1.Introduction
Perovskites-type oxides have been extensively studied. They represent an important class of materials of great technical values in many device applications. Most of them show some excellent properties such as colossal magnetoresistance 1, sensors and catalysts 2, multiferroic devices 3, ferromagnetic 4, ferroelectric capacitors 5, ceramic fuel cells and other properties 7,8. In this work, we are interested in the BaRuO3 polytype known for its adoption to different crystal structures. These structures can be hexagonal-4H 9,10, hexagonal-6H 11, hexagonal-10H 12, rhombohedral-9R 13 or cubic Pm-3m 14,15. We are interested also in the ortho-perovskite GdRuO3 that it adopts an orthorhombic structure with space group Pnma 16. These functional perovskite materials that exhibit a wide range of electrochemical properties including ferroelectricity, ferromagnetism, magnetoresistance and spin-orbit effect have attracted a considerable attention last years. In particular, BaRuO3 perovskite oxide was studied carefully in our last works. BaRuO3 was given to gather with SrRuO3 and BaRuO3 oxides. These materials adopt different structures (Cubic (Pm-3m), Four-Layered hexagonal 4H (P63/mmc), and two-Layered Hexagonal (2H) (P63/mmc), orthorhombic (Pnma) as given in Ref. 15. This work has proved that only BaRuO3 oxide stabilized in the (2H) phase and showed a ferromagnetic role. We recall that during our previous publications in which we have studied the optoelectronic and magnetic properties of BaRuO3 and BaRuO3; the results obtained have affirmed that the BaRuO3 with cubic structure and space group Pm-3m was a half-metallic ferromagnetic 17 and the GdRuO3 with orthorhombic structure and space group Pnma was semiconductor antiferromagnetic A-type 18. Alloys are produced in order to improve the materials properties or to give them exceptional properties. For this reason, we have realized an ab-initio predictive study of the heterovalent alloy GdxBa1-xRuO3 in which, we will explain the crystal structure changes by Gd-substitution in Ba site, the physical mechanism for suppression of ferromagnetism and finally ferromagnetism and antiferromagnetism coexistence induced by Gd-doping.
Similar studies have been carried like that of Kobayashi et al. 19 who have shown that the mechanism of the
extraordinary Hall effect in the system
Ba1-xSrxRuO3 is basically the same as that of
the ordinary ferromagnetic metals. They have also inferred that the difference of
the Fermi surface resulting from the different layered-structure yields the distinct
magnetic and transport properties of the system
Ba1-xSrxRuO320. On the other hand, Xu et al., have asserted that the
Sr1-xLaxRuO3 gives a FM ground state at
2.Computational method
Ab-initio calculations of GdxBa1-xRuO3 alloy (x = 0.0; 0.125; 0.5; 0.875; 1.0) have been performed within Density Functional Theory (DFT) implemented in the electronic structure calculation code Wien2k (0K, 0 Gpa) 22 based on the hybrid full-potential L/APW+lo method 23. In this method the unit cell is divided into non-overlapping muffin-tin (MT) spheres, inside of which the basic functions are expanded in spherical harmonics functions and the basic functions in the interstitial region, outside the MT spheres, are plane waves.
The exchange-correlation potential was treated with the (GGA+U+SO) approximation 24. This method is applied to enhance predictions of structural, electronic and magnetic properties of the GdxBa1-xRuO3 alloy taking into account electronic correlation and spin-orbit coupling (SOC). The Hubbard formalism 25 was used to treat the strong Coulomb repulsion between the localized electrons Ru -4d and Gd-4f.
To model our GdxBa1-xRuO3 alloy, we have used two
supercells 2 x 2 x 2: one is cubic (parent structure of the BaRuO3
compound 17) (Fig.1a) and the other is orthorhombic (parent structure of the
GaRuO3 compound 18)
(see Fig. 1b), each having 8x Gd atoms, 8 (1-x)
Ba atoms, 8 Ru atoms and 24 oxygen atoms (
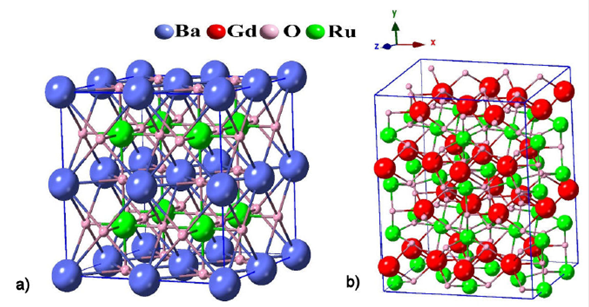
Figure 1 A 40-atom Crystal structure for: a) the cubic BaRuO3 compound and b) the ortho-perovskite GdRuO3.
We have treated the electronic states of the atoms Gd (4f75d16s2), Ba(6s2), Ru (4d7 5s1) and O (2s2 2p4) as configurations of valence states and adopted as muffin-tin (MT) radii the values of 2.4, 2.2, 1.8 and 1.6 Bohr for respectively Gd, Ba, Ru and O elements. The number of plane-wave energy cutoff RMT*KMAX was 8.0. The magnitude Gmax and the cut-off energy were chosen to be 12 and -6 Ry. Total energy was determined using a set of 68 k-points in the irreducible sector of the Brillouin zone equivalent to a 4 x 3 x 4 grid of Monkhorst-Pack 26 mesh for 40 atoms cubic and orthorhombic structures. The energy selected was 0.0001Ry during self-consistency cycles. We recall that all the calculations were completed with the Hubbard U = 6 eV applied on the Gd-4f and Ru-3d orbitals.
3.Results and discussion
3.1.Structural stability
In general, perovskites oxides adopt the cubic phase with (Pm-3m) space group, this structure has a three-dimensional net of corner sharing (BO3) octahedral with A2+ ions in twelve-fold cavities between the polyhedral as represented in (Fig.1). We know that the A and B ions in these perovskite oxides are very flexible and can be varied to related structure (Hexagonal or orthorhombic phases, ect.). In the current paper, we studied the cubic phase with five different concentrations x in comparison with orthorhombic phase. Table I shows the results of the calculated structural parameters carried out for the 5 concentrations (x = 0; 0.125; 0.5; 0.875; 1) in Vegard’s law using the structural data of the parent compounds BaRuO317 and GdRuO318 with the GGA + U + SO approach (U = 6 eV). We can see that when 𝑥 increases the cell volume decreases linearly in the cubic phase and increases linearly in the orthorhombic phase. This has an explanation: to preserve electrical neutrality, the Ru3+ ions in GdRuO3 must replace the Ru4+ ions in BaRuO3 after substituting Ba2+ by Gd3+. As the ionic radius of Ru3+ (0.77 Å) is larger than that of Ru4+ (0.63 Å), the cell volume systematically increases with the increase of x in the orthorhombic phase and decreases systematically with the increase of 𝑥 in the cubic phase as long as the ionic radius of Gd3+ (0.94 Å) is smaller than that of Ba2+ (1.35 Å).
Table I Calculated structural parameters made for the 5 concentrations 0 ≤ x ≤ 1 in Vegard’s law using the GGA+U+SO approximation (U = 6 eV) of GdxBa1−xRuO3 alloy for the ferromagnetic Cubic (FM) phase and the antiferromagnetic (A-AFM) orthorhombic phase
| Alloy | Concentration “x” | a (Å) | b (Å) | c (Å) | Vol (Å 3) | |
| 0.000 | 8.0897 | / | / | 529.4162 | ||
| 0.125 | 8.0021 | / | / | 512.4033 | ||
| Cubic | 0.500 | 7.9730 | / | / | 506.8335 | |
| 0.875 | 7.9437 | / | / | 501.2663 | ||
| GdxBa1−xRuO3 | 1.000 0.000 | 7.934 11.9669 | / 15.2003 | / 10.4770 | 499.4323 1905.7712 | |
| 0.125 | 11.9384 | 15.3672 | 10.5673 | 1938.6745 | ||
| Orthorhombic | 0.500 | 11.8361 | 15.8461 | 10.8234 | 2029.9939 | |
| 0.875 | 11.7337 | 16.3251 | 11.0795 | 2122.3206 | ||
| 1.000 | 11.6996 | 16.4847 | 11.1649 | 2153.3117 |
Figure 2 shows the variation of the equilibrium energy of the GdxBa1-xRuO3 alloy as a function of the concentrations x in cubic and orthorhombic phases. It also indicates a structural stability of this alloy between the ferromagnetic cubic structure (FM) and the antiferromagnetic orthorhombic structure (AFM) A-type. The GdxBa1-xRuO3 alloy therefore undergoes a transition between a cubic phase and another orthorhombic at x = 0.5. It is clear that at this point our alloy (Gd0.5Ba0.5RuO3) is at the same time FM and AFM A-type, in another way, we can say that A-AFM and FM configurations coexist in our alloys. It should be remembered that the cubic equilibrium energy is taken as a reference. We note that in our last work 17, the orthorhombic GdRuO3 (Pnma space group) is considered to be a wide-band gap semiconductor, for this oxide the A-AFM phase is more stable than others (FM, C-AFM, and G-AFM). The cubic BaRu03 oxide (Pm3m space group) is found to be Ferromagnetic (FM) in different scientific works (experimental and theoretical) 15: Y. J. Song et al., 19 and J. Am. et al., 20 had noted that cubic BaRuO3 remains metallic down to 4.2 K. However, the ferromagnetic transition temperature Tc is 60 K.
Eeva-Leena et al., 21
investigated by transmission electron microscopy the
La0.5Ba0.5CoO3: perovskite alloy for which
they confirmed that the considered 0.5 concentration allows a ferromagnetic
character and noted also that the latter develops strain fields and,
consequently, local lattice distortions which explain the high coercivity of
this hard ferromagnet (
The value of 0.5 concentration is the common point between the two phases studied here (cubic and orthorhombic). This type of alloys is also given by J. G. Cheng et al., 22, they noted that ferromagnetism and its evolution in the orthorhombic perovskite system Sr1-xCaxRuO3 is correlated with structural distortion. All these results can explain accurately the coexistence of the FM and A-AFM behaviors in the supercell of GdxBa1-xRuO3 alloy considered in the present work. The origin of these two different behaviors is due essentially to the ferromagnetic character which exists naturally in the cubic BaRuO3 designated with (Pm-3m space group); on the hand we found also the antiferromagnetic A-type character given by the orthorhombic (Pnma space group) GdRuO3 oxide. This result is consolidating in the following part by the the optimization of the total energy as a function of the concentrations “x” using the GGA+U+SO approach (U = 6 eV) where the two-character FM and A-AFM is also present.
3.2.Magnetic stability
The structural phase diagram of the GdxBa1-xRuO3
alloy shown in 2 revealed that this alloy is stable in the cubic structure for
the concentrations x = 0; x =0.125 and x = 0.5, while in the orthorhombic
structure, it appears clearly that the concentrations decreased from x = 0.0 to
x = 1. Table I has given us the results
of the structural parameters performed for the 5 concentrations
For the concentrations: x = 0, x = 0.125 and x = 0.5, we have studied the ferromagnetic (FM) configuration and the 3 antiferromagnetic (AFM) configurations of the GdxBa1-xRuO3 alloy. For the concentrations x = 0.875 and x = 1, we have also studied four configurations: antiferromagnetic A-type (AF-A), C-type (AF-C), G-type (AF-G) and the ferromagnetic state (FM).
We remark that the concentration of 0.5 is the result obtained, after the optimization of the total energy as a function of the concentrations “x” using the GGA+U+SO approach (U = 6 eV), is shown in Fig. 3 which clearly shows that the ferromagnetic state (FM) is most suitable for the concentrations in the cubic phase while the AFM A-type configuration is favoured for the rest of the concentrations of the alloy GdxBa1-xRuO3 in the orthorhombic phase.
3.3.Magnetic properties
Table II includes the results of the magnetic moments (in Bohr magneton) of the Gd x Ba 1-x RuO3 alloy using the GGA+ U + SOC approximation. It is clear that the origin of magnetism comes from the two atoms, gadolinium and ruthenium, this is mainly due to the orbitals Ru-3d and Gd-4f. The values of the magnetic moments were around 1.27 μB/atom for Ru and 7 μB/atom for Gd in the case of the parent perovskite compounds BaRuO317 and GdRuO318.
Table II Results of the magnetic moments (in Bohr magneton, μB) of the Gd x Ba 1-x RuO3 alloy using the GGA+U+SOC approximation
| Concentrations | µ Cell (µB ) | µ Gd (µB ) | µ Ba (µB ) | µ Ru (µB ) | µ interst. (µB ) | |
| GdxBa1−xRuO3 | 0 | 15.99 | / | 0.00043 | 1.27 | 1.90 |
| 0.125 | 21.98 | 7.00 | 0.00056 | 1.33 | 2.10 | |
| 0.5 | 39.95 | 7.00 | 0.00061 | 1.35 | 1.91 | |
| 0.875 | 0 | 6.99 | 0.00064 | 1.39 | 0.025 | |
| 1 | 0 | 6.99 | / | 1.40 | 0.37 |
In the case of our Gd x Ba 1-x RuO3 alloy, we can see that the total magnetic moment increases linearly with the concentrations “x” since it has passed from 15.99 μB for x = 0 to 39.95 μB for x = 0.5, this is valid in the cubic phase. In the orthorhombic phase, its value remains zero regardless of the concentration because we are in an antiferromagnetic configuration. Table II also shows how the magnetic moment of Gd decreased from 7.00 for x = 0.5 to 6.99 for x = 0.875 and x = 1, making it clear that the values of the magnetic moment decrease and tend to be stable according to the phase transition that the alloy may undergo. The magnetic moment of spin of the Ru atom increases also linearly with increasing x, as opposted to that of Gd, which decreases slightly. The same case of spin of the Ba atom.
3.4.Electronic properties
3.4.1.Density of states (DOS)
Figure 4 represents the total and
partial densities of states (DOS) of the perovskite alloy in the cubic
(4(a,c)) and orthorhombic (4(b,d)) phases for all concentrations x
(
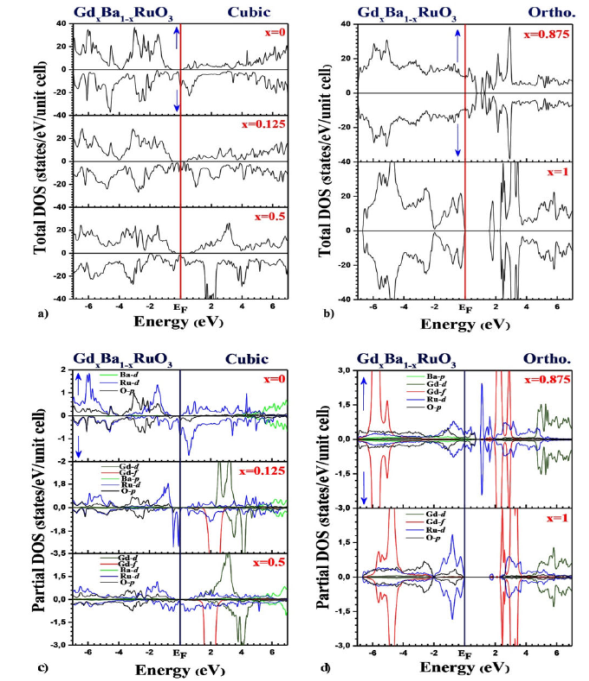
Figure 4 Total density of states of the Gd x Ba 1-x RuO3 alloy in a) the cubic and b) orthorhombic, phases using the GGA+U+SOC approximation for all concentrations x (0 · x · 1).
In cubic phase, as shown in Fig. 4a), for x = 0, x = 0.25 and x = 0.5, we notice from the majority and minority-spin that our alloy exhibits a half-metallic character. It is clear that the difference between these results lies in the position of the Fermi level which varies with the gadolinium substitutions: the bandwidth below Fermi level decreases linearly as x increases. The bandwidths of TDOS in Fig. 4a) are 0.58 eV, 0.63 eV and 0.82 eV at x = 0.00, 0.125 and 0.5, respectively. This would suggest that Gd x Ba 1-x RuO3 has more strongly correlated properties with increasing x as correlation effects is in proportion to an interaction strength to a bandwidth.
In orthorhombic phase, Fig. 4b) shows that the Gd x Ba 1-x RuO3 alloy behaves as being a Mott insulator for x = 0.875. The suppression of ferromagnetism was at the origin of the displacement of the valence band towards the conduction band. Figure 4b) shows also a semiconductor behavior of our alloy (GdRuO3) with 1.57 eV direct gap at the Y point of the Brillouin zone for x = 1. This result is in good agreement with another theoretical calculation 18.
Figure 4c) and 4d) give us precise details of the electronic states of the Ba1-xGdxRuO3 alloy in the cubic and orthorhombic phases:
When x = 0 (absence of Gd atoms), the Ba1-xGdxRuO3 alloy is quite simply the parent perovskite compound BaRuO3. Our analysis shows that in its valence band which extends from -7 eV to the Fermi level, the contributions are dominated essentially by a hybridization of the 3d-Ru and 2p-O states. Note that an insignificant contribution from the 3d-Ba states should be noted. In the conduction band, the contribution comes mainly from 3d-Ru states in the interval (0 to 5.2 eV) and 3d-Ba states in the interval (5.2 to 7eV). These results are valid for the 2 channels (spin-up and spin-down).
Regarding the concentration x = 0.125 (presence of one Gd atom), the DOS of our Ba0.875Gd0.125RuO3 alloy shows that in the valence band, the hybridization of 3d-Ru states with 2p-O is dominant with an advantage to 3d-Ru states from -1.6 eV to the Fermi level. Note the existence of 2 peaks at -0.4 eV and -0.1 eV in the “spin-down” configuration. In the conduction band, the contribution of the 3d-Ru and 3d-Ba states in the interval (0 to 5.2 eV) remains unchanged in the 2 spins compared to the concentration x = 0, however the 4f-Gd and 3d-Gd states contributed effectively in the intervals (1.6 eV to 2.8 eV) in the spin-up and (2 eV to 4.8 eV) in the spin-down.
When x = 0.5 (Ba0.5Gd0.5RuO3), the contribution is similar compared to the previous case (x = 0.125) except that there has been a narrowing of the conduction band responsible for the increase in the gap and the fusion of the 2 peaks into one.
This is valid in spin-up. In the spin-down, there hasn’t been a big change apart from merging the 2 peaks into one in the valence band. In general, the contribution of states 4f-Gd remains unchanged in the conduction band. we notice that it is dominant in relation to the other elements (Ru and O), although the Ba element also underlines an important contribution.
In the case of the concentration x = 0.875 (Ba0.875Gd0.25RuO3) for the orthorhombic phase, the contribution of the 4f-Gd states is predictable in the intervals (-7 eV to -5 eV) of the valence band and (2 eV to 3.4 eV) of the band conduction. Hybridization of 3d-Ru states with 2p-O is dominant in the valence band to Fermi level. It continues in the conduction band up to the point 0.8 eV. Note the presence of a peak at point 1.2 eV. From this point, up to 5 eV, a collaboration of 3d-Ru states is dominant. It is followed by domination of the 3d-Gd states until the end of the conduction band.
Finally, when we replace 8 atoms of Ba by as many atoms of Gd (x = 1), we fall back on our parent compound GdRuO3. It is clear that the contribution of the states 4f-Gd, 3d-Gd and 2p-O remains identical to the previous concentration. The change lies in the decline of the 3d-Ru states at Fermi level with the appearance of a peak at the point -1 eV in the valence band and the narrowing of these states in the conduction band. That allowed the appearance and confirmation of the gap calculated in the band structure.
In summary, we can say that the collaboration of the 3d-Ru and 2p-O states was the most regular in the 2 bands respectively: the conduction band and the valence band in the 2 cubic and orthorhombic phases. We also note the mixed collaboration of the states 3d-Ba. On the other hand, the contribution of 3d-Gd states was only effective in the band of conduction, at the time when that of the 4f-Gd states was noticed especially in the orthorhombic phase. The mixture between the 3d-Ru states and the 4f-Gd states makes the coexistence of the two characters Ferromagnetic (FM) and (A-AFM).
4.Conclusion
Perovskite (ABO3), double-perovskite structures and their alloys exhibit several desirable physical properties related closely to their element’s composition/concentration or transition phases. These three factors at the same time reveal other specific and unique properties as magnetoresistance, dielectric, conductivity, charge spin order and many others. We investigated in current work the structural, magnetic and electronic properties of Ba1-xGdxRuO3 (x = 0, 0.125, 0.25, 0.5, 0.875, 1) based on generalized gradient approximation (GGA+U+SO) by first-principles calculations. It was been found that the Ba1-xGdxRuO3 alloy is a half-metallic in the cubic phase, and, Mott insulator for x = 0.875 and semiconductor for x = 1 in the orthorhombic phase. The current results reveal that both FM and A-AFM coexist in our considerable Gd0.5Ba0.5RuO3 alloys. The GdxBa1-xRuO3 alloy therefore undergoes a transition between a cubic phase and another orthorhombic at x = 0.5. We predict also this phenomenon in all concentration classification (x = 0, 0.125, 0.25, 0.5, 0.875, 1) given here for Ba1-xGdxRuO3 alloy because it appears clearly that the collaboration of the 3d-Ru and 2p-O states is plays an important role for the ferromagnetism in the considered alloy. Also, we found that both the total and Ru magnetic moments increases linearly with the concentrations “x” while the magnetic moment of Gd decreases slightly, which means that despite the change in the concentration (growth or decrease) for 2 elements in this alloy does not influence the magnetic behavior, i.e. the coexistence of the two configurations FM and A-AFM at the same time is due especially to the contribution of the d-Ru states which play the most important role to show the electromagnetic properties in our Ba1-xGdxRuO3 perovskite alloy.











 nueva página del texto (beta)
nueva página del texto (beta)




