1. Introduction
Andiroba is the popular name of Carapa guianensis Aublet. From Amazonian origin, vegetable oil rich in polyunsaturated fatty acids (PUFA) is extracted from it [1-5]. Such compounds in natural oils are often labeled with phytotherapy [6, 7], associated with anti-inflammatory, antibacterial and antifungal properties [8-10]. With such characteristics, andiroba oil has enormous potential for application. In view of this, the study for the development of oil-based nano emulsions of vegetable origin is highly encouraged [11, 12]. Nano emulsions are dispersions of two or more immiscible fluids where at least one of their components is on a nano-scale. Among their characteristics, they can present good versatility and high interaction with external magnetic fields, such as those constituted by a polymeric matrix doped with nanoparticles, based on ferrite magnets and diluted in aqueous solution. This type of compound generates interest in permeation and vectoring studies in the delivery of drugs, in addition to other biological applications [13-18]. In this study, Raman spectroscopy (Raman) and Fourier transform infrared spectroscopy (FTIR) in essential oil of Carapa guianensis Aublet in natura (CGEO) and polymerized (CGPO) and nano emulsion (CGNE) developed from Polymerized material was applied. FTIR and Raman are important tools in determining functional groups linked to molecules and atoms, besides having as an advantage, fast data acquisition, with high precision and complementarity of the active modes in each of them [19-23]. A computational approach was added by incorporating the oleic acid molecule. The Density Functional Theory (DFT) was the method chosen. Thus, molecular optimization, the calculated frequencies and spectra of the oleic acid molecule (OA) were obtained, which were compared with experimental results. OA was chosen because it was a major component in a higher proportion for CGEO [24]. The B3LYP functional and 6-311+G** basis were used, since they best fit the types of atoms involved [25-27]. In addition, additional information has been obtained in VEDA software. The calculation generated theoretical spectra and vibrational signature data that were compared with experimental results. The experimental results indicate that all samples present bands related to the OA molecule, especially for bands of C=C and C=O. These results express that the components of CGEO are still present in the studied samples. The DFT method was successful in predicting important bands of the molecule studied. The importance of the study is directed to the field of applied sciences, since there are no reports of the application of this methodology to the study of CGEO based nano emulsions. The study is a pioneer in combining experimental techniques and computational modeling in the development of a CGEO-based nano emulsion.
2. Materials and methods
2.1. Synthesis of nanoemulsion
The CGEO was obtained from a producers cooperative (Associação de Produtores Agroextrativistas da Colônia do Sardinha, Lábrea-AM, Brazil). The essential oil-based polymer was synthesized using the polycondensation technique, where the triglyceride chain was broken using 1N Koh and free fatty acids polymerized using the ligand monomer C2H6O4 [28-31]. The nanoparticles were synthesized by a chemical precipitation technique using 1:2 FeCl3-6H2O and FeCl2-4H2O [32, 33]. Using an ultrasonic homogenization, an oil-in-water nanoemulsion was prepared, where nanoparticles are homogenized with the essential oil-based polymer, adding, in proportion 1:4, water and 0.4 g Tween 80 in an Ultrasonic Sonicator under 60 W and 20 kHz for 10 min.
2.2. Experimental
The Raman spectrum was acquired at Raman Labram HR 800 spectrometer, with 633.5 nm laser, in the range from 80 to 3400 cm-1 at the Light spreading Laboratory of the Federal University of Mato Grosso - UFMT. The FTIR acquired spectra were acquired using a SHIMADZU FTIR-IR-Prestige-21 spectrometer, in total attenuated reflectance (ATR) mode with the samples in liquid state. For analysis, one drop of each sample (without dilution) is deposited in a diamond crystal and the spectra were obtained in the region between 4.000 to 400 cm-1, with a resolution of 4 cm-1 and 60 scans. Measurements were performed at the Laboratory of Characterization and Microscopy of Materials of Federal University of Alagoas UFAL. The experimental spectra were treated with Savitzky-Golay smoothing function in Origin software [34, 35].
2.3. Computational methods
Computational calculations were performed using the Gaussian 09 pack age, optimizing the structure and predicting the harmonic vibrational Raman and IR frequencies [36]. Functional B3LYP combined with a base set 6-6-311+G** or 311+G(d,p) was used. B3LYP is a three parameter function (B3) used for the Lee-Yang Parr (LYP) functional correlation exchange. The LYP correlation is a more cost-effective approach to calculating the molecular structure, vibrational frequencies, and energy of optimized structures [37,39]. The polarization used allows good optimization results in organic molecular groups. The 3 description of normal oleic acid modes was performed in terms of analysis of potential energy distribution (PED) using the VEDA software. The description of normal oleic acid modes was performed in terms of analysis of potential energy distribution (PED) using the VEDA 4 software [40]. The theoretical vibrational spectra were reduced with a scale factor of 0.967 to compensate for the resulting error of the vibrational disharmony and the incomplete electronic correlation of the treatment.
3. Results and discussion
3.1. Experimental
Figure 1 and 2 show FTIR and Raman of CGNE, CGPO and CGEO spectra (Also the calculated spectra of OA in line blue). Figure 1 shows the region between 4000and 500 cm-1. The CGEO spectrum is seen in red, CGPO in blue and CGNE in black. FTIR bands present markings for OH, CH, COC, C=O and CH3 groups. FTIR of CGEO shows bands in 2935, 2853, 1743 cm-1 and in 1235 e 725 cm-1. The spectrum of CGPO Fig. 1 shows bands in 3299, 2935 and 2853 cm-1 (associated with CH2 and CH3), 1411 cm-1 (linked to vibrations CH3), 1161 cm-1 and 1093 cm-1 (OCC group). For CGNE the main bands are in 3299, 1550 and 680 cm-1 regions. The results show that CGPO and CGNE contain bands at 3299 cm-1, a broad and intense band common to OH stretching. We can also see bonded bands CH2 and CH3 (methyl group) in 2935 and 2853 cm-1 for CGPO and CGEO.
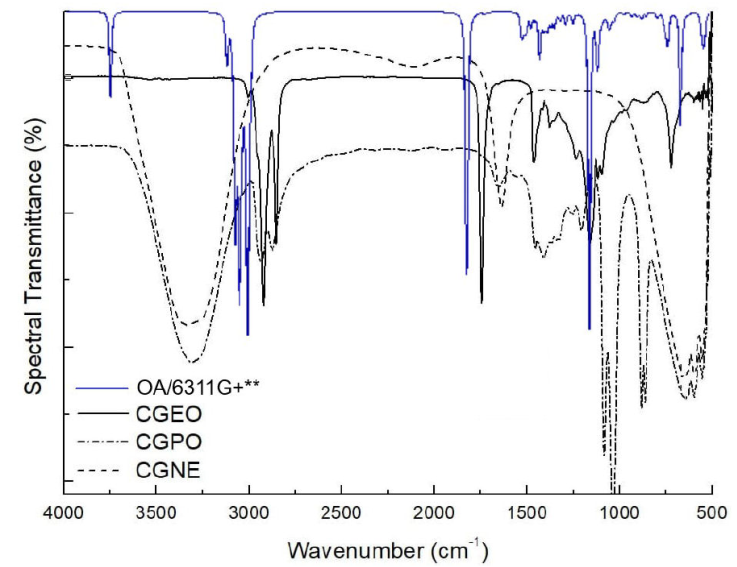
Figure 1 Fourier transformed infrared spectra of the Carapa guianensis Aublet oil, Carapa Guianensis polymer and magnetic nanoemulsion in continuous line, dash-dot and dash respectively. Calculate FTIR spectrum of the oleic acid molecule in blue. The B3LYP/6-311G+** or 6-311G+(d,p) was used.
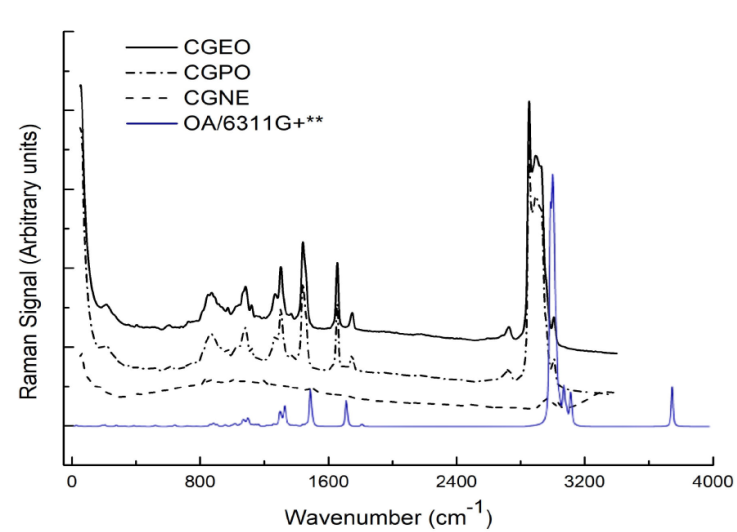
Figure 2 Raman spectra of the Carapa guianensis Aublet oil (CGEO), Carapa Guianensis polymer (CGPO) and magnetic nanoemulsion (CGNE). Calculate Raman spectrum of the oleic acid molecule In blue. The B3LYP/6-311G+** or 6-311G+(d,p) was used.
The region between 4000-2800 cm-1 shows that CGEO does not contain OH band (3299 cm-1) and CGNE does not show CH2 and CH3 bands. In the region between 2000-1000 cm-1 we have an intense and thin band at 1743 cm-1 for CGEO, indicating the presence of C=O stretch. Just below, in the region of 1628 cm-1, CGPO and CGNE show bands of the C-O grouping. Below 1250 cm-1, the CGPO shows a band combined with marking at 1090 cm-1 and 1030 cm-1 associated with C-O-C groups vibrations The bonding of CO esters are indicators on 1235 cm-1 of CGEO [41, 43]. The region between 750-500 cm-1, shows a CH band at 725 cm-1 for CGEO. Considering carboxylic acid a long chain grouping, the band is associated with 725 cm-1, present in this type of structure [42, 44, 50]. Whereas for CGPO and CGNE, we see a broad band between 900-500 cm-1 that may be associated with oxygen interactions with other compounds in dispersion, as is the case with ferrite magnets. As the CGPO is the fruit of the polymerization process, we can see that the FTIR spectrum shows that few bands were preserved in relation to essential oil, except for the CH bands in 2935, e 2853 cm-1. The new bands found are related to the new synthesized polymer. In this case, the ligand monomer used in the CGEO polymerization process was Ethylene Glycol (EG), whose synthesized compound is a derivative of Polyethylene Glycol (PEG) [45]. The main bands are OH (3299 cm-1), COO (1628 cm-1) and COC (1090 and 1030 cm-1). When analyzing the data obtained from FTIR of the synthesized polymer, it was observed that the predominant bands are similar to the PEG visited. The FTIR spectrum of CGNE follows this trend. In CGNE there are bands associated with OH and CO bonding, as in CGPO, in addition to vibrations bands in the region ranging from 750 to 500 cm-1 [43]. In this case, the absence of CH bands around 2850 cm-1 and the appearance of a band centered at 1550 cm-1 and a band between 750-500 cm-1, which may be associated with the interactions of FeO oxygen with other compounds in dispersion. This may explain the band enlargement, as reported for magnets ferrites [46]. Figure 2 shows the Raman of the CGEO, CGPO and CGNE samples. It is observed that the results bring in the region of digital printing, a set of 5 to 6 medium intensity bands for CGEO and CGPO, very common vegetable oils spectra [47]. These bands are at 1080 cm-1, 1302 cm-1 and 1438 cm-1, referring to groups of -CH2. In 1657 cm-1 a typical band for C=C and C-H bonds, common in unsaturated fatty acids, is associated with symmetrical stretching modes [48, 49]. In 1749 cm-1 for CGEO and CGPO samples we have a band associated with carbonyl group stretching (C=O). The band at 1749 cm-1 can be easily noticed in the spectra in the FTIR measurements in Fig. 1, because it is an intense and narrow band. Bands common to CH groups appear above 2500 cm-1. Thus, bands in 2724 cm-1, 2848 cm-1, 2893 cm-1 and 3007 cm-1 are associated with -CH and -CH3 bonding [50, 51]. In the Raman spectrum of CGNE there is a total or partial suppression of the bands for a wide fluorescence curve, as seen mainly in the region between 300-1800 cm-1, which indicates the enveloping of the bands present. In the vicinity of 3000 cm-1, a set of bands are noted that refer to the existing CH groups.
Still in CGNE, above 3200 cm-1, a mild elevation, may indicate the presence of OH modes. In studies already mentioned [52-54], there are no references of a broadband in this region for vegetable oils, even if they contain fatty acids, which have the hydroxyl radical (Fig. 3), something appears for the calculated spectra (see Fig. 1). Below 500 cm-1, all the spectra show a band with common characteristics. For CGEO and CGPO we have the band at 238 cm-1 and CGNE is at 210 cm-1 region. All the samples show a band at 372 cm-1, associated with CC deformations.
3.2. Calculation
Figure 3 is the oleic acid structure, belonging to the group of C1 points of symmetry.
It has 156 fundamental vibrations expressed by 53 stretching (υ), 52 bending (δ) and 51 torsions (τ), which have been assigned with the VEDA software. The vibrational assignment of normal molecule modes was performed based on PED analysis. Only PED values greater than 10% are provided [40]. Table I shows the acquired vibrational data, including the calculated fundamental harmonic frequencies and their corresponding scale values are compared with the experimental Raman and FTIR wavenumber. The calculated spectra of OA with functional B3LYP and 6-311G+** base set are also shown in Figs. 1 and 2 (blue line) for facilitate comparison. In Fig. 1 (blue line) the intense band of C=O bonding appears at 1832 cm-1 for FTIR spectrum. This band appears less intense in Raman (Fig. 2 - blue line). This situation shows how symmetric or non-symmetric groups can be distinct in both techniques. The situation is similar when checking the band at 1714 cm-1 in calculated Raman. It appears narrow and intense in calculated Raman, but in calculated FTIR this band is practically omitted. In Fig. 1, around 3000 cm-1 the bands related to CH groups have similar, intense and fractionated profiles. The highlight is the band centered at 3083 cm-1 which is intense in both spectra. Still in Fig. 1, in 3759 cm-1 we have bands that indicate symmetrical stretching modes of OH. In the experimental spectra this band is not present for CGEO and CGPO. Whereas for CGNE, the presence of broadband may be evidence of the sample’s OH groups [23, 42, 50]. The bands in 636 and 647 cm-1 are linked to carboxyl grouping with formation mode and torsion respectively, as seen in Table I. These groups may indicate that in CGEO and CGPO, they may be responsible for the low intensity band in the region of 636 cm-1.
Table I FTIR and Raman experimental and theoretical modes of the B3LYP/6-311G+** parameters calculated oleic acid molecule and assignments. Percentage (%) of vibrational contribution given by VEDA software.
| Experimental | Theoretical | Assignments (PED%) | ||
|---|---|---|---|---|
| ω IR | ω Raman | ω calc | ω scal | |
| 3299 | - | 3759 | 3635 | υ s OH(1 54) (100) |
| - | - | 3123 | 3020 | υ s CH cis (17 48) (58) |
| - | - | 3098 | 2996 | υ as CH cis (16 47) (56) |
| - | 3007 | 3083 | 2981 | υ as CH3 (19 52) (74) |
| - | - | 3078 | 2976 | υ as CH3 (19 51) (43) |
| - | - | 3077 | 2975 | υ as CH2 (12 40) (63) |
| - | - | 3054 | 2953 | υ as CH2 (18 49 50) (27) |
| 2935 | - | 3037 | 2937 | υ s CH2 (14 43) (37) |
| - | - | 3017 | 2917 | υ s CH3 (19 51) (37) |
| - | - | 3009 | 2910 | υ s CH2 (8 32 31) (27) |
| 2853 | 2893 | 2993 | 2894 | υ s CH(3 22) (38) |
| - | 2724 | - | - | Undefined |
| 1743 | 1749 | 1812 | 1752 | υ s C=O(2 20) (85) |
| 1628 | 1657 | 1714 | 1657 | υ s C=C(17 16) (72) |
| 1460 | - | 1514 | 1464 | δ sci CH2 (26 5 25) (19) |
| - | 1438 | 1488 | 1439 | δ sci CH2 (46 15 45) (23) |
| 1411 | - | 1414 | 1367 | δ sci CH2 (52 19 51) (31) |
| - | 1302 | 1352 | 1307 | δ w CH(40 12 16 17) (21) |
| - | - | 1325 | 1281 | δ t CH2 (27 6 9) (14) |
| 1235 | - | 1237 | 1196 | δ r CH(41 13 17) (13) |
| - | - | 1143 | 1105 | υ s OC(1 20) (31) |
| 1093 | 1080 | 1099 | 1063 | υ s CC(18 14) (11) |
| 1030 | - | 1071 | 1035 | υ s CC(11 5) (27) |
| 937 | - | 957 | 925 | υ s CC(13 17) (27) |
| 725 | - | 647 | 626 | τOHCC(54 1 20 18) (55) |
| - | 636 | 636 | 615 | δ sci OC=O(2 20 1) (60) |
| - | 372 | 380 | 367 | δCCC(4 8 12) (24) |
| - | 238 | 200 | 193 | δCCC(8 12 16) (23) |
| - | 210 | 182 | 176 | δCCC(9 13 17) (10) |
Symmetrical (υ s ), asymmetrical (υ as ), scissor (δ sci ), twist (δ t ), wagg (δ w ), rock (δ r ) and torsion (τ).
Table I presents a combination of experimental and calculated frequencies. The frequencies are accompanied by the vibrational signatures and percentage of contribution given by PED. Frequencies are labeled with ω. PED contributions with a value of 10% or more were indicated. In the PED analysis performed automatically by VEDA for the oleic acid, the EPM parameter reached 27.43. The EPM is the optimization parameter value, which is the arithmetic mean of the maximum elements of each column of the PED matrix [55]. The calculated frequencies were chosen to be consistent with the experimental data. In addition to experimental and calculated frequencies, Table I also brings staggered frequencies. The theoretical vibrational spectra were reduced with a scale factor of 0.967 to compensate for the resulting error of the vibrational disharmony and the incomplete electronic correlation of the treatment, as indicated in CCCBDB [56, 57]. The DFT method was successful in predicting important bands of oleic acid, allowing the assignment of vibrational modes to Carapa guianensis Aubl.
4. Conclusion
Raman and FTIR spectra showed bands of CGEO and CGPO and CGNE specific to each sample. CGEO bands were preserved in CGPO and CGNE. FTIR spectra are clear for all the samples indicating the stage each of them has been subjected to. In FTIR of CGEO, the esterification process determined the predominance of PEG bands combined with CGEO. In Raman, the CGNE data are not clear. However, bands between 2300 and 2500 cm-1 regions that indicate the presence of CH2 and CH3 bands are preserved. The results indicate that all samples present bands related to the OA molecule, especially for bands of C=C and C=O. These results express that the components of CGEO are still present in the studied samples. The study may subsidize future research on nano emulsions, since natural polymer nano emulsions are of great scientific and community interest.











 nueva página del texto (beta)
nueva página del texto (beta)




