INTRODUCTION
Physical disturbances are important in structuring natural ecosystems (Hobbs and Huenneke 1992, Worm et al. 2006, Pickett and White 2013). They can result in increased mortality, changes to the physical environment, altered food web structure, and reduced ecosystem functioning. Although disturbances of moderate strength and/or frequency can result in enhanced biodiversity (Connell 1978), severe and/ or frequent disturbances can result in near-complete mortality of some species (e.g., Edwards and Estes 2006) and ultimately lead to lower biodiversity. Disturbances can be especially important if they result in the loss of foundation species, which provide primary habitat and refuge for other species, enhance biodiversity, modify the environment, and regulate energy flow (Jones et al. 1994). The loss of a foundation species can therefore result in altered ecosystem function (Ellison et al. 2005), which includes controlling rates of primary productivity by the autotrophs, and respiration by the autotrophs, heterotrophs and microbes (del Giorgio et al. 1997, Duarte and Agustí 1998, Edwards et al. 2020). In the coastal marine environment, conspicuous foundation species create coral reefs (Luckhurst and Luckhurst 1978), mangrove habitats (Nagelkerken et al. 2008), kelp forests (Graham 2004, Graham et al. 2007), seagrass beds (Orth et al. 1984), and rhodolith beds (Graham et al. 2016, Tompkins and Steller 2016, Gabara et al. 2018, McConnico et al. 2018).
Rhodoliths (also referred to as maerl) are free-living, coralline red algae (Corallinales) that occur in all the world’s oceans (Bosence 1983, Foster 2001). They provide habitat for numerous species on their thallus exteriors (i.e., epiflora and epifauna), within and among their calcified branches (i.e., cryptofauna), and within the underlying sediments they cover (i.e., infauna) (Steller et al. 2003, Grall et al. 2006). When they form large aggregations or “beds” over otherwise soft sedimentary seafloor, they sequester carbon (Mao et al. 2020), increase structural complexity of the environment (Foster 2001, Steller et al. 2003, Gabara et al. 2018), increase larval settlement, retention, and metamorphosis (Steller and Cáceres-Martínez 2009), reduce predation stress (Kamenos et al. 2004a, b), and aggregate food resources for associated consumers (Grall et al. 2006, Gabara 2020). Consequently, they act as foundation species that create important habitat that supports highly diverse communities of commercially and ecologically important finfish and invertebrate species (Foster 2001, Steller et al. 2003), and rhodoliths are there fore increasingly recognized for their conservation value (Airoldi et al. 2008). Further, these invertebrate communi ties can exhibit high rates of respiration (Newell 1973), and reductions in the abundance and/or body sizes of these spe cies due to habitat degradation can therefore potentially alter net ecosystem productivity and respiration (Brey 2010).
Despite being highly calcified, rhodoliths are branched algae that fragment and grow slowly at a rate of less than 1 mm·y-1 (Foster 2001). Although rhodoliths require low to moderate physical disturbance for normal development (Steller and Foster 1995), they are susceptible to strong dis turbances, from which they are thought to recover slowly. Indeed, anthropogenic impacts from fishing, boat moorings, pollution, anchoring, eutrophication, and sedimentation are all known to have negative effects on rhodolith bed commu nities (Hall-Spencer and Moore 2000, Tompkins and Steller 2016). In particular, chronic crushing from boat mooring chains has been identified as particularly detrimental to rhodolith beds on Catalina Island, California, USA, in that it reduces live rhodolith cover and habitat structural com plexity, increases sedimentation, and reduces biodiversity, all with long-lasting effects on habitat quality (Tompkins and Steller 2016, Gabara et al. 2018). What remains unclear is how this crushing impacts other aspects of ecosystem func tioning, such as primary productivity and respiration. To address this, we ask 3 integrated questions: (1) Do produc tivity and respiration differ between “intact” rhodoliths from undisturbed locations and rhodoliths that have been crushed into fragments by mooring chains, (2) Does crushing of the rhodolith thalli alter their productivity and respiration, and (3) What are the respiration rates of the most common inver tebrate epifauna that inhabit the rhodolith beds? We combine our estimates of invertebrate respiration for the 5 most com monly observed epibenthic species with general estimates of their abundances in Catalina rhodolith beds and in adja cent mooring-disturbed (degraded) habitats to model how reductions in their abundance may contribute to changes in ecosystem respiration. Our overarching goal is to better understand how chronic disturbance from boat moorings affects productivity and respiration of rhodoliths and the species that they support.
MATERIALS AND METHODS
Study Site
Catalina Island, USA (33.40º N, 118.40º W), is one of California’s top recreational boating destinations, with about one million visitors each year (https://www.catalina tours.com/catalina-island-by-the-numbers/), over 50,000 of whom stay on private boats and use the moorings provided by Catalina’s harbors. The island is roughly 32 km south of the city of Los Angeles and is part of the California Channel Islands. It is oriented Northwest-Southeast and experiences the warm water from the Southern California Countercur rent. While the nearshore habitat around the island is pri marily rocky reef dominated by kelp and rockweed forests that are interspersed with sandy habitats, 7 rhodolith beds were recently identified in shallow (5-25 m) soft-bottom, protected bays where high densities of recreational boat mooring systems also occur (Tompkins 2011, Tompkins and Steller 2016). The chains on these moorings have led to crushing of the rhodoliths and severe degradation of the rhodolith beds over large swaths of the benthos (Gabara et al. 2018). The result of chronic crushing by these chains is visible areas of white, crushed carbonate rhodolith sand that support lower abundances of invertebrate species (Gabara et al 2018). Crushed rhodolith fragments (hereafter CRF), which are pigmented and alive but otherwise reduced in size relative to intact rhodoliths (Kim et al. 2020), occur at the edges of these disturbed areas, where they abut the undis turbed rhodolith beds. CRF hereafter consists of both broken rhodolith branches and rhodolith core areas.
Sample collection
Approximately 200 intact rhodoliths (Lithothamnion australe) (Foslie) and approximately 100 g wet weight (ww) of CRF were collected from 4-10 m water depth at the Isthmus Reef rhodolith bed in January 2019 (see map in Tompkins and Steller 2016). The intact rhodoliths were collected from within the center of a living bed (i.e., where there was approximately 100% cover of undisturbed rhodoliths) and the CRF were collected at the interface of this bed in an area where boat mooring chains physically contact the benthos and crush the living rhodoliths (Fig. 1). In addition, 10 haphazardly selected individuals of the 5 most common epibenthic invertebrates observed in the rhodolith beds at Catalina Island were collected from within 3 rhodolith beds at Isthmus Reef, Emerald Bay, and Cherry Cove (see map in Tompkins and Steller 2016). These included wavy turban snail (Megastraea undosa), cone snail (Californiconus californicus), top shell snail (Lirularia sp.), white urchin (Lytechinus pictus), and the California aglaja (Navanax inermis). All organisms were transported to the Wrigley Institute for Environmental Science (WIES) in darkened coolers and immediately placed in flow-through seawater tables in an indoor laboratory, where they were held under ambient temperature conditions until processing (within 24 h).

Figure 1 Photo showing undisturbed (intact) rhodolith bed and mooring-disturbed crushed rhodolith fragments (CRF). The undisturbed rhodolith bed is pictured with inset showing intact rhodoliths on the left panel, and the mooring-disturbed CRF habitat is pictured with inset showing CRF on the right panel. The interface between the 2 habitats is clearly delineated.
Effects of crushing on rhodolith productivity and respiration
To examine if productivity and respiration differ between naturally occurring undisturbed rhodoliths and rhodoliths that have been crushed into fragments by boat mooring chains, we compared maximum net productivity and respiration between intact rhodoliths and CRF. Specifically, 9 replicate intact rhodoliths of similar size (1.80 ± 0.91 g ww) (mean ± SD) and 10 replicate similarly sized (1.71 ± 0.76 g ww) sam ples of CRF were obtained from the laboratory holding tank, blotted dry with a paper towel, and weighed. Then, working with one replicate at a time, each rhodolith or CRF sample was placed into a 96-mL water-jacketed respiration chamber that was connected to the flow-through seawater system at WIES, which kept the chamber at ambient seawater tem perature during the measurements (15.9 ± 0.5 ºC). The res piration chamber was connected to a YSI OBOD probe (self-stirring Optical BOD probe) that measured seawater dissolved oxygen (DO) within the chamber every 5 s using a ProODO optical DO meter (YSI; Yellow Springs, OH, USA). The respiration chamber was placed in front of a halogen light source (PAR38 Wide Flood 60 deg. soft white light bulb) that provided an irradiance of approximately 350 μmol photons·m-2·s-1 to the chamber’s interior, which exceeded L. australe’s saturation irradiance and allowed for estimates of maximum photosynthesis without inducing photoinhibi tion (Kim et al. 2020). To first estimate initial dark respira tion, the chamber was covered with an opaque black cloth and decreases in DO within the chamber were recorded for 15 min. The cloth was then removed, exposing the rhodo liths and/or CRF to saturating irradiances, and net produc tivity was subsequently measured by recording increases in DO over the following 15 min. Following measurement, the rhodoliths and/or CRF were removed and weighed. Respira tion and net productivity for each rhodolith and CRF sample were determined by calculating the rate of change in DO (Δ mg O2·g thallus ww-1·s-1) within the chamber using sepa rate linear regressions. These estimates were scaled by each rhodolith’s biomass and then expanded to reflect hourly rates.
To examine if physical crushing of the rhodolith thalli affects their maximum net productivity and/or respiration, we experimentally crushed intact rhodoliths in the laboratory and measured changes in their metabolisms. Specifically, 6 rhodoliths of similar size (1.51 ± 0.66 g ww) were obtained from the laboratory holding tank, blotted dry with a paper towel, and weighed. Each rhodolith was placed in the respi ration chamber and its respiration and net productivity was measured as described above. Following this, each rhodo lith was removed from the respiration chamber and exper imentally crushed into small (<0.25 cm) CRF by dropping a 60-g lead fishing weight on it over approximately 1 min. These CRF, including the core, were returned to the respi ration chamber and their respiration and net productivity was re-measured. The differences in productivity (ΔProd) and respiration (ΔRes) between the pre-crushing (first) measure ments and post-crushing (second) measurements were then determined for each intact and crushed rhodolith. To ensure any differences observed between the 2 physiology mea surements were indeed due to crushing and not simply due to the rhodoliths and/or CRF being measured a second time (i.e., a handling effect), the 9 intact rhodoliths that were used to compare undisturbed rhodoliths and mooring disturbed CRF (discussed above) were also measured a second time, which served as a procedural (handling) control. To do this, each of those rhodoliths were removed from the respiration chamber immediately after measurement, left out for 1 min (the approximate time it took to crush the rhodoliths with the lead weight), and then returned to the chamber, where its res piration and net productivity were re-measured. This deter mined there were little-to-no differences between the first and second measurements (see Results), which informed us that any differences observed in the CRF were indeed due to crushing the thalli.
Quantifying rhodolith-associated invertebrate respiration
Respiration rates of M. undosa, C. californicus, Lirularia sp., L. pictus, and N. inermis were quantified using a respiration chamber in the laboratory. Specifically, 10 indi viduals of each species were chosen haphazardly from the holding tank and placed individually in a 1-L water-jacketed respiration chamber similar to the one described above. The chamber was connected to the WIES flow-through seawater system, which kept the chamber seawater at ambient tem perature (15.9 ± 0.5 ºC). Respiration for each organism was estimated by measuring changes in DO within the chamber for 15 min under ambient light (>100 μmol photons·m-2·s-1). The organism was then removed from the chamber, blotted dry of excess water using a paper towel, and weighed as grams ww. To evaluate if any observed changes in DO within the chambers resulted from factors other than invertebrate respiration (i.e., microalgae and/or microbe metabolism), the chamber was filled with seawater as above and changes in DO were measured for 15 min within the chamber in the absence of the invertebrates. This was repeated 5 times, which indi cated that little-to-no changes in DO occurred within the chamber in the absence of invertebrates. Therefore, to model how the loss of these 5 species due to rhodolith habitat degra dation affects ecosystem respiration, we combined our respi ration measurements with estimates of each species’ density in rhodolith beds and adjacent mooring-degraded habitats as described by Gabara et al. (2018) (see Table 1).
Table 1 Median respiration rates for 5 common Catalina Island rhodolith bed invertebrate taxa, their densities in undisturbed and mooring-disturbed habitats, extrapolated median contribution within undisturbed and mooring-disturbed habitats, and percent respiration difference lost from undisturbed to mooring-disturbed habitat.
| Taxa | Median respiration rate (mg O2·h-1·ind-1) | Density in undisturbed rhodolith beds (ind·m-2) (Gabara et al. 2018) | Density in mooring-disturbed rhodolith beds (ind·m-2) (Gabara et al. 2018) | Median respiratory contribution in undisturbed habitat (mg O2·d-1·m-2) | Median repiratory contribution in disturbed habitat (mg O2·d-1·m-2) | Differnce in repiratory contribution (mg O2·d-1·m-2) | Percent of respiratory contribution lost |
| Californiconus californicus | 0.111 | 0.110 ± 0.03 | 0.050 ± 0.02 | 0.292 | 0.133 | 0.159 ± 0.133 | 55.55% |
| Megastrea undosa | 0.656 | 0.120 ± 0.05 | 0.003 ± 0.002 | 1.890 | 0.050 | 1.840 ± 0.818 | 97.50% |
| Lirularia sp. | 0.175 | 0.070 ± 0.30 | 0 | 0.293 | 0.000 | 0.293 ± 0.126 | 100% |
| Navanax inermis | 0.440 | 0.030 ± 0.01 | 0.004 ± 0.003 | 0.317 | 0.042 | 0.275 ± 0.137 | 86.67% |
| Lytechinus pictus | 0.097 | 0.030 ± 0.01 | 0.010 ± 0.01 | 0.069 | 0.023 | 0.046 ± 0.046 | 67.70% |
Statistical analysis
Variation in net productivity and respiration among the undisturbed (intact) rhodoliths, the mooring-disturbed CRF, and laboratory-crushed rhodoliths were evaluated with sepa rate analyses of variance (ANOVA). Following this, a priori comparisons between pairs of the 3 treatments were evalu ated with Fisher’s least significant difference (LSD) post hoc tests. Differences in net productivity (ΔProd) and respiration (ΔRes) between pre- and post-crushing in the laboratory were each evaluated with separate paired t-tests. Prior to anal yses, all data were checked for normality using probability plots and for equal variances using Bartlett’s test (for respira tion) and Levine’s test (for productivity), with the latter used because data for productivity in the laboratory-crushed rhod oliths exhibited minor departures from normality. All statis tical analyses were done using R-Studio v.1.1.463, R v.3.5.2, and SYSTAT v.6.0.
RESULTS
Effects of laboratory crushing on rhodolith productivity and respiration
Net productivity was significantly different among the undisturbed (intact) rhodoliths (0.032 ± 0.023 mg O2·g-1·h-1), the mooring-disturbed CRF (-0.002 ± 0.030 mg O2·g-1·h-1), and the laboratory-crushed rhodoliths (0.026 ± 0.023 mg O2·g-1·h-1) (ANOVA: f2,21 = 8.468, P = 0.002). Specifically, net productivity was significantly lower in the mooring-disturbed CRF than in the undisturbed (intact) rhodoliths (Fisher’s LDS: P = 0.001) and in the laboratory-crushed rhodoliths (P = 0.048), but it did not differ between the undisturbed (intact) rhodoliths and the laboratory-crushed rhodoliths (P = 0.129) (Fig. 2). In fact, the mooring-disturbed CRF exhibited net respiration, while the intact rhodoliths and the rhodoliths that were crushed in the lab both exhibited net productivity (Fig. 2). In addi tion, respiration was also significantly different among the undisturbed (intact) rhodoliths (0.040 ± 0.094 mg O2·g-1·h-1), the mooring-disturbed CRF (0.069 ± 0.019 mg O2·g-1·h-1), and the laboratory-crushed rhodoliths (0.062 ± 0.016 mg O2·g-1·h-1) (ANOVA: F2,21 = 4.512, P = 0.023). Specifically, respiration was significantly greater in the mooring-disturbed CRF than in the undisturbed (intact) rhodoliths (Fisher’s LSD: P = 0.007), but it did not differ between the undis turbed (intact) rhodoliths and the laboratory-crushed rhodo liths (P = 0.322), or between the mooring-disturbed CRF and the laboratory-crushed rhodoliths (P = 0.112) (Fig. 3). Fur ther, although a general trend was observed in that crushing of the rhodoliths in the laboratory appeared to lead to slight decreases in net productivity and slight increases in respi ration (Fig. 4), no significant differences were observed between pre- and post-crushing for either net productivity (paired t-test: t7 = -1.894, P = 0.100) or respiration (t7 = 0.265, P = 0.799).
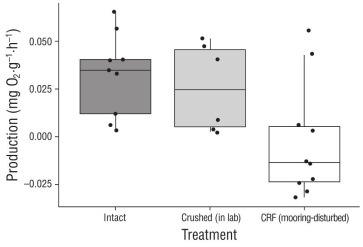
Figure 2 Box plots showing maximum net productivity (mg O2·g-1·h-1) for undisturbed (intact) rhodoliths, rhodoliths that were experimentally crushed in the laboratory, and mooring-disturbed crushed rhodolith fragments (CRF). Positive values reflect net productivity (i.e., oxygen production) and negative values reflect net respiration (i.e., oxygen consumption). Horizontal lines represent median values.
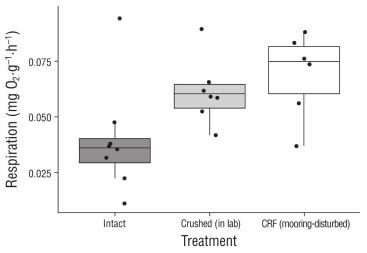
Figure 3 Box plots showing respiration (O2·g-1·h-1) measured for undisturbed (intact) rhodoliths, rhodoliths that were experimentally crushed in the laboratory, and mooring- disturbed crushed rhodolith fragments (CRF). Although respiration is often displayed as negative values (i.e., oxygen consumption), we report positive values here for ease of interpretation; thus, greater respiration is reflected as being elevated along the y-axis. Horizontal lines represent median values (n = 6 to 10 each), and vertical whiskers reflect the range where 75% of all values are expected to fall.
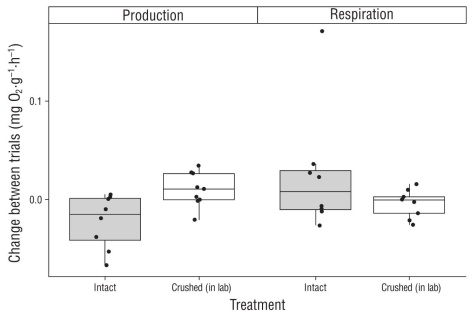
Figure 4 Box plots showing the changes in net productivity and respiration in rhodoliths following experimental crushing in the laboratory (i.e., crushed) and between the first and second measurements for undisturbed (intact) rhodoliths. The intact rhodoliths were measured a second time to serve as a procedural control for the crushing experiment (i.e., to evaluate if any changes in productivity or respiration between pre- and post-crushing were simply due to re-measuring the rhodoliths). Negative values reflect a loss of productivity and/or respiration and positive values reflect an increase in these measures. Black horizontal bars reflect median rates (n = 6 to 10 each).
Quantifying rhodolith-associated invertebrate respiration
Respiration rates differed among the 5 epibenthic inver tebrate taxa studied (Fig. 5). When considered on an indi vidual basis, respiration rates ranged between 0.097 mg O2·g-1·ind-1·h-1 (L. pictus) and 0.656 mg O2·g-1·ind-1·h-1 (M. undosa) (Table 1). These respiration values are significant given that the rhodoliths used in this study averaged in size between 1.51 ± 0.66 g ww and 1.80 ± 0.91 g ww (means ± SE) and exhibited respiration values between 0.040 ± 0.094 mg O2·g-1·h-1 (for uncrushed) and 0.069 ± 0.019 mg O2·g-1·h-1 (for CRF) (see above), and therefore should be considered when modelling ecosystem respiration.
DISCUSSION
Our results show that the chronic crushing of rhodolith thalli by boat mooring chains alters patterns of rhodolith metabolism. Crushed rhodolith fragments (CRF) collected from the habitat where mooring chains repeatedly disturbed the benthos respired at a higher rate than intact rhodoliths that were collected from the adjacent undisturbed rhodolith bed. While the reason for this remains unclear, this may be due to alterations to their three-dimensional structure, which is lost when the rhodoliths are crushed into fragments. As a result, the photosynthetic pigments within their interiors are exposed to high light, and photorespiration likely increases as reported by Kim et al. (2020). Consequently, maximum net productivity rates were lower in CRF than in intact rhod oliths. However, our laboratory experiments suggest that changes to rhodolith productivity and respiration were not detectable immediately following crushing. This leads us to conclude that crushing of the rhodolith thalli alone does not result in immediate changes to the net productivity or respiration. Instead, repeated disturbance to the rhodoliths by chronic crushing from mooring chains over time leads to their mortality and degrades the habitat. This results in a reduction in net productivity and an increase in respiration by the rhodoliths. Further, our findings also suggest that over time, with frequent mooring disturbance, rhodolith habitats likely shift to becoming increasingly respiration dominated, which may alter DO levels within the ecosystem. This pat tern is likely enhanced when the entire benthic communities, including rhodolith-associated microbes (e.g., Cavalcanti et al. 2018), are considered (del Giorgio et al. 1997, Duarte and Agustí 1998). Consequently, similar to what was found in kelp forest ecosystems (e.g., Castorani et al. 2018), dis turbance frequency may be more important than disturbance magnitude in determining the physiological response of rhodoliths to mooring chain impacts.
Our study found that the epibenthic invertebrates inhab iting rhodolith beds have respiration rates that differ both on individual and mass-specific levels. This suggests that inver tebrate consumers differentially contribute to respiration (oxygen consumption) and overall community metabolism. When individual-based respiration rates of the invertebrate taxa studied were combined with estimates of each species’ abundance in the rhodolith beds and the degraded rhodo lith habitats (see Gabara et al. 2018), our data suggest that disturbance-related reductions in the abundance of these 5 species resulted in reduced ecosystem respiration by approximately -2.613 mg O2·m-2·d-1, with individual spe cies contributing between -0.046 mg O2·m-2·d-1 (L. pictus) and -1.84 mg O2·m-2·d-1 (M. undosa) (Table 1).
The gastropod C. californicus and the echinoderm L. pictus had the lowest individual respiration rates, while the gastropod C. californicus had the lowest mass-specific respiration rate. However, despite their low respiration values, at high densities these taxa may alter ecosystem respiration within the rhodolith beds. Both of these organ isms may be able to tolerate low oxygen levels in rhodolith bed sediments, as they are often found within the infaunal communities. This also suggests that the organisms with the highest respiration rates, namely N. inermis and M. undosa, are likely constrained to the surface epibenthic habitat where they are commonly found. This is supported by Grall et al. (2006), who found that rhodolith-associated organisms par tition themselves with depth, and by Diaz and Rosenberg (1995) and Altieri and Diaz (2019), who observed that in soft sediment systems like the Chesapeake Bay, hypoxia (<2.8 mg O2·L-1) occurs annually during summer, causing mortality and emigration by many of the organisms that live there. However, it remains unknown if the epibenthic invertebrates in the rhodolith beds on Catalina Island partition themselves within the rhodolith bed matrix based on oxygen availability and/or their oxygen consumption. What is known is that these taxa are not generally replaced by other taxa in the crushed rhodolith habitat who would compensate for this lost respiration, but rather the rhodoliths beds support higher overall organism abundances than the crushed rhodo lith habitats (Gabara et al. 2018).
This study takes steps towards creating a more individualized community model for rhodolith primary productivity and respiration in both undisturbed and mooring-disturbed (degraded) rhodolith beds. Thus, our estimates for rhodolith production and respiration and invertebrate respiration can give a preliminary context for field-based community estimates. Field-based estimates of production and respiration are needed that incorporate both flora and fauna within communities in undisturbed and mooring-disturbed habitats under natural conditions. Future studies could determine if biomass-specific estimates of productivity and respiration in the lab can be scaled by each species’ biomass in the field to match production and respiration rates measured in situ (e.g., using benthic chambers), such as described by Middelboe et al. (2006). A better understanding of the individual contributions of production and respiration by the dominant taxa relative to whole-community estimates will also reveal if all contributions are characterized properly. If not, less conspicuous members of the community (e.g., microbes and rarer taxa, or smaller but numerically abundant taxa) may have greater contributions to community metabolism than previously thought. For example, the microbiome associated with live rhodoliths from Catalina Island differs from that of dead rhodoliths (e.g., crushed rhodoliths sands), with live rhodo liths supporting a diverse array of taxa that includes Cyanobacteria, Actinobacteria, Betaproteobacteria, Clostridia, and Gammaproteobacteria, and dead rhodoliths being dominated by Gammaproteobacteria (Cavalcanti et al. 2018).
Future studies can build off our methods for measuring temporal effects of mooring crushing by monitoring these effects in the lab and/or the field over greater frequencies and over longer time scales. In addition, future studies can quantify respiration rates for additional taxa that inhabit the rhodolith beds, specifically macro- and microscopic infaunal invertebrates such as tanaids, ostracods, caprellids, and gam marids. These organisms can occur in the densities of over 10,000 per square meter and therefore may make significant respiratory contributions despite their small size and biomass (Gabara et al. 2018). Given that rhodolith physiology varies seasonally (Martin et al. 2007) and over wide temperature ranges (Steller et al. 2007), expanding these experiments over wider temporal scales will broaden the application of these results.











 nueva página del texto (beta)
nueva página del texto (beta)



