1. Introduction
Our aim in here is to get an improvement of chitosan as adsorbent of pollutant agents as CO2 by modifying porosity in its structure, which can be changed to a nanocomposite by adding some form of carbon ring. This is important due to air pollution is a mixture of solid particles and gases in the air. Car emissions, chemicals from factories, dust, pollen, and mold spores suspended as particles, contribute to air pollution. We have previously been working using chitosan to clean water [1-10]. At this time our searching is for taking profit of chitosan and carbon rings as new nanocomposite to be applied for cleaning of air pollutant gas as carbon dioxide (CO2). The capture of atmospheric carbon dioxide has increased due to a global increase in temperature. Atmospheric CO2 levels have contributed to distinguish the need for ambient air cleaning. In this case a new material is proposed for pollutant gas capture. There are poisonous air pollutants as ozone gas, which is a major part of air pollution in cities. Ozone is a combination between pollution, heat, and sunlight, which is called smog. Among the effects of the air pollution in the environment are the following problems: global warming, climate change, acid rain, smog effect, deterioration of fields, extinction of animal species, respiratory health problems, deterioration in building materials.
Atmospheric carbon dioxide (CO2) capture research has been increased due to a global rise in temperature. Levels of atmospheric CO2 have contributed to distinguish need for capture processes. To reduce CO2 emissions is a worldwide research matter, from emission sources as power plants burning fossil fuels, carbon capture and storage have been widely proposed [11]. The CO2 capture has been achieved through chemical absorption (using aqueous amine solutions) for removing it from natural gas since long time ago [12]. Then, it is considered that already exists a technology readiness level (TRL) of 9. This technology use to be utilized in two post-combustion capture facilities in coal-fired power plants [13,14,15,16]. Advances in polymeric membranes have allowed technology to successfully reach demonstration scale (TRL 7). According to this, with our nanocomposite, new polymeric membranes might be constructed. Lithium ortho-silicate is solid sorbent for capturing CO2. Characteristics of the absorption reaction studied by thermogravimetric and volumetric methods exhibit influence of heat and mass transfer limitations in packed bed. This method measures absorption reaction characteristics in experiments. The CO2 absorption rate depends on the CO2 pressure and reactor temperature. The maximum absorption rate observed is at 670°C when the conversion fraction is 0.3 [17].
Nanoparticles (NP) prepared with chitosan and chitosan derivatives possess a positive surface charge and mucoadhesive properties such that can adhere to mucus membranes and release the drug payload in a sustained release manner. Chitosan-based NP have various applications i14n non-parenteral drug delivery for the treatment of cancer, gastrointestinal diseases, pulmonary diseases, drug delivery to the brain and ocular infections according to the review [18]. Chitosan is a cationic biopolymer with potential applications in the food industry because of its unique nutritional and physiochemical properties. These properties depend on its ability to interact with anionic surface-active molecules, such as phospholipids, surfactants, and bile acids. [19]
Chitosan characteristics as adsorbent have been shown in literature. Monomer chitosan (C6H13O5N) interacting with hBN nanosheet (B48N48H24) in the armchair geometry with mono-hydrogenated ends yield chemisorption. The monomer is adsorbed parallel to the nanosheet inducing torsion in the nanosheet. The system at hand exhibits high polarity (3.076 D) like the interaction of chitosan and BN nanotube, and the amine group and hBN nanosheet as reported in literature [20]. The aflatoxins interaction with chitosan is computationally investigated by DFT-B3LYP functional with 6-31g(d) basis set. Through a total energy calculation, the most negative charge is on oxygen atoms of aflatoxins, being the preferred site to interact with chitosan. Aflatoxins are physisorbed and confirmed by the simulated IR spectra [21]. To represent the graphene or boron nitride nanosheet as a CnHm-like cluster to study the adsorption of the monomer of chitosan, represented by the monomeric unit (C6H13O5N), on the graphene surface (G + MCh). As another case, the adsorption of MCh on the G nanosheet functionalized with boron (G + B) has been explored [20,22-25].
The nanocomposite is built joining one carbon double ring to one unit of copolymer chitosan.
It is well known that geometry optimization of two parallel linear carbon chains of
same size provide one polygonal carbon ring after interaction of these two chains
[26]. A carbon double ring molecule
appears in this case, for two parallel C
2. Methodology
VAMP software in BIOVIA Materials Studio is a semiempirical method for calculating the heat of formation at 298°K, in contrast to ab initio methods, which compute electronic energy. The heat of formation is determined from a combination of computed and parameterized data as discussed by Stewart [27], and it is obtained using VAMP for a molecular configuration geometry which depends on computational parameters. After building a structure, it needs refining for getting a stable geometry [27-29].
Geometry optimization is applied using gradient norm of 0.4 kcal/mol/Å, partial general Hessian, Hamiltonian NDDO-AM1, spin restricted Hartree Fock, and CISD configuration interaction type. Using this methodology, and according to single point calculations shown in Fig. 1, the calculated energy for breaking one oxygen from CO2 molecule is 147.28 kcal/mol while the experimental value of the dissociation energy (O-CO) of the CO2 molecule is 125.749 kcal/mol [30]. The difference corresponds to 14.6 %. On the other hand, frequency calculation for geometry optimization accomplished on a CO2 molecule gives four vibrational normal modes:
<> Heat of Formation : -79.820491 kcal/mol
<> Zero Point Energy : 7.459 kcal/mol
<> Translations and Rotations (cm-1)
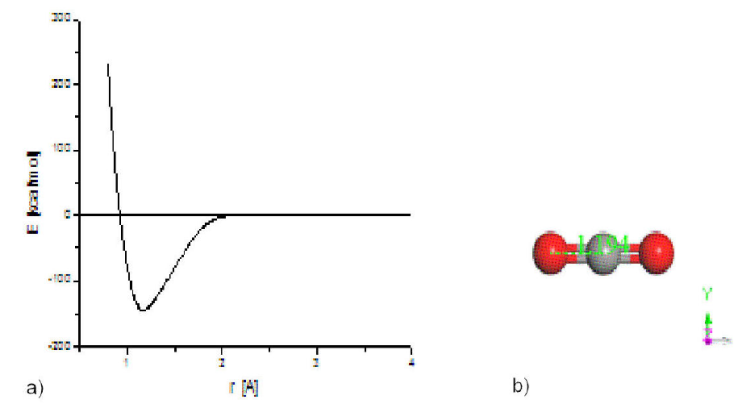
Figure 1 a) Potential energy curve for OELUMO-CO breaking, showing the size of the well of potential. b) OCO after geometry optimization. Oxygen atoms in red color, and carbon atom in gray color.
The heat of formation 79.82 kcal/mol obtained using frequency calculations does not near of
the experimental value with 36.52 % of error; however, frequency values converted
from cm
Along with the previous frequency calculation, frontier orbitals HOMO and LUMO are obtained as another option in the properties of this VAMP methodology.
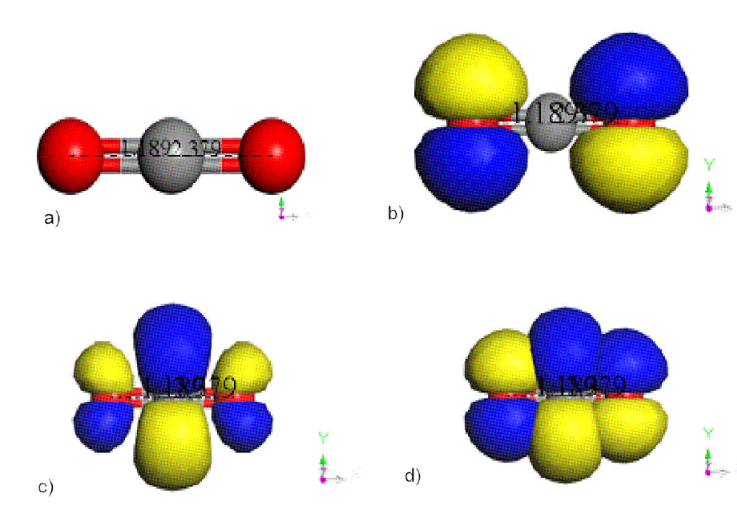
Figure 2 Energy of HOMO-LUMO on a
CO2 molecule. a) CO2 molecule of size 2.379 Å. b)
Calculations are carried out using cero global charge. However, as an example a comparison between Coulson and Mulliken atomic charges for the C
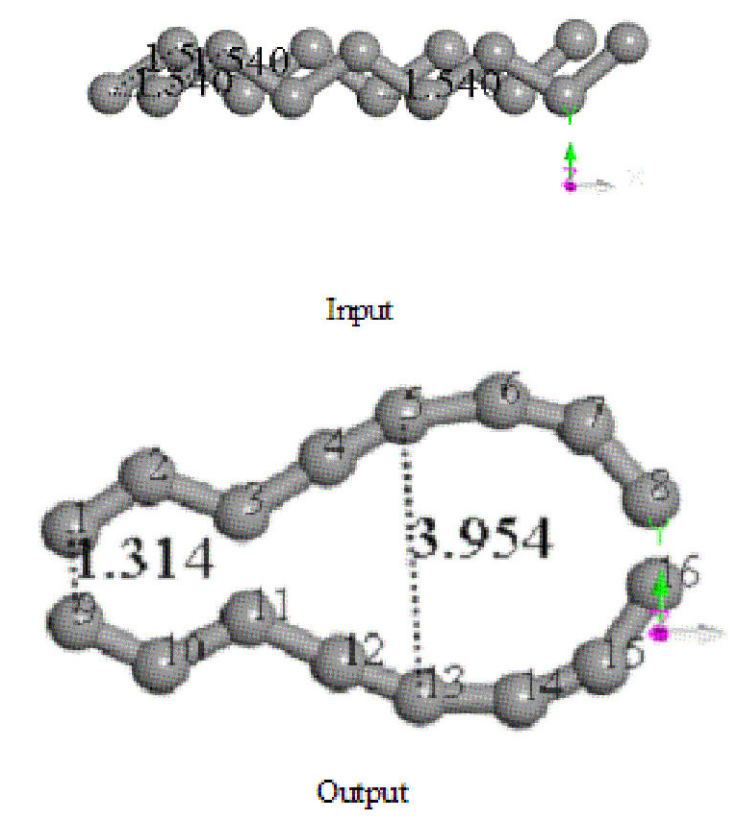
Figure 3 Input and output of the geometry optimization on two C8 carbon chains. Two C8-carbon chains separated at distance of one C-C bond length 1.54 Å.
Table I Coulson atomic charges.
| Atom | Type | Charge | Atom | Type | Charge |
|---|---|---|---|---|---|
| 1 | C | 0.025 | 2 | C | 0.093 |
| 3 | C | 0.131 | 4 | C | 0.068 |
| 5 | C | 0.008 | 6 | C | 0.009 |
| 7 | C | 0.006 | 8 | C | 0.000 |
| 9 | C | 0.024 | 10 | C | 0.092 |
| 11 | C | 0.131 | 12 | C | 0.068 |
| 13 | C | 0.009 | 14 | C | 0.009 |
| 15 | C | 0.007 | 16 | C | 0.001 |
Table II Mulliken atomic charges.
| Atom | Type | Charge | Atom | Type | Charge |
|---|---|---|---|---|---|
| 1 | C | -0.000 | 2 | C | -0.074 |
| 3 | C | 0.125 | 4 | C | -0.056 |
| 5 | C | 0.013 | 6 | C | -0.009 |
| 7 | C | 0.006 | 8 | C | -0.003 |
| 9 | C | -0.001 | 10 | C | -0.074 |
| 11 | C | 0.125 | 12 | C | -0.057 |
| 13 | C | 0.013 | 14 | C | -0.009 |
| 15 | C | 0.006 | 16 | C | -0.004 |
By symmetry in the C16 carbon double ring, we see that atoms 1 and 9, 2 and 10, and so on, have very similar charges, both for the Coulson and Mulliken atomic charge. In both cases the largest charge corresponds to atoms 3 and 11, while the smallest charge corresponds to charges 8 and 16 in the Coulson case, and 1 and 9 in the Mulliken case. Connectivity calculations according to Estrada inferences [31] on no bonding to s- and f -shell scheme, bond type, and converting representation to Kekulé are accomplished for bond length tolerances from 0.6 to 1.15 Å.
3. Results and discussion
Two parallel C8 single-bond carbon chains at a separation distance of 1.54 Å (the experimental value of C-C bond is 1.535 Å) have been set in the input for geometry optimization, as observed in Fig. 3 with certain chirality. After interaction of these carbon chains through geometry optimization (four times using the methodology mentioned previously), the output exhibits a carbon double ring, one C6 ring and one non-circular C12 ring sharing one C-C single bond, on which after applying connectivity and bond type for no bonding to s- and f -shell, the biggest ring is double bond, and the smallest ring has two triple and four single bonds (see Fig. 4).
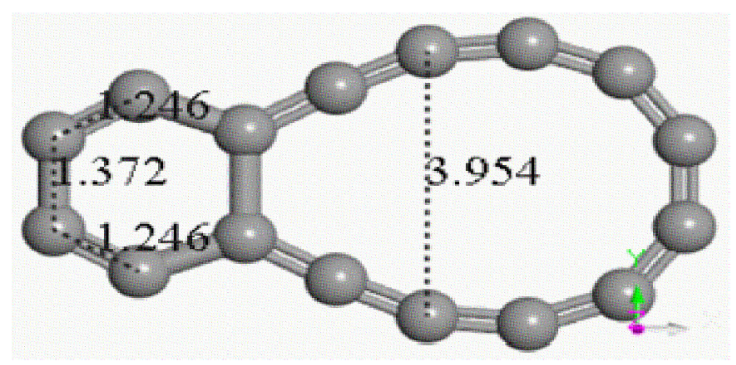
Figure 4 Conectivity applied on the output of Fig. 3. C16 carbon double ring (C6 and C12) sharing one bond length after geometry optimization. Gray color for carbon atoms.
Carbon rings alternating single and triple bonds (-C
The heat of formation of the C16 carbon double ring is 669.41468 kcal/mol. The output is also calculated to show the HOMO-LUMO for bonding (blue orbitals) and antibonding (yellow orbitals), thus, as it can be seen in Fig. 5, blue bonds between atoms in the molecule are the places for insertion of other atoms of another molecule according to the electron sharing between them.
To continuation a sketch of one unit of copolymer chitosan (C14H24N2O9) has been built to be taken as Input for geometry optimization, on which cleaning has been applied as first approximation to bond lengths and angles, as shown in Fig. 6.
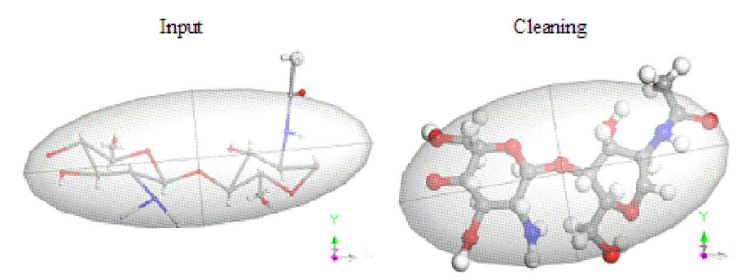
Figure 6 Input: Building a copolymer chitosan structure plus a rough cleaning. Gray, white, red, and blue color for carbon, hydrogen, oxygen, and nitrogen atoms, respectively.
After this, the Output of geometry optimization using VAMP is accomplished. This result exhibits HOMO-LUMO for the corresponding bonding and antibonding places, as shown in Fig. 7. Such molecular structure has: Chemical Formula = C14H24N2O9, NetMass = 364.351, NumAtoms = 49, NetCharge = 0.00000 e,
Then, to get a chitosan-carbon nanocomposite, these were put together in the input with a configuration on which one carbon atom of the non-circular carbon ring is close to a C-N bond length. A new configuration is obtained in the output, where the non-circular carbon ring is perpendicular to one unit of copolymer chitosan and one oxygen atom was captured by the non-circular carbon ring, and this whole system has been inserted into chitosan forming a nanocomposite with C30H24N2O9 chemical formula. We see in the output of the geometry optimization (Fig. 8) the insertion of the C16 double carbon atom in the C14H24N2O9 copolymer molecule of chitosan which has been rotated 90° approximately. Chitosan has been modified because one of its oxygen atoms stays now adsorbed in the smallest ring of C16 carbon double ring. Then, the C30H24N2O9 configuration of the chemical structure is the new product, which is one nanocomposite unit.
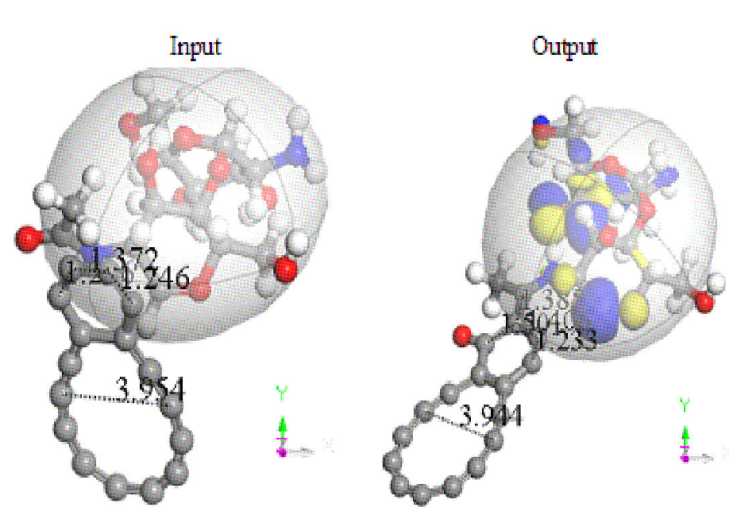
Figure 8 Input-Output about geometry optimization on interaction between chitosan and carbon double ring, with the C6 carbon ring bonded to chitosan at the end.
The adsorption when non-circular C12 carbon ring is the nearest one to chitosan copolymer, according to the output after geometry optimization remains as observed in Fig. 9 in two perspectives. This Figure clearly exhibits the facilities given by HOMO-LUMO for providing reaction sites in this interaction, which in this case corresponds to attractive forces. Furthermore, this nanocomposite has: Chemical Formula = C30H24N2O9, NetMass = 556.527, NumAtoms = 65, NetCharge = 0.00000 e,
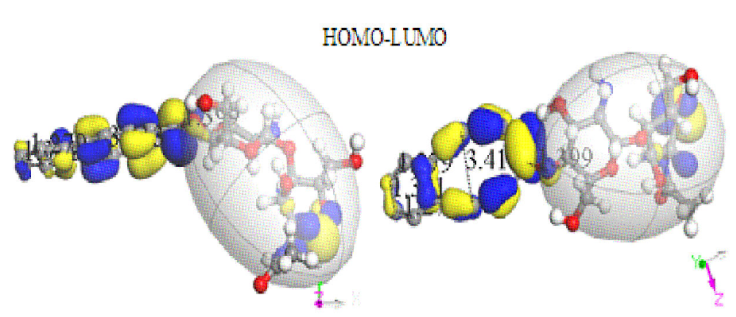
Figure 9 Two perspectives of geometry optimization on interaction between chitosan and carbon double ring, with the greatest ring bonded to chitosan in one side.
To confirm that non-circular carbon ring is responsible of this chemical adsorption, we extract one of its interaction sites, which is the C-C carbon molecule in Fig. 10a). According to C-C bond length of Fig. 5, it has antibonding orbitals. The other part of Fig. 10a) has been extracted from Chitosan molecule. The whole Fig. 10a) has been extracted from the Input of Fig. 8, and it is now the Input on which a geometry optimization is applied, obtaining a new conformation shown in Fig. 10b). In the latter, we observe C-C bond as head on perpendicular to C-H bond length greater than the initial one C-C bond side on perpendicular to C-H, meaning that in Fig. 10b) one carbon atom of C-C is inserting in the bond of the C-H. Applying connectivity from BIOVIA MS Modeling, chemical adsorption is clearly observed in Fig. 10c).
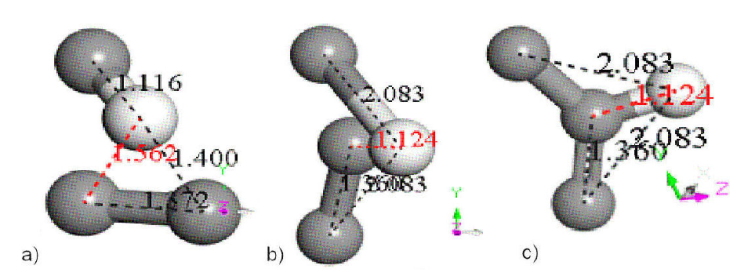
Figure 10 Interaction between reaction sites and of C16 carbon double ring and C14H24N2O9 copolymer chitosan, respectively. This notation implies that C2 is part of the C16 carbon ring, and HC is part of the C14H24N2O9 chitosan molecule.
Antibonding orbitals carry at to repulsive effects, then in Fig. 11, which exhibits orbitals of Fig. 10a), it is observed that bonding is located between C-H carbon atom and the bond length of C-C, as expected.
One CO2 molecule can be independently captured by either C16 non-circular ring or C14H24N2O9 chitosan copolymer. An example of the former is shown in three steps of Fig. 12 (input, output, and connectivity); however, in Fig. 12b), it is observed a potential energy curve of the conformation shown in Fig. 12a). This interaction between linear CO2 with heat of formation of -99.029128 kcal/mol and planar C16 carbon double ring (having a heat of formation of 611.621 kcal/mol) considers rigid molecules and it exhibits a well of potential with a small size of energy around 2 kcal/mol as in Fig. 12b). The output in Fig. 12c) exhibits a conformation where the CO2 is non-linear, given that it considers the same input of Fig. 12a), now with relaxed molecules, and Fig. 12d) exhibits the connectivity between the molecules in Fig. 12c), which is the insertion of CO2 in the C16 carbon double ring. now with carbyne oxide C17O2 (with heat of formation of 535.445 kcal/mol) structure, due to the alternating single and triple bonds. The adsorption energy of Fig. 12a) is calculated as:
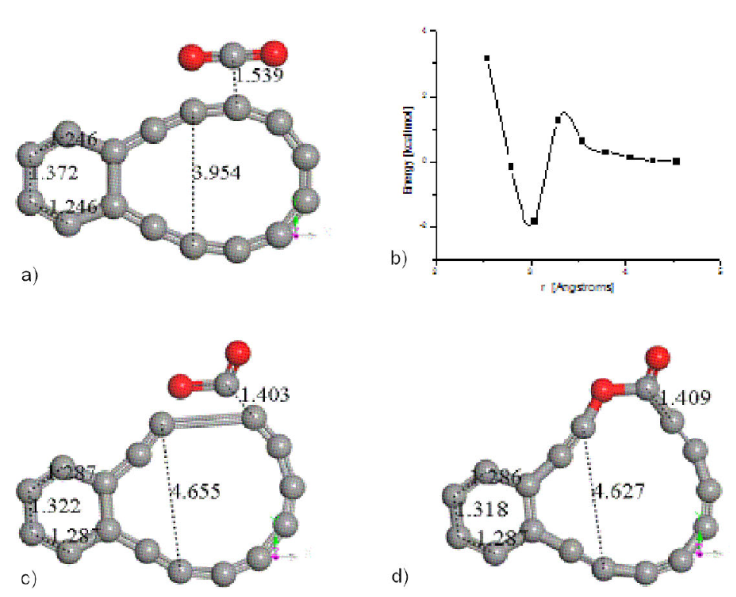
Figure 12 CO2 attacking C16 carbon double ring. a) Input, for geometry optimization, b) Interaction for the previous configuration, c) Output, of the geometry optimization, d) Connectivity.
In the latter result for a relaxed optimization, the heat of formation provides a chemisorption, while the rigid case provides a physisorption, according to the range handled by Atkins [36] and others [37-39], around 5 kcal/ mol for physisorption and greater than 10 kcal / mol for chemisorption, approximately because it depends on the elements in the system. Then, through chemisorption CO2 is captured by C16 forming a new C17O2 molecule as observed in Fig. 12.
In case of more than one CO2 molecules interacting with C16 carbon double ring, in an input arrangement to the geometry optimization shown in Fig. 13a), we see in the output for the geometry optimization shown in Fig. 13b), that at least 50% of CO2 gas are molecules adsorbed on this carbon ring, and the other 50% CO2 molecules have been repulsed when 16 CO2 molecules interact with C16 carbon double ring as shown in Fig. 13c). The input exhibits a separation of 1.539Å between CO2 molecules in a geometrical ordered arrangement. The electrostatic attractions and repulsions among these molecules provide around 50 % of CO2 molecules tending to move away, and three carbon rings added to the C16 carbon double ring. Then a new molecule of five rings has one carbon ring of ten atoms and another of six atoms, two new rings of six and one more new ring of four atoms. The latter has one oxygen and three carbon atoms, and in the two new rings of six atoms there are two oxygen atoms alternating with carbon atoms as shown in Fig. 13c). One carbon dioxide molecule is broken in an oxygen atom which stay bonded to the C16 carbon double ring molecule, and a CO molecule moves away, consequently there is certain percentage of formation of carbon monoxide, while the carbon dioxide considerably decreases as pollutant.
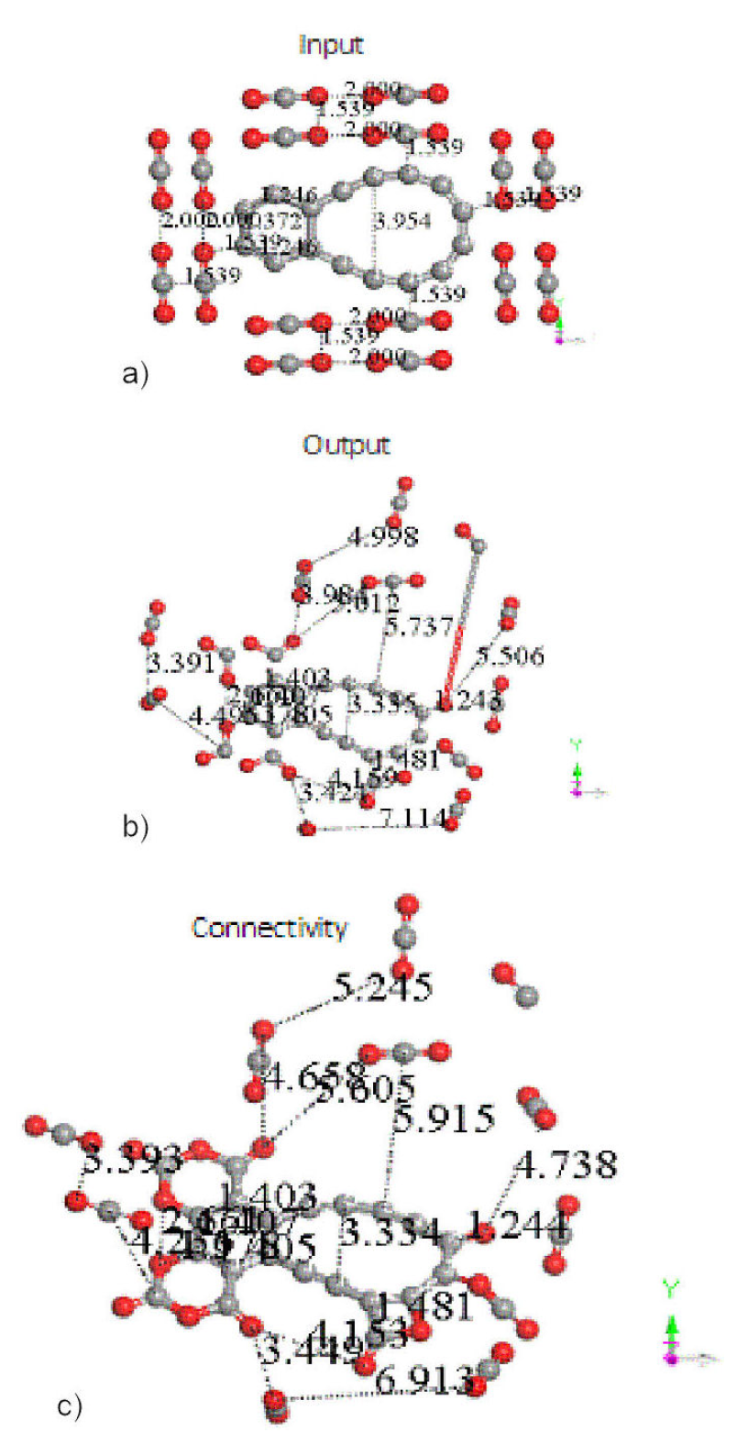
Figure 13 C16 carbon double ring is attacked by 16 CO2 molecules. After minimum energy optimization around 50% of CO2 molecules are adsorbed on C16 carbon double ring.
An example of interaction between one molecule of CO2 and one molecule of copolymer chitosan is shown also in three steps in Fig. 14 (input, output, and connectivity): From these two cases using VAMP calculations, while CO2 is captured by carbon double ring in the former, in the latter CO2 is broken giving CO as new product. Then, among reactions between CO2 and the nanocomposite C30H24N2O9, the CO2 can be captured near to carbon atoms, and can be broken near to gas atoms.
4. Conclusions
The multiplicity of one unit of
Carbon dioxide CO2 molecules can be adsorbed by either nanocomposite or independently by C16 double-carbon-ring or copolymer chitosan. The confidence of this semiempirical model in this case is about 15 % due to the comparison between experimental dissociation energy of CO2 and the breaking of one oxygen atom from the CO2 molecule.
The generated C30H24N2O9 nanocomposite has an improved superficial area with respect to the pristine either C16 or C14H24N2O9, and for increasing CO2 catching. In this case, the C16 carbon molecule is that acting to degrade the oxygen atoms in molecules such as CO2. Then, as much carbon molecules added to the copolymer the better is the adsorption of CO2 in nanocomposite of this composition, given that porosity of chitosan has been changed to be adsorbent of pollutant agents.
We applied different methodologies with different results on the size of the carbon double ring. However, with the chosen Hamiltonians, it seems as if C16 carbon double ring were activated in the insertion process on a chitosan unit, which promotes it to be in charge for catching CO2, because the results for catching CO2 on chitosan indicate that repulsion is easier than attraction.











 nueva página del texto (beta)
nueva página del texto (beta)






