyamine and scopolamine in Atropa belladonna (Hashimoto et al.1991) and rhizathalene, a diterpene from Arabidopsis thaliana (Vaughan et al. 2013). Metabolites may be synthesized and stored at the same plant organ or be transported from where is synthesized to another part of the plant where it is stored. It is known that there is a great complexity in the biosynthetic processes of secondary metabolites, and it has been reported that in the same tissue there are groups of cells specifically responsible for transcripts of the biosynthetic genes, while other cells store the final metabolites (Jochum et al. 2007, Bartwal et al. 2013).
The low content of secondary metabolite production in plants, and the lack of knowledge about the dynamics of accumulation, are the main bottleneck in biosynthetic studies. Knowing the optimal ontogenetic stages yielding the highest amounts of a specific metabolite is crucial, particularly for SMs that do not have an established in vitro culture production model, as it is the case for urechitol A. This tri-nor-sesquiterpene is an intriguing molecule due to its unusual carbon skeleton, not related to any other group of sesquiterpenes reported in literature, and to date its biosynthetic origin remains unknown. The increased production of urechitol A in the root tissue of flowering plants provides valuable information about the production of the tri-nor-sesquiterpene, while the use of flowering plants of P. andrieuxii represents a reasonable model for biosynthetic studies of this unique metabolite. Furthermore, and considering that the biosynthesis of SMs show organ-tissue specificity, our results suggest that the roots of P. andrieuxii are the site of biosynthesis and accumulation of urechitol A; future studies will confirm this hypothesis and provide information about the biosynthetic origin of the sesquiterpene.
We conclude that temperature is an important environmental signal to flower, reflecting the adaptation of P. andrieuxii to a tropical environment; furthermore, the results also show that the flowering stage of P. andriuexii positively influences the accumulation of urechitol A and that the content of this metabolite in the roots of the plant is determined not only by genetic factors, but also by the developmental stage of the plant and its interaction with environmental cues.











 nova página do texto(beta)
nova página do texto(beta)





