INTRODUCTION
The wide diversity of marine environments on the costas of Mexico, from temperate oceans to tropical waters, favors the development of a wide diversity of marine algal species. Nearly 1,000 species of macroalgae can be found on these coasts (Robledo 1998). It is common for the floristic pattern of a locality to be affected by the accidental or deliberate introduction of new macroalgae species (invasive algae) (Pedroche and Sentíes 2003, Williams and Smith 2007). That is the case of the red alga Acanthophora spicifera (Børgesen 1910), a native species from the Caribbean and Florida coasts. It was recorded as an invasive species on the Central Pacific Islands (Russell 1992, Tsuda et al. 2008). Recently, A. spicifera, was found on the southwestern costas of the Gulf of California, where it has formed extensive beds with considerable biomass (Fig. 1) (Riosmena-Rodríguez et al. 2009). On the other hand, marine algae are an important source of valuable soluble polysaccharides such as agar, carrageenan, and alginates (Painter 1983, Craigie 1990, Lobban and Harrison 1994). Generally, the composition and properties of the different polysaccharides in seaweeds are related to the physiological function in the corresponding taxonomic algal classification (Kloareg and Quatrano 1988, Miller 1997, Usov and Zelinsky 2013). Red algae, in particular, are known for producing polysaccharides belonging to the family of sulfated galactans. With a few possible exceptions, the structures of all the galactans in this group have a common, simplifying feature: they use as basis linear chains of β-D-galactopyranose residues glycosidically linked through positions 1 and 3 (A units) and α-galactopyranose residues glycosidically linked through positions l and 4 (B units) in either the agar (levogyre [L] B unit) or the carrageenan (dextrogyre [D] B unit) (Painter 1983, Craigie 1990).

Figure 1 Acanthophora spicifera overgrowing on Pocillopora and Porites coral colonies off Point Roca el Caimancito, La Paz, Baja California Sur, Mexico.
In the case of A. spicifera seaweed, which belongs to the order Ceramiales, previous studies have reported that it contains highly sulfated galactans, and studies using mainly Fourier-transform infrared (FTIR) spectroscopy suggest that it produces polysaccharides belonging to the carrageenan family, particularly the lambda type (λ) (Table 1a) (Parekh et al. 1989, Cajipe 1990, Gomaa and Elshoubaky 2016). However, this is controversial as other more complete studies have shown that A. spicifera polysaccharides belong to the agaran family (Gonҫalvez et al. 2002, Duarte et al. 2004, Seixas et al. 2007). Since A. spicifera is a new species in the La Paz Bay area, Baja California Sur, Mexico, no studies have been conducted on the characteristics of the polysaccharides it contains. The purpose of this research was to assess the seasonal content and chemical characteristics of the soluble polysaccharides in A. spicifera, native and alkali-treated, to determine the potential use for this species.
Table 1 Disaccharide repeating units of lambda (λ)-carrageenan (a), native agaran (b), and ideal agarose (c).
| Name | Repetitive idealized disaccharide structure (A-B) |
Full IUPAC* name |
| (a)
λ-Carrageenan |

|
3-linked
β-D-galactopyranose 2-sulfate (A) and 4-linked α-D-galactopyranose 2,6-disulfate (B) |
| (b)
Repetitive dimer in the agaran family (native) |

|
3-linked
β-D-galactopyranose (A) and 4-linked α-L-galactopyranose 6-sulfate (B) |
| (c) Repetitive dimer of agarose in the agaran family (alkali-treated) |

|
3
linked β-D-galactopyranose (A) and 4-linked α-L-3,6-anhydro-galactopyra nose (B) |
* International Union of Pure and Applied Chemistry.
MATERIALS AND METHODS
Collection of biological material
Samples of A. spicifera were collected monthly from February to December 2013 off Point Roca El Caimancito (24º12´12.91 N, 110º18´3.43 W), in La Paz Bay, Baja California Sur, Mexico. The samples were first sun-dried and milled to Ф ≤ 2 mm, then stored in plastic bags in a cool, dry place until analysis.
Extraction of soluble polysaccharides
Native soluble polysaccharides
Done in triplicate, 3 g of dried and milled seaweed simples were extracted by stirring at boiling point for 2 h in 90 mL of 0.1 M phosphate buffer at 6.5 pH. Diatomaceuous earth was added as a filter aid and was vacuum filtered. To increase the ionic strength in the solution, sodium chloride (NaCl) crystals were added up to 0.1 M, and soluble polysaccharides were precipitated by changing the solvent with 2 volumes of 96% ethanol. To remove the salts and water, the coagulum was first rinsed twice with 70% ethanol, then once with 96% ethanol, and then it was dried for 24 h at 60 ºC and weighed (Craigie and Leigh 1978).
Alkali-treated soluble polysaccharides
Done in triplicate, 3 g of dried and milled seaweed were treated with a solution of 6% potassium hydroxide (KOH) and then heated at 90 ºC for 60 min. The liquid was drained after cooling. To remove excess alkali, seaweed samples were first rinsed with running tap water, then with 0.05 N H2SO4 for 3 min, and finally with distilled water. The extraction was carried out following the procedure described for the native extraction (Craigie and Leigh 1978).
Physicochemical characterization
Total carbohydrates were quantified by the phenolsulfuric acid colorimetric method (DuBois et al. 1956) using galactose as standard. 3,6-anhydrogalactose content (3,6- AG) was determined by the resorcinol-acetal method of Yaphe and Arsenault (1965) modified by Craigie and Leigh (1978) using fructose as standard. To determine sulfate content, about 20 mg of dry samples of native and alkali-treated polysaccharides were hydrolyzed with 2N HCl at 100 ºC for 2 h. Sulfate content was then determined by the turbidimetric method of Tabatabai modified by Craigie and Leigh (1978) using potassium sulfate (K2SO4) as standard. The molar ratio of components, galactose, 3,6-anhydrogalactose, sulfates (Gal:3,6-AG: sulfates), was calculated from the native and alkali-treated polysaccharides specially extracted for this determination. The values of the molecular weight of each component were used in the calculation, where sulfates were calculated as sodium sulfate (SO3Na). The absolute viscosity of native and alkali-treated polysaccharides was measured in millipascals per second (mPa·s) in 3% (w/v) solutions at 20 ºC with a Brookfield Mod LVT viscometer with spindle No. 1 at 30 rpm.
Infrared spectroscopy
Fourier-transform infrared spectroscopy (FTIR) was applied. About 1 mg of dry polysaccharide samples was mixed and pressed with potassium bromide (KBr) crystals to form a translucid pellet. The spectra were then acquired in a Perkin-Elmer Spectrum 100 spectrophotometer after 10 scans at a resolution of 4 cm-1 scannings, between 400 and 4,000 cm-1 wavelengths, in transmittance mode.
Statistical analysis
All analyses were performed in triplicate. A priori tests were performed to define normality and homoscedasticity. Since the data were not normal, an analysis of variance was applied to detect significant differences among treatments (P < 0.05). A rank analysis (Kruskal-Wallis) and a posteriori Tukey tests were used with the non-parametric data (Zar 1999).
RESULTS
The applied alkaline treatment affected the yield and composition of the soluble polysaccharides from A. spicifera. Overall, the yields of native extractions were higher (24.7% to 39.3%) than those of extractions with the alkaline treatment (9.1% to 14.9%). In the case of native extractions, the highest yield (39.4%) was obtained in July and the lowest (26.2%) in September (Fig. 2). When using the alkaline treatment, the highest yield (14.9%) was obtained in April and the lowest (9.1%) in August. Only the yield of native polysaccharides showed significant differences throughout the year (P < 0.05) (Fig. 2).
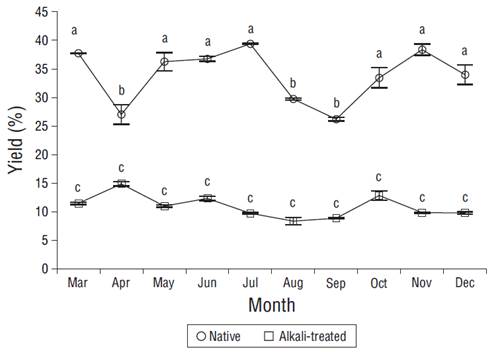
Figure 2 Monthly variation of native and alkali-treated polysaccharide yields from Acanthophora spicifera. Vertical bars are standard deviations (n = 3). Different letters correspond to significant differences between months.
In addition to the changes in soluble polysaccharide yields, there was an increase in the 3,6-AG content and a decrease in the sulfate contents when applying the alkaline treatment before extraction. The 3,6-AG yields for native polysaccharides ranged from 16.48% to 20.26%, and the yields for alkali-treated polysaccharides ranged from 13.80% to 25.70%. Significant differences occurred throughout the year (P < 0.05) (Table 2). Moreover, sulfate content was higher for native polysaccharides (8.80%-13.80%) than for alkali-treated polysaccharides (4.50%-8.90%). Similarly, significant differences were observed between sampling months (P < 0.05) (Table 2).
Table 2 Monthly variation of the 3,6-anhydrogalactose (3,6-AG) and sulfate contents in native and alkali-treated polysaccharides from Acanthophora spicifera. Data are presented as the mean of triplicate assays (n = 3). Different letters indicate significant differences between months.
| 3,6 AG (%) | Sulfate (%) | |||
| Month | Native | Alkali-treated | Native | Alkali-treated |
| March | 19.70 ± 0.37e | 19.40 ± 0.14b | 13.80 ± 0.15e | 7.00 ± 0.12d |
| April | 19.40 ± 0.13e | 19.50 ± 0.20b | 11.50 ± 0.18d | 6.50 ± 0.05c |
| May | 19.60 ± 0.11e | 13.80 ± 0.02a | 10.50 ± 0.07c | 4.50 ± 0.07a |
| June | 16.48 ± 0.13 a | 25.70 ± 0.92f | 8.80 ± 0.05a | 8.90 ± 0.10e |
| July | 20.26 ± 0.01d | 22.29 ± 0.10e | 13.70 ± 0.07e | 6.80 ± 0.14c |
| August | 18.19 ± 0.09c | 20.64 ± 0.07c | 10.30 ± 0.03c | 5.40 ± 0.07b |
| September | 17.17 ± 0.32b | 19.52 ± 0.40b | 10.20 ± 0.28c | 6.80 ± 0.01c |
| October | 18.23 ± 0.13c | 21.03 ± 0.09d | 11.10 ± 0.13d | 7.80 ± 0.12d |
| November | 19.96 ± 0.12e | 20.13 ± 0.53c | 10.90 ± 0.17c | 6.70 ± 0.05c |
| December | 17.20 ± 0.09b | 19.71 ± 0.47b | 9.92 ± 0.06b | 7.60 ± 0.18d |
The FTIR spectra of the soluble polysaccharides obtained from A. spicifera revealed the characteristic signals for sulfated galactans from red algae. A strong band was detected between 1,250 and 1,230 cm−1, with additional bands at 930, 890, 836, and 771 cm−1. The spectrum for the alkali-treated polysaccharide showed more and better-defined signals than the corresponding spectrum for native polysaccharide (Fig. 3).
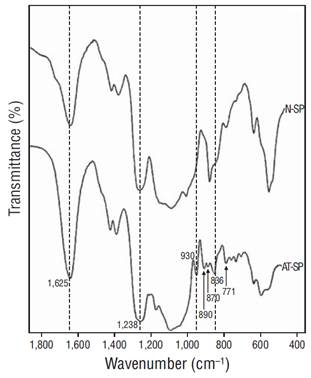
Figure 3 Fourier-transform infrared spectra of native (N-SP) and alkali-treated (AT-SP) soluble polysaccharides from Acanthophora spicifera.
Physicochemical properties
The polysaccharide obtained from A. spicifera is nongelling and produced low-viscosity solutions, which were lower than 41 mPa·s in all cases. The absolute viscosity of the native polysaccharide was significantly higher than that of the alkali-treated polysaccharide. The highest viscosity was obtained with the native process in October (41 mPa·s), while the lowest (4 mPa·s) was obtained with the alkaline treatment in December. An increase in the viscosity of the native polysaccharide was observed from mid summer to the beginning of fall; significant differences were observed mainly for the native polysaccharide (Fig. 4).
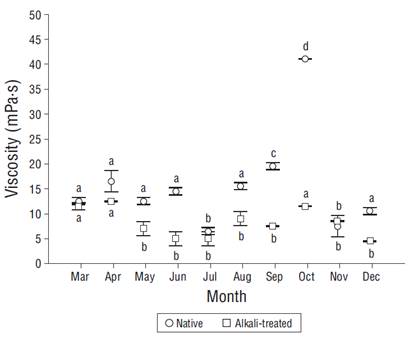
Figure 4 Absolute viscosity of native and alkali-treated polysaccharides measured with a Brookfield viscometer with spindle 1 at 30 rpm. Vertical bars are standard deviations (n = 3). Different letters indicate significant differences between months.
The molar ratio of components (Gal:3,6-AG:sulfates) showed that the A. spicifera polysaccharide is a sulfated galactan with low proportion of 3,6-AG (Table 3). The proportion of hemiester sulfate groups in the native polysaccharide was about 1 sulfate group for every 3 disaccharide units (A-B), while with the alkaline treatment, it decreased to 1 sulfate group for almost every 4 disaccharide units (Table 3).
Table 3 Composition, molar proportion (galactose [Gal], 3,6-anhydrogalactose [3,6-AG], sulfates), and molar ratio of native and alkali-treated polysaccharides from Acanthophora spicifera.
| Content (%) | Molar proportion | Molar ratio | |||||||||
| Treatment | Gal | 3,6-AG | Sulfate | Gal | 3,6-AG | Sulfate | Gal | 3,6-AG | Sulfate | ||
| Native | 70.74 | 18.96 | 10.30 | 0.44 | 0.13 | 0.10 | 1.00 | 0.30 | 0.23 | ||
| Alkali-treated | 72.47 | 19.62 | 7.91 | 0.45 | 0.14 | 0.08 | 1.00 | 0.30 | 0.17 | ||
DISCUSSION
Seaweeds belonging to the Rhodophyceae family are important sources of sulfated polysaccharides, either the agar or the carrageenan types (Painter 1983, Craigie 1990, Lobban and Harrison 1994). To isolate and characterize soluble polysaccharides from red seaweeds, usually in addition to obtaining the native polysaccharide, it is also necessary to apply an alkaline treatment, as it produces more chemically homogeneous molecules and, in some cases, enhances gelforming properties (Hoffmann et al. 1995, Usov 2011).
In this study native and alkali-treated soluble polysaccharides from the red seaweed A. spicifera from La Paz Bay, Baja California Sur, Mexico, were extracted and characterized. As expected, when using the alkaline treatment on the raw material, given the high temperature and high alkali concentration, the 4-linked α-galactopyranose 6-sulfate (B unit) (Table 1b) transformed into 3,6-anhydro α-galactopyranose (Table 1c). The alkaline treatment modifies the composition and properties of polysaccharides in red seaweeds (Kloareg and Quatrano 1988, Craigie 1990, Usov 2011); however, it also entails some degradation and loss of the polysaccharide (Stanley 1987, Myslabodski 1990). Therefore, in this study, the native extraction yields (24.7% to 39.3%) were higher than the extractions with the alkaline treatment (9.1% to 14.9%) (Fig. 2); by contrast, there was an increase in the 3,6-AG content and a concurrent decrease in sulfate content (Table 2). The same trend, lower yields and increased 3,6-AG content, was observed for some alkali-treated agar-producing species (Freile-Pelegrín and Robledo 1997, Arvizu-Higuera et al. 2007, Orduña-Rojas et al. 2008) and for carrageenanproducing seaweeds (Craigie 1990, Freile-Pelegrín et al. 2006).
Using FTIR spectra, some authors have classified the polysaccharides from A. spicifera as carrageenans, particularly of the lambda (λ) type (Parekh et al. 1989, Cajipe 1990, Pickering and Mario 1999, Gomaa and Elshoubaky 2016). λ-Carrageenan is a highly sulfated galactan that yields high viscous solutions but has no gelling properties; it has been chemically characterized (data not shown) and its composition has high contents of sulfate groups (35% to 40%) and only traces of 3,6-AG. In this study, we found that A. spicifera polysaccharides also have no gelling properties, and unlike carrageenans they produce solutions with very low viscosity, even after the alkaline treatment (Fig. 4). Compared to λ-carrageenan, the chemical composition of the A. spicifera polysaccharide (native) has lower sulfate content (8.8% to 13.8%) and higher 3,6-AG content (17.0% to 20.0% for native polysaccharides) (Table 2). Futhermore, the molar ratio (G:3,6-AG:sulfates) for λ-carrageenan is 1.00:0.13:1.10 (experimental data, not shown), whereas for the A. spicifera polysaccharides it is 1.00:0.30:0.23 for the native polysaccharide and 1.00:0.30:0.17 for the alkali-treated polysaccharide (Table 3); however, the A. spicifera polysaccharide composition also differs from true agar, which has higher 3,6-AG content and lower sulfate content, with an ideal molar ratio of 1.00:1.00:0.00 (Armisen and Galatas 1987, Craigie 1990).
On the other hand, studies on A. spicifera polysaccharides have shown that the soluble polysaccharides in this species belong to the agaran family (Gonҫalvez et al. 2002, Duarte et al. 2004). They are known to be highly sulfated on C-2, with pyruvic acid linked as a cyclic ketal bridge at 4,6-O-(1′-carboxyethylidene groups) in the 1-3linked A unit (Duarte et al. 2004). This is in agreement with the fact that the polysaccharides found in other algae belonging to the order Ceramiales biosynthesize sulfated agarans (Painter 1983, Craigie 1990, Usov 2011).
In this study, the FTIR spectra of the A. spicifera polysaccharides showed that these are highly sulfated polysaccharides with pyruvic acid, as shown by the presence of a strong signal between 1,240 and 1,260 cm-1 (asymmetric stretching of O=S=O) (Stancioff and Stanley 1969) and a double signal at 1,637 and 1,417 cm-1 for the pyruvic acid carboxylic group (Prado-Fernández et al. 2003). The spectra of the alkali-treated polysaccharides showed better-defined signals between 1,000 and 700 cm-1 because of the loss of the labile sulfate in C-6 of the B unit and the concurrent 3,6-AG formation. This revealed the signal at 930 cm-1 because of 3,6-AG (Rochas et al. 1986) and a signal at 890 cm-1 for desulfated galactose (Greer and Yaphe 1984). Both signals are absent in λ-carrageenan but are characteristic of agars (Armisen and Galatas 1987, Barros et al. 2013, Gómez-Ordóñez and Rupérez 2011). There was also a strong signal at 830 cm-1, which corresponded to the equatorial sulfate in galactose 2-sulfate (Rochas et al. 1986), and signals at 790 and 717 cm-1, which are characteristic of agar (Matsuhiro and Miller 2002) (Fig. 3). Our results suggest that the soluble polysaccharides in A. spicifera belong to the agaran family; however, given their composition and properties, they must be considered deviants of the true agar (agaroids) (Craigie 1990). On the other hand, despite the deficient viscosity and gelling properties of some polyssacharides, it should be noted that sulfated galactans in red seaweeds usually show potent antiviral activity (Carlucci et al. 1997, Damonte et al. 2004), where the inhibitory effect is attributed to their negatively charged sulfated polysaccharides because of the possible conformational similarity between disaccharide repeating units of sulfated galactans and heparan sulfate, which serves as a negatively charged cell-surface target for most viruses (Chen et al. 1997, Duarte et al. 2004, Usov 2011). Considering mainly the composition and properties of the polysaccharides found in this study, A. spicifera cannot be considered a good source of agar; however, it could alternatively be used for its possible antiviral activity (Duarte et al. 2004, Bouhlal et al. 2011, Gomaa and Elshoubaky 2016) or its possible anti-inflammatory properties, as described for Acanthophora muscoides (Quinderé et al. 2013).











 nueva página del texto (beta)
nueva página del texto (beta)


