Introduction
The spotted sand bass, Paralabrax maculatofasciatus, is a potential species for aquaculture along the northwest coast of Mexico. It is a gynogenetic hermaphrodite. Several aspects of this species's cultivation have been developed in our laboratory, including larval culture (Álvarez-González et al. 2000), juvenile nutrition (Álvarez-González et al. 2001), and growout in sea cages (Grayeb-del Alamo 2001). However, sexual maturation occurs precociously, before commercial size is reached (unpublished data). This is considered a major problem because sexual maturation is accompanied, in many species, by a decrease in survival and growth rate (Felip et al. 2001, Piferrer et al. 2009).
Triploidy induction is the most common type of chromosome manipulation in fish when the goal is to produce sterile fish for aquaculture (Piferrer et al. 2009). Triploidy is achieved by forcing the retention of the second polar body shortly after fertilization, using one of several methods, including thermal shock (cold or heat) and hydrostatic pressure (Dunham 2004). Most studies using chromosome set manipulation in marine species have focused on finding the right combination of treatment variables to induce triploidy, and examining triploid growth during the juvenile and adult stages, hematological dynamics, performance under stress, and gonad development (Piferrer et al. 2000, Felip et al. 2001, Alcántar-Vázquez et al. 2008). However, embryogenesis and survival through the larval period in triploid marine fish have received little attention to date.
Some studies report an increase in the number of dead embryos and low hatching percentages in triploids (Piferrer et al. 2003, Peruzzi et al. 2007); however, none have reported which alterations in the embryonic development are causing mortality. Early embryogenesis consists of a series of mitotic divisions known as cell cleavage. Each division produces new cells called blastomeres. Embryos containing unusual arrangements of blastomeres are considered to exhibit 'abnormal cleavage'. Abnormal cell cleavage early in embryogenesis is detrimental (Avery and Brown 2005). An abnormality in one or more blastomeres may have a greater impact on development than deformity in 1 or 2 cells later in development (Kjørsvik et al. 1990). Positive correlations between the rates of normal early cleavage and the subsequent hatching success have been reported for different species (Kjørsvik et al. 2003, Rideout et al. 2004, Penney et al. 2006). Little is known about the effects of triploidy induction on cellular cleavage and how it affects survival at hatching (Sultana 2005).
Higher mortality in triploids than in diploids is also observed in larval culture (Piferrer et al. 2003, Opstad et al. 2013). Different explanations have been proposed to explain this early mortality in triploids: the timing or magnitude of the treatment, the duration of that treatment (Johnstone 1985, Piferrer et al. 2003), and the newly acquired triploid condition, which involves the presence of a third set of chromosomes (Felip et al. 1997, Piferrer et al. 2000).
A cold shock method for inducing triploidy in the spotted sand bass has been developed in our laboratory (Alcántar Vázquez et al. 2008). However, cold shock treatment does not always induce 100% triploidization. In the aftermath of cold shock treatment, the remaining diploid individuals are known as cold-shocked diploids. In a previous experiment of triploidization in spotted sand bass, the ratio of triploids to cold-shocked diploids was 9:1 at hatching; but after 10 months of culture, that ratio had changed to 1:3, showing a 27 times higher mortality rate in triploid larvae than in coldshocked diploids (unpublished results). Similar results have not been reported for other species.
The objectives of this study were to look for some alterations in embryogenesis of spotted sand bass that could explain the high mortality of triploids during this period and to know if the low triploidy percentage observed in 10-month juveniles was repeatable and when it happened. Experiment 1 recorded the occurrence of abnormal cleavage in diploid and cold-shocked spotted sand bass embryos. Experiment 2 examined differences in hatching success and early survival between normal and abnormally cleaved embryos. Experiment 3 evaluated triploidy percentages during the larval period in individual spawns (control and cold-shocked groups).
Materials and methods
Broodstock management, spawning, and triploidy induction
Wild broodstock (females: 150-300 g; males: 300-500 g) of spotted sand bass were captured in the southern part of the Gulf of California (Bahía de La Paz, Mexico, 24o9'N, 110o19'W) during the reproductive season. Upon arrival at the laboratory, fish were anesthetized with 2-phenoxyethanol (400 mg L-1) and assessed for gonadal maturity. Spawning was induced with LHRHa as described in Alcántar-Vázquez et al. (2008). Hydrated oocytes were collected by manual stripping. Approximately 1 h prior to female cannulation, sperm was collected using a 1-mL syringe and kept on ice at 4oC. Triploidy was induced as described in Alcántar Vázquez et al. (2008). Five minutes after fertilization, the embryos were subjected to 8 ± 0.3 oC cold shocks for 20 min.
Experiment 1: Effect of cold shock treatment on early cell cleavage
Spawns from 13 females were used: 7 in 2008 and 6 in 2009. Each spawn was fertilized with sperm from 3 to 4 males. Fertilized eggs from each female were divided into 2 groups: control (2 replicates) and cold-shocked (3 replicates). After the cold shock treatment, approximately 50 cold-shocked eggs and 50 eggs from the control group were placed in 12-well microplates (4 eggs per well) filled with sterile seawater. Normal or abnormal cleavage was determined under a dissecting optical microscope. Because development occurs rapidly in spotted sand bass, some spawns were observed as early as the 4-cell stage, while others were observed as late as the 32-cell stage, but never later. Any embryo that was not divided in a normal 'boxed formation' was considered abnormal (Fig. 1a, b). Embryos at the 32-cell stage were considered normal or abnormal based on the structural organization of the blastomeres (Fig. 1c, d). Following this evaluation, the 12-well microplates were placed in a temperature-controlled chamber (25oC) for incubation. After hatching, the number of dead embryos, and live and dead yolk-sac larvae were recorded. Yolk-sac larvae that were bent and slightly opaque were considered dead (Fig. 1e, f).
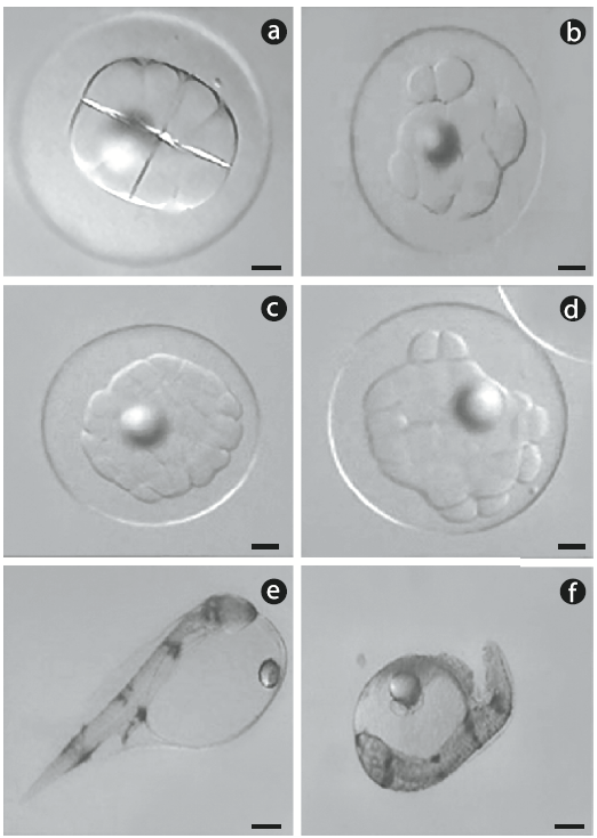
Figure 1 Digital photographs of representative spotted sand bass (Paralabrax maculatofasciatus): (a) normal cleavage and (b) abnormal cleavage in embryos at the 8-cell stage, (c) normal cleavage and (d) abnormal cleavage in embryos at the 32-cell stage, (e) normal hatched larvae, and (f) dead larvae. Scale bar = 90 μm.
Experiment 2: Link between abnormal cleavage and hatching success
The above procedure was repeated in 2009. This time, in order to determine whether the hatching results were linked to the abnormal cleavage, embryos were separated into 4 groups: (1) normally and (2) abnormally cleaved embryos from the control group, and (3) normally and (4) abnormally cleaved embryos from the cold-shocked group. They were placed separately in 12-well microplates and incubated as described above.
Experiment 3: Effect of triploidy on survival during the larval period
The triploidy percentage was examined at different moments during the larval period. Hormone-induced spawns of spotted sand bass are very small so it was impossible to use replicates. For this reason, the experiment was performed with 3 individual spawns. Four to 6 males were used to fertilize the eggs from each female. Cold shock was applied as described above. Spawns with triploidy percentages around 80% were selected to get larvae of both ploidy (triploid and cold-shocked diploid) in order to follow the ploidy percentages during larval culture. Eggs were immediately transferred for incubation at 25 oC and 35 salinity to 2 aerated 120-L conical tanks with water circulation. Twenty-four hours after hatching, the number of yolk-sac larvae was estimated using volumetric methods. Larval density was set using the number of larvae that had survived the cold shock treatment. Control and cold-shocked larvae were transferred to 2 separate 100-L tanks. A photoperiod of 13:11 (light:dark) was used. Temperature and oxygen were measured daily using an oxymeter (±0.1) (YSI model 58, Yellow Springs Instrument Co., Inc., Ohio). Salinity was measured using a refractometer (Aquafauna, Tokyo).
The feeding schedule during the culture trial was as follows: S-type rotifers (Brachionus plicatilis) enriched with Selco (Artemia System, Ghent, Belgium) were offered from 1 to 18 days after hatching (DAH) at a density of 4-15 rotifers per milliliter. From 15 until 30 DAH, larvae were fed Artemia franciscana nauplii enriched with Selco at a density of 4-12 nauplii per milliliter. From 25 to 30 DAH, laboratory-grown Artemia were also added to the tanks. Samples of 30 larvae per tank were taken at hatching, and at 5, 15, and 30 DAH. They were kept at -80 oC until the ploidy analysis was performed. The number of live larvae at day 30 (NL30) in the cold shock treatment and control groups were counted.
The number of triploid larvae at day 30 (T30) and the number of cold-shocked diploids at day 30 (CSD30) were evaluated using the following formulae:
T30 = NL30 × (% triploids on day 30) (1)
CSD30 = NL30 - T30 (2)
The percentage of triploids to survive (ST) and the percentage of cold-shocked diploids to survive (SCSD) to day 30 were calculated as follows:
ST = [(NL30 × % triploids on day 30)/IT] × 100 (3)
SCSD = [(NL30 - no. triploids on day 30)/ICSD] × 100 (4)
where IT is the initial number of triploid larvae and ICSD is the initial number of cold-shocked diploid larvae.
Survival at hatching was calculated as the number of hatched larvae relative to the number of initial eggs and expressed as a percentage. The number of larvae used for ploidy determination was added to the total number of hatched larvae. The proportion of live yolk-sac larvae was calculated as the number of live yolk-sac larvae per hatched larvae. Ploidy was determined using flow cytometry (Partec Ploidy Analyser, Münster, Germany) as described by Alcántar-Vázquez et al. (2008). Triploidy was analyzed on a pool of 30 larvae, except on days 15 and 30 after hatching during the larval culture, when a muscle sample was taken from each larva and analyzed separately.
Statistical analysis
All percentage data were arcsine transformed prior to statistical analysis (Sokal and Rohlf 1998). The normality of the data was verified using the Kolmogorov-Smirnov test, while the homogeneity of variance was examined using the Levine test. For experiment 2, a general linear model that included year and treatment as fixed factors and the percentage of triploidy per spawn as a covariate was used (Y = arcsine of %triploid + year + treatment + year*treatment + e). When normal embryos were separated from abnormal embryos in the control and cold-shocked groups, we applied the same model, using treatment (control and cold-shocked eggs) and cellular division (normal or abnormal) as fixed factors. For cold-shocked eggs, the percentage of triploidy was used as a continuous predictor to assess its effects on cell cleavage, hatching success, and live yolk-sac larvae. Pearson's correlation coefficient was used to determine if there was a significant association between abnormal cleavage and survival at hatching or in the proportion of live yolk-sac larvae in the control and treated groups. In experiment 3, a one-way analysis of variance was used to identify differences in the percentage of triploidy during the larval period. A Tukey test was used when a significant difference was detected. The established level of significance for all analyses was P < 0.05.
Results
Effect of triploidy on cell cleavage
A significant negative Pearson's correlation coefficient was observed between arcsine-transformed percentages of abnormal cleavage and hatching (r = -0.63; P < 0.05) and the proportion of live yolk-sac larvae (r = -0.58; P < 0.05). The proportion of abnormally cleaved embryos was significantly higher (P < 0.05) in the cold-shocked group during both experimental years, while the hatching percentage and the proportion of live yolk-sac larvae were significantly lower (P < 0.05). The percentage of triploidy was significantly higher during the first year (experiment 1) than during the second year (experiment 2) (Table 1). The arcsinetransformed percentage of triploidy was not a significant covariate (P = 0.83) for survival at hatching, the proportion of abnormal cleavage, or the proportion of live yolk-sac larvae. There was no interaction between treatment and experimental year for any of these variables.
Table 1 Percentages of abnormal cleavage (AC), hatching (H), liveyolk larvae (LL), and triploidy (T) (mean ± standard error) for the control (C) and cold-shocked (CS) spotted sand bass (Paralabrax maculatofasciatus) eggs in experiments 1 (n = 7) and 2 (n = 6).
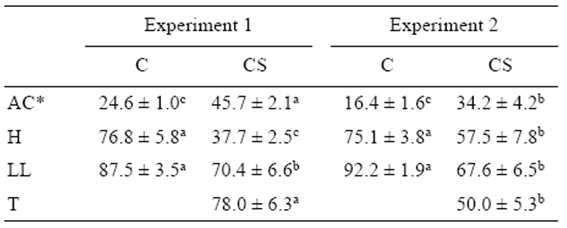
*Means for untransformed data. Superscript letters indicate significant differences in each row (P < 0.05).
Link between abnormal cleavage and hatching success
When the normally cleaved embryos of the control and cold-shocked groups were incubated separately from the abnormally cleaved embryos, significant differences (P < 0.05) were observed in the hatching percentage and the percentage of live yolk-sac larvae (Table 2). Hatching percentages (P = 0.017) were significantly different due to the significantly lower hatching percentage for abnormally cleaved embryos from the cold shock treatment group (P < 0.05) compared to normally cleaved embryos. No significant difference was observed between normal and abnormal embryos in the control group. A significant higher number of live yolk-sac larvae (P < 0.05) were observed in normally cleaved embryos from the control group, while a significantly lower number was observed in the abnormally cleaved embryos from the cold shock treatment group (Table 2).
Percentages of triploidy during the larval period
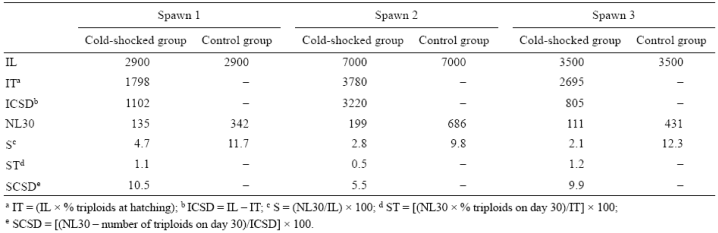
A significant decrease in triploidy percentages (P < 0.05) was observed throughout the larval period in the 3 individual spawns (Table 3). The number of pre-juveniles on day 30 (NL30) was higher in the control than in the cold-shocked group (Table 4). In the cold-shocked group, the percentage of diploids to survive to day 30 (SCSD) was higher than that for triploids, but close to that observed for the control group for the 3 spawns. On day 30, the number of diploid cold-shocked pre-juveniles was always higher than the number of triploids for the 3 spawns (Table 4).
Table 3 Percentage* of triploidy at hatching (TH) and at 5 (T5), 15 (T15), and 30 (T30) days after hatching. Data from individual spotted sand bass spawns (S1, S2, S3).
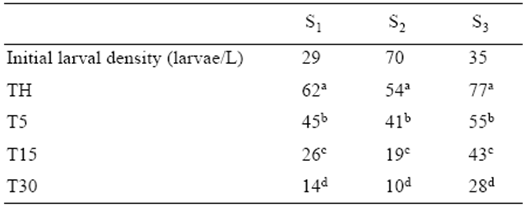
*Means of untransformed data. Superscript letters indicate significant differences in each column (P < 0.05).
Table 4 Initial number of larvae (IL), initial number of triploid larvae (IT), initial number of cold-shocked diploid larvae (ICSD), number of pre-juveniles at day 30 (NL30), survival percentage at day 30 (S), triploid survival percentage at day 30 (ST), and cold-shocked diploid survival percentage at day 30 (SCSD). Data from individual spotted sand bass (Paralabrax maculatofasciatus) spawns.
Discussion
In this study, higher mortality related to abnormal cleavage in cold-shocked spotted sand bass embryos was observed. Moreover, decreasing percentages of triploids were recorded during larval rearing.
Abnormal cell cleavages were observed in the control and cold-shocked groups; however, while the rate of abnormal cleavage was 25% in the control group, hatching success exceeded 90%. These results are consistent with those of Vallin and Nissling (1998), who argue that deviations in undifferentiated early-stage cod cells (4 to 32 cells) allow further development to proceed normally until hatching. Rideout et al. (2004) note that viability varies in abnormally cleaved embryos and depends on the type of blastomeric abnormality. Moreover, proteins and repair mechanisms are constantly working to reduce abnormal blastomeres and embryo mortality, acting as checkpoints during cell division (Gerald 2005). However, when normally and abnormally cleaved embryos in the control and treated groups were separated, a significant negative correlation was observed between abnormally cleaved embryos and hatching only in the cold-shocked eggs. Hence, a high proportion of abnormally treated eggs did not hatch. Self-repair mechanisms are probably disrupted during the cold shock treatment due to changes in intracellular proteins. Felip et al. (1997) and Piferrer et al. (2003) suggest that the increase in abnormal cell cleavage observed following cold shock treatment may be the result of aneuploidy. The addition of a third set of chromosomes may interfere during the anaphase. These additional chromosomes may affect the alignment of the mitotic spindle in the early stages of mitosis, changing the dynamics of the microtubules during cell division and, consequently, modifying the distribution of genetic material and cytoplasmic volume between daughter cells (Gerald 2005, Voronina and Wessel 2006). On the other hand, abnormal cleavage may be due to the cold shock intensity. Intracellular proteins involved in the formation of microtubules may be negatively impacted, resulting in aneuploidy or a disruption of the action and distribution of specific proteins in each half of the dividing cells (Downing and Allen 1987, Voronina and Wessel 2006).
In Atlantic cod, which inhabit cold waters, Sultana (2005) did not find any differences in the percentage of embryos showing abnormal cleavage between cold-shock and control groups. In fact, the percentage of abnormal cleavage in embryos was very low, as was the hatching percentage for both groups. The temperature differential between maintenance temperature and cold shock temperature seems to be more important than the shock temperature itself (Diaz et al. 1993). The difference was only 5 oC in the Atlantic cod experiment; in our experiment it was 14 oC. This difference may explain the high proportion of abnormal cleavage in our study.
Larval rearing in our study resulted in poor survival of triploid spotted sand bass larvae compared with the control and cold-shocked diploid groups. Survival in the control diploid group was similar to that reported by Álvarez-González et al. (2000) in a previous study with the same species in our laboratory. Higher mortality of triploid larvae compared with control diploids has been reported by Solar et al. (1984). They suggest that this gradual decrease in survival may be the result of a degree of inbreeding caused by the retention of the second polar body.
However, the negative effect of the intensity of the cold shock cannot be overlooked. Individuals not responding to thermal shock treatment when triploidy induction is not 100% can serve as controls to determine the effect of shock intensity on the survival and growth rates during the larval period (Piferrer et al. 2009). Maxime (2008) suggests that it seems unlikely that temperatures used during the triploidy induction treatment are intense enough to disrupt development beyond early embryogenesis. The percentages of coldshocked diploids in the 3 spawns were close to that observed in the control group, suggesting that cold shock intensity was not responsible for the low survival of triploids.
We have no conclusive evidence regarding the differential mortality between triploid and cold-shocked diploid larvae. Many differences between triploid and diploid fish have been reported that may affect larval performance and survival. Abnormal behavior, including unusual swimming and feeding behavior (Solar et al. 1984), has been reported for triploid larvae of some species (Piferrer et al. 2009). A less aggressive behavior has been reported for triploid juveniles (Galbreath et al. 1994). Lower jaw (Lijalad and Powell 2009), gas bladder, and skeletal deformities (Tiwary and Ray 2004) have also been observed in triploid fish. The reduction of the cell surface area related to the elevation of ploidy status and its potential relation to several negative biological and physiological characteristics, including the reduction in the cell number in some organs (Swarup 1959), circulating blood cell number, oxygen carrying capacity (Saddler et al. 2000), metabolic pathways (Hyndman et al. 2003, Atkins and Benfey 2008), and stress response (Beyea et al. 2005) have also been proposed.
In conclusion, this study showed that mortality in embryos of cold-shocked treated spotted sand bass can be attributed to abnormal cell cleavage resulting from the 8 oC cold shock, leading to lower hatching than that observed in abnormally cleaved embryos from the control group. Also, a differential survival rate was observed between triploid and cold-shocked diploids, translating into low triploidy percentages at the end of the larval period. This represents a serious impediment for the production of triploid spotted sand bass. A possible solution might be to conduct larval culture exclusively with cold-shock treated spawns that have resulted in 100% triploidity. Important research remains to be done to ascertain the viability of triploid spotted sand bass culture. A logical next step would be to focus on investigating the sexual maturity of triploid spotted sand bass.











 texto en
texto en 



