1.Introduction
Nanostructured spinel ferrites with chemical composition MFe2O4 (M=Fe, Co, Ni, Mn, Zn, etc.) have attracted enormous attention from the research community for several decades because of their remarkable electrical and magnetic properties. Spinel ferrites are cost-effective materials that possess smaller Hc, smaller saturation magnetization (Ms), and higher Mr. The unusual magnetic behavior of spinel ferrites diversely associates them with various significant technological applications involving the production of multifunctional appliances such as transformer cores, microwave devices, high-density recording media, gas sensors, ferrofluids, read out heads, treatments of contaminated water, organic dye degradations, bactericides and medical equipment 1. In nanostructured spinel ferrites, electrical and magnetic properties prove to be a function of composition, cations distribution, particle size, surface coating, and synthesis methodology 2. The structure of a spinel unit cell is based on an fcc oxygen lattice comprising 64 tetrahedral (A-sites) and 32 octahedral (B-sites) interstitial sites. In a spinel structure, the metal cations can occupy only 8 out of 64 available A-sites and 16 out of 32 available B-sites. A spinel structure can be categorized into normal, mixed, and inverse spinels based on cation distribution over two types of interstitial sites. In a normal spinel, all the 8 divalent cations occupy 8 A-sites, and all the 16 trivalent cations occupy 16 B-sites. In an inverse spinel, all the 8 divalent cations occupy 8 of the filled 16 B-sites, and the 16 trivalent cations occupy the left-over 8 A and 8 B sites. Any other intermediate arrangement of cations in the structure makes the ferrite compound a mixed spinel. Spinel ferrites exhibit ferrimagnetism originated from the interactions of magnetic moments of metal cations with anti-parallel spins located at A and B-sites 3. An application-driven control on the evolution of microstructure and physical properties of spinel compounds is of utmost importance to tune the electromagnetic properties suitable for numerous potential applications. The ferrite compounds like NiFe2O4 and CoFe2O4 are inverse spinels, and their application-driven designs are met by adopting appropriate synthesis methodologies besides tailoring of surface morphology of their nanostructured constituents. The synthesis methods commonly used to prepare nanostructured spinel ferrites comprise the techniques of sol-gel auto-combustion, co-precipitation, sonochemistry reaction, reverse micelles, flow injection, micro-emulsion, electrospray, hydrothermal reaction, microwave combustion, solid-state reaction, and high energy ball milling 4,5. A sol-gel auto-combustion technique can yield pure phase spinel ferrites at nanoscale under optimal reaction conditions. This technique involves a self-ignited, self-sustained course of combustion, and a ferrite powder is yielded by mixing an aqueous solution of metal salts with a fuel of citric acid in the presence of a catalyst content ammonia solution and ethylene glycol which is an oxidizing agent and a surfactant. The organic materials generated and employed during the sol-gel chemical reaction are eliminated by elevated temperature treatments and thermal annealing in an oxygen-rich environment 6. The physical properties of a single-phase ferrite compound synthesized by sol-gel technique can be controlled by reaction parameters like stoichiometry of surfactants, solvents, and metal salts as well as by time and temperature of gel-formation, pH of the reaction, and time and temperature of post-growth annealing. An organic solvent and surfactant in a reaction controls the size and shape-morphology of yielded nanostructured ferrite powder. A complete removal of organic contents from interacting surfaces of nanostructured ferrites is extremely important for optimum display of magnetic parameters like Ms and Hc7,8.
A size growth is induced by an elevated temperature treatment or a prolonged
annealing time yielding the nanostructured ferrite particles with larger sizes.
Contrarily, the quantum confinement effects like exchange interactions in
nanoparticles are optimized by ensuring lager intact surface area among them. For
the sake of optimum soft magnetic behaviour, the nanostructured ferrite particles
with smaller sizes can be obtained by a low-temperature sol-gel auto-combustion
technique involving a shorter post growth annealing time at lower temperature 9,10. An incorporation of Co2+ cations in
NiMn0.5Fe1.5O4 compound can widen its
application potential causing an enhanced magneto-crystalline anisotropy, higher
magnetization and coercivity. So far, a significant research work has been devoted
to synthesize Co0.5Ni0.5Fe2O4
nanoparticles and their composites 10-12. There is no published data available on the
investigations of nanostructured
Ni1-xCoxFe1.5Mn0.5O4
spinel ferrites synthesized by sol-gel auto-combustion technique. Herein, we report
an investigation of microstructure and magnetic properties of nanostructured
Ni1-xCoxFe1.5Mn0.5O4
(x = 0,0.25,0.5,0.75,1) spinel ferrites synthesized by sol-gel
auto-combustion technique involving a lower annealing temperature
2.Experimental procedures
2.1.Materials and methods
A series of nanostructured CoxNi1-xMn0.5Fe1.5O4(x = 0,0.25,0.5,0.75,1) spinel compounds was prepared by sol-gel auto-combustion technique. Analytical grade metal salts including cobalt nitrate (Co(NO3)2⋅6H2O), nickel nitrate (Ni(NO3)2⋅6H2O), iron nitrate (Fe(NO3)3⋅9H2O), manganese nitrate Mn(NO3)2 , citric acid (C6H8O7⋅H2O), ethylene glycol (C2H6O2) and ammonia solution (NH3H2O) were obtained from Sigma Aldrich and used without further purification. In the stoichiometric calculations, the molar ratio of citric acid to metal nitrates was kept 2: 1 whereas, the molar ratio of ethylene glycol to metal nitrates was set 1:1. The citric acid was used in the proposed chemical reaction as a fuel and ethylene glycol was employed as a surfactant and oxidizing agent for metal cations whereas, the ammonia solution was used as a catalyst. A general synthesis methodology involved the dissolution of corresponding stoichiometric ratios of all the metal nitrates, citric acid, and ethylene glycol in deionized water. The pH of the solution of metal nitrates and organic compounds was adjusted to a neutral value 7 by a dropwise addition of ammonia solution. Later on, the mixed solution was vigorously stirred and heated on a hot plate at a temperature of 80oC. The process of heating and stirring of the mixed solution was continued maintaining a pH ∼ 7 until a wet gel was obtained. The removal of water and organic materials from the wet gel was carried out by drying it in an oven at gradually increasing temperature in the presence of air to yield a fluffy powder by self-ignited and self-propagated combustion approach. Later on, the powder product was kept in the oven at a constant temperature of 120 for 2 h for calcination purposes to obtain a loose spinel ferrite powder. The calcined ferrite powder was ground by agate mortar for 10 min for better homogeneity, and the ground powder was annealed at 600 for 1 h in a muffle furnace for better crystallinity in the obtained ferrite compounds.
2.2.Characterization
The obtained CoxNi1-xMn0.5Fe1.5O4 ferrite compounds were characterized by powder XRD, SEM, EDX, TEM, HRTEM, SAED, and VSM. An investigation of phase formation of the nanostructured compounds was carried out by recording XRD diffractograms using a Siemens Bruker D-5000 diffractometer equipped with Cu-Kα radiation in an angle range 2θ = 20 -80 operated at 40 kV and 30 mA with a measurement step 0.02/sec. The XRD diffractograms of all the nanostructured compounds were analyzed to investigate the phase formation and microstructure of the compounds in addition to a Rietveld structural refinement using MDI Jade 6.5 computer software with JCPDS database. A crystallite size D for each spinel compound was estimated using the XRD data of the strongest reflection peak (311) in each pattern employing Debye-Scherer’s formula expressed as under:
For Cu-Kα radiation, the wavelength λ was 1.5406 Å, 2θ was the position of the maximum of a diffraction peak measured in degrees whereas, β was the full width at half maximum of the peak measured in radian. The lattice parameter α of the fcc unit cell of compounds was calculated using an expression described as under:
Here, h, k, l represented the Miller indices corresponding to the planes causing reflections of notable intensity.
The shape-morphology of CoxNi1-xMn0.5Fe1.5O4 powder compound and size of the powder agglomerates was investigated employing a JEOL JSM-6490 SEM operated at 25 kV equipped with an energy dispersive X-ray detector (EDX). An EDX detector was employed to confirm the elemental composition of the CoxNi1-xMn0.5Fe1.5O4 ferrite compound. A JEOL JEM-2010F TEM with an accelerating voltage of 200 kV was employed to record a bright-field TEM micrograph, a high-resolution TEM (HRTEM) micrograph, and a selected area electron diffraction (SAED) pattern of nanostructured CoxNi1-xMn0.5Fe1.5O4 compound, which were investigated to study the morphology, crystallite size and microstructural details of the compound. Magnetic measurements of the nanostructured Ni1-xCoxFe1.5Mn0.5O4 compounds were carried out by recording the M-H hysteresis loops at room temperature under a maximum applied field of 15 kOe using a Lakeshore-735 VSM.
3.Results and discussions
3.1.XRD analysis
Figure 1 shows the powder XRD patterns of
annealed series of
CoxNi1-xMn0.5Fe1.5O4
(x = 0,0.25,0.5,0.75,1) nanostructured compounds. The XRD patterns were indexed
by matching each pattern with the standard patterns of
NiFe2O4 (PDF#74-2081) and
CoFe2O4 (PDF#22-1086) using MDI Jade 6.5 software. It
was found that the recorded patterns of the series of compounds were consistent
with the standard patterns, and indexing of the recorded patterns led to the
Miller indices corresponding to the diffracting planes (220), (311), (222),
(400), (331), (422), (511), (440) and (620) of an fcc spinel structure belonging
to an Fd-
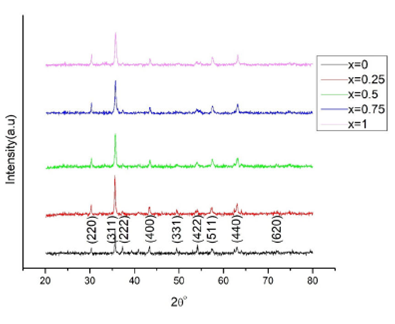
Figure 1 Powder X-ray diffraction patterns of CoxNi1-xMn0.5Fe1.5O4 (x = 0,0.25,0.5,0.75,1) spinel ferrites.
It was found that no impurity peak was evident in the recorded patterns, which
indicated that the prepared ferrite compounds were purely single-phase solid
solutions 13. A gradual
broadening of the strongest diffraction peak (311) in the series of recorded
patterns merely indicated that the structural parameters like crystallite size
D and lattice parameter α of the obtained compounds were a
function of composition. It was noted that the ferrite samples with increasing
Co2+ concentration showed a steady decline in crystallite size
D. The crystallite size was estimated by Debye-Scherer’s
formula using the XRD data of the strongest peak (311) in each XRD pattern which
led to a crystallite size range
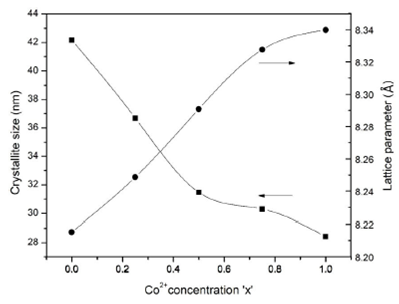
Figure 2 A plot of crystallite size D and average lattice parameter a versus Co2+ concentration x in CoxNi1-xMn0.5Fe1.5O4 spinel ferrites.
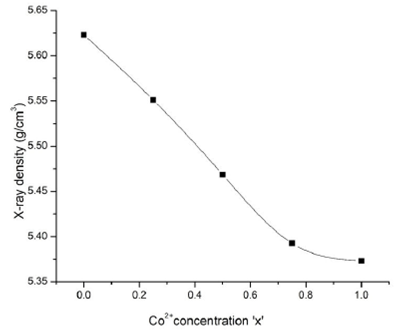
Figure 3 A plot of X-ray density Dx versus Co2+ concentration x in CoxNi1-xMn0.5Fe1.5O4 spinel ferrites.
Table I The crystallite size D (nm), average lattice parameter a (Å) and X-ray density Dx (g/cm3) versus Co2+ concentration in CoxNi1-xMn0.5Fe1.5O4 spinel ferrites.
| S.No | Co2+ concentration (x) | D (nm) | a (Å) | Dx (g/cm3) |
| 1 | 0 | 42.16 | 8.215 | 5.623 |
| 2 | 0.25 | 36.68 | 8.248 | 5.551 |
| 3 | 0.50 | 31.47 | 8.290 | 5.468 |
| 4 | 0.75 | 30.30 | 8.327 | 5.392 |
| 5 | 1 | 28.42 | 8.339 | 5.372 |
3.2.SEM analysis
A characteristic SEM micrograph of nanostructured NiMn0.5Fe1.5O4 compound annealed at 600oC for 1 h is shown in Fig.4a). The micrograph revealed the formation of almost octahedral-shaped grains as agglomerates of fine magnetic particles with sizes of the order of several microns 22-25. The size of the grains of a compound NiMn0.5Fe1.5O4 under investigation was estimated from the SEM micrograph shown in 4b). It was revealed that the grain size widely ranged from 3-15 μm. The diverse size distribution of grains was attributed to a varying size-dependent chaining tendency of the nanostructured ferrite particles, which constituted the grains by a huge collection of fine magnetic particles 26. The morphological shape of the grains of NiMn0.5Fe1.5O4 compound was studied from the SEM micrograph shown in Fig. 4c). The micrograph clearly depicted an overwhelming trend of the octahedral-shaped morphology of the grains comprising fine magnetic ferrite particles 27. A representative EDX spectrum recorded for the NiMn0.5Fe1.5O4 spinel ferrite composition is shown in Fig. 5. The main objective of EDX characterization was to endorse the purity and surety of the proposed chemical composition of the spinel compounds. On the investigation of the recorded spectrum, it was found that all the three kinds of cations Ni, Mn, and Fe, involved in constituting the ferrite composition under investigation were detected appropriately as the major constituent elements along with a representative peak for oxygen anions. The EDX spectrum also indicated an absence of any kind of impurity elements in the compound. Additionally, the EDX pattern reflected a good crystallinity in the nanostructured spinel ferrite compound, besides revealing that the chemical precursors had entirely undergone a chemical reaction to yield the ferrite compound 28.
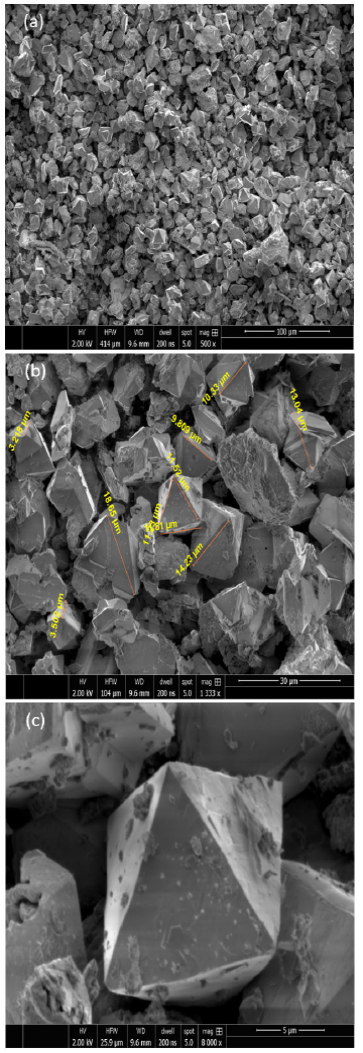
Figure 4 SEM micrographs of nanostructured Ni1-xCoxFe1.5Mn0.5O4 spinel ferrites with a) 100 μm scale, b) 30 μ m scale, c) 5 μm scale.
3.3.TEM analysis
As the particle size of a nanostructured compound is a rational measure of the diameter of the constituent particles offering conclusive remarks on key features necessary to define a domain of the characteristics of the compound. A TEM micrograph merely offers a very first choice to exactly evaluate the particle size of a nanostructured compound under investigation. Moreover, a TEM micrograph also gives an account of the ranges of size distribution, uniformity of dispersion by perceiving the agglomeration tendency of fine particles along with the supplementary morphological details. In literature, the nanostructured spinel compounds are usually found displaying cubic, spherical, octahedral, or star-like symmetric shapes 29,30. Herein, the TEM investigations were carried out to study the microstructure and morphology of the nanostructured Ni1-xCoxFe1.5Mn0.5O4 compound. Figure 6a) shows the TEM micrograph of calcined Ni1-xCoxFe1.5Mn0.5O4 ferrite powder. The micrograph revealed no specific morphology of the nanostructured particles reflecting a poor crystallinity and an overwhelming chaining tendency of the particles, which resulted in the formation of huge agglomerates of nanoparticles. Figure 6b) represents a TEM micrograph for the annealed nanostructured Ni1-xCoxFe1.5Mn0.5O4 ferrite compound. An investigation of the recorded micrograph revealed that the compound particles were monodispersed with nanoscale sizes possessing a closer size distribution with a display of an almost homogeneous spherical morphology. These features led to a conclusion that there was a controlled evolution of particle sizes and shape morphology of the nanostructured ferrite compounds. From the micrograph, it was also learned that the size of the particles of nanostructured Ni1-xCoxFe1.5Mn0.5O4 compound ranged from 20-50 nm, which was in good agreement with the size of crystallites as determined from the corresponding XRD data employing Debye-Scherer’s formula 31,32. A high-resolution TEM (HRTEM) micrograph interprets the microstructure of nanoparticles under investigation and facilitates an inspection of the thickness of crystal defects, surface orders, and polydispersity. Figure 7 shows the HRTEM micrograph of nanostructured Ni1-xCoxFe1.5Mn0.5O4 compound. The micrograph revealed an ordered pattern of diffraction fringes of the spinel lattice, and the fringe separations were noted to be 4.8 and 2.9 Å which corresponded to the reflection planes (111) and (220) of the standard diffraction pattern of NiFe2O4 (PDF#74-2081), respectively 26. An inset in 7 shows the selected area electron diffraction (SAED) pattern of the nanostructured Ni1-xCoxFe1.5Mn0.5O4 compound. The SAED pattern involved two distinctly spotted diffraction rings indicating a polycrystalline nature of the ferrite compound under investigation. A spotted appearance of the diffraction rings in the SAED pattern was attributed to the good crystallinity of the obtained compound, and the diffraction rings were indexed to (220) and (400) reflection planes of the standard diffraction pattern of NiFe2O4 (PDF#74-2081) 27,33.
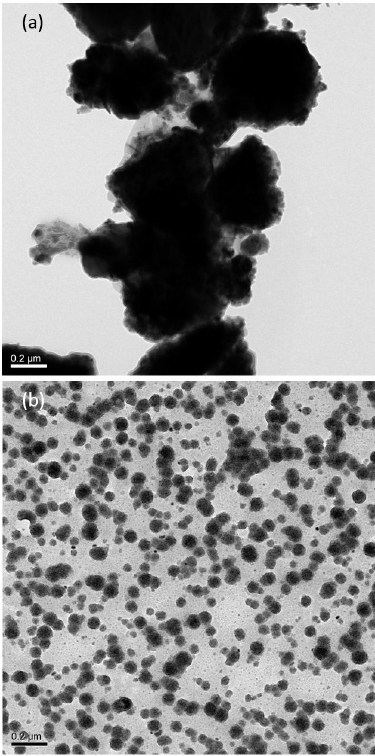
Figure 6 Bright field TEM micrograph of Ni1-xCoxFe1.5Mn0.5O4 spinel ferrite a) calcined at 120±C for 2 h b) calcined at 120±C for 2 h and annealed at 600±C for 1 h.
3.4.VSM analysis
The magnetic measurements of a nanostructured ferrite compound solely depend on the chemical composition, crystalline structure, particle size distribution and morphology (34). A set of M-H hysteresis loops of the nanostructured compounds CoxNi1-xMn0.5Fe1.5O4 is shown in Fig. 8(a, b, c, d, e) for Co1-xNixMn0.5Fe1.5O4 compounds with x = 0, x = 0.25, x = 0.5, x = 0.75 and x = 1, respectively. Several magnetic parameters deduced from the recorded M-H hysteresis loops are listed in Table II.
Tabla II The coercivity H c , maximum magnetization Mmax and remanent magnetization M r versus Co2+ concentration in CoxNi 1-x Mn05Fe1.5O4 spinel ferrites.
| Sr. No. |
Co2+ concentration (x) |
Hc (kOe) |
Mmax (emu/g) |
Mr (emu/g) |
| 1 | 0 | 0.67 | 19.98 | 12.88 |
| 2 | 0.25 | 0.68 | 20.97 | 12.32 |
| 3 | 0.50 | 0.70 | 21.34 | 15.9 |
| 4 | 0.75 | 0.72 | 31.98 | 20.22 |
| 5 | 1 | 0.75 | 36.86 | 22.30 |
In ferrimagnetic materials like spinel ferrites which follow the Neel’s two-sublattice model, there can be three types of exchange interactions among the cations distributed in tetrahedral (A-type) and octahedral (B-type) sublattices represented by A-A, A-B, and B-B interactions. The strength of the A-B interaction is greater than the rest of the two A-A and B-B interactions, and the net magnetic moment for a spinel compound is taken as M=MB-MA. A larger value of M results in a higher saturation magnetization, Ms of the spinel compound 35,36. In the series of recorded M-H hysteresis loops, it was noted that the Ni1-xCoxFe1.5Mn0.5O4 ferrite (x = 0) showed a maximum magnetization, Mmax of 19.98 emu/g, and on the substitution of Co2+ into the next successive composition (x = 0.25), the maximum magnetization, Mmax experienced a slight rise (20.97 emu/g). This rise in Mmax was attributed to the cation concentration at B-site, which influenced the exchange interactions between A and B-site cations and positively led to a higher maximum magnetization, Mmax, and thereafter, it kept the trend steadily on further increased concentrations. In intermediate compositions (x = 0.25,0.5,) of CoxNi1-xMn0.5Fe1.5O4(x = 0,0.25,0.5,0.75,1) mixed spinel compounds, such a dependent response of Mmax was ascribed to the higher magnetic moment of Co2+ besides its dominating occupational affinity of the octahedral site in comparison with Ni2+ in the spinel lattice. A dominant substitution of Co2+ cations in replacement of the host cations in spinel compounds (x = 0.75,1) caused a change in structure type from a mixed spinel to an inverse spinel, and hence an evident rise in magnetization was noted 37. Due to a stable valance state and ionic size, the Mn3+ cation is intended to occupy the tetrahedral interstitial site. A compound with maximum Co2+ concentration (x = 1) exhibited a maximum coercivity of 0.75 kOe, which reflected that the entire series of ferrite compounds could easily be magnetized without a considerable loss of magnetic flux, and hence the ferrite compounds preserved a character of soft magnets. A significant variation in the shape of hysteresis loops on changing concentration of Co2+ cations enabled the ferrite compounds to be effectively applied in magnetic storage devices, microwave appliances, transformer cores, and magnetic field sensors. The series of ferrite compounds under investigation exhibited a significant soft magnetic character devised by enhanced exchange interactions among the nanostructured particles based on a larger surface to volume ratio, which in turn induced the mechanisms of spin canting, magneto-crystalline anisotropy, and super-exchange interaction 38. An increase in Co2+ concentration in ferrite compounds caused a reduction in particle size at the nanoscale, boosting the surface area intact among ferrite particles. Subsequently, a quantum confinement effect in the nanostructured ferrites led to a superior magnetic character 39. It was concluded that the interplay of the three aforementioned major interactions, i.e., spin canting, superexchange interaction and magneto-crystalline anisotropy prevailed in the nanostructured compounds and the magnetic behavior of the ferrites was an outcome of the said interactions. The values of the magnetic parameters were found consistent with those reported earlier for the same family of nanostructured ferrites 40,41.
4. Conclusions
A series of nanostructured
CoxNi1-xMn0.5Fe1.5O4(x =
0,0.25,0.5,0.75,1) spinel ferrites was successfully synthesized by low temperature
processing using sol-gel auto-combustion technique which involved a shorter
post-growth annealing of 1 h at lower temperature of 600oC. The XRD
patterns of the ferrite compounds confirmed the formation of pure single-phase
spinels, and indexing of the patterns led to an fcc structure of ferrites belonging
to the Fd-











 nueva página del texto (beta)
nueva página del texto (beta)





