INTRODUCTION
Sea urchins are important ecosystem engineers throughout the world’s oceans due to their effects on community struc ture and food webs as grazers and habitat providers (Harrold and Reed 1985, Hartney and Grorud 2002, Nishizaki and Ackerman 2007, Ling et al. 2015, Lowe et al. 2015). Many sea urchin species are macrophyte-dependent, living within the depths that support seaweed and seagrass growth, known as the macrophyte zone. However, the export of macrophyte biomass can support sea urchins hundreds of kilometers from the location of macrophyte production and alter their effects on local benthic ecosystems (Vanderklift and Kendrick 2005). Red sea urchins (Mesocentrotus franciscanus) are found throughout the rocky reefs of the Northeast Pacific Ocean and are generally associated with the macrophyte zone (Tegner 2001, Rogers-Bennett and Okamoto 2020). Red sea urchins directly consume kelp and, like other sea urchin species, act as shredders that process algae biomass into smaller particles through messy feeding and fecal pellet pro duction (Mamelona and Pelletier 2005). Unlike other species of sea urchins, M. franciscanus rarely aggregate on accumu lations of detrital kelp, herein ‘drift’, but instead remain sed entary and catch drift transported by water motion or gravity (Britton-Simmons et al. 2009). The sedentary drift catching behavior and messy feeding may facilitate trophic transfer of kelp carbon to benthic food webs (Dethier et al. 2019, Yorke et al. 2019) and alter transport of carbon to the deep sea (Wernberg and Filbee-Dexter 2018), particularly below the euphotic zone, where algal biomass is limited.
Much of our understanding of red sea urchin natural his tory has come from studies in shallow, open coastal habi tats dominated by Macrocystis pyrifera in California or mixed kelp assemblages in Alaska, where they feed on avail able drift or destructively graze standing algae when drift is limiting (Duggins 1981, Harrold and Reed 1985, Kato and Schroeter 1985, Rogers-Bennett et al. 1995). In these habi tats red sea urchin abundance decreases precipitously below the macrophyte zone (Rogers-Bennett et al. 1995). How ever, vast areas of the rocky reef habitat preferred by red sea urchins exist outside of these well-studied regions. Indeed, more than 90% of the ~84,000 km of intertidal rocky shore lines from Oregon to Alaska lies within the protected coast of the Salish Sea, British Columbia, and Alaska (Starko et al. 2019). Analogous estimates of the rocky shore length from Baja California (Mexico) and California (USA) are not avail able, but including the ~3,000 km of total shoreline along the Pacific coast of Baja California and California does little to change this percentage. These inland, protected waters differ from open coastal habitats in that tidal currents, rather than ocean swell, generate most water motion. For example, the tidal range in the Salish Sea often exceeds 3 m, generating tidal currents >2 m·s-1 (Mofjeld and Larsen 1984). The gla cially carved fjords of the Northeast (NE) Pacific are often deep, meaning sea urchin habitat could extend to depths below the photic zone (Syvitski et al. 1987, Britton-Simmons et al. 2012). Furthermore, the steep, fjord-like features common to the Salish Sea, British Columbia, and Alaska allow rapid transportation of drift to outer-shelf upper-slope depths, potentially facilitating a depth range expansion of red sea urchins to rocky reefs at depths beyond which the available food supply would support reproductive popu lations on the outer coast. While the precise area of avail able subtidal rocky habitat has not been quantified, one thing remains clear: the majority of our understanding of red sea urchins comes from less than 10% of their geographic range and an area that is potentially an outlier in terms of the most common habitat they occupy. The hydrodynamics and topog raphy of these less-studied habitats exist in stark contrast to outer coast kelp beds, potentially eliciting dramatic differ ences in the ecology of benthic habitats with red sea urchins owing to effects of wave or tidal energy (Dayton 1971).
The transfer of energy across ecosystem boundaries is an important process structuring ecosystems (Polis and Hurd 1996). Seaweed forests are one of the most productive eco systems on earth, exporting most of the carbon fixed there to adjacent terrestrial (Polis and Hurd 1996), nearshore marine (Britton-Simmons et al. 2012, Krumhansl and Scheibling 2012,), and deep-sea ecosystems (>1,000 m; Krause-Jensen and Duarte 2016). Spatial subsidies of seaweed carbon and nutrients from the shallow photic zone influence deep subtidal food webs (Vetter 1994, Britton-Simmons et al. 2012), con sumer behavior (Lowe et al. 2015), and carbon sequestration (Krumhansl and Scheibling 2012, Krause-Jensen and Duarte 2016). The drivers of energy subsidies are predicted to vary between open coast, swell-dominated habitats and protected, tidal-dominated habitats directly via physical transport and indirectly by influencing animal-mediated transfer of organic matter (Berglund et al. 2003). This contrast becomes partic ularly important for drift-feeding sea urchins living in these habitats. Sea urchins in open coast habitats are important shredders of drift that accumulates in depositional habitats in kelp forests (Yorke et al. 2019), whereas sea urchins in tidal-dominated habitats may trap drift in non-depositional habitats and make it available to other consumers (e.g., Duggins 1981). Lowe et al. (2015) hypothesized the strength of red sea urchin trophic facilitation should increase with depth below the macrophyte zone, but understanding of the magnitude and depth to which this relationship occurs has been limited by the difficulty of access to these habitats.
Methodological limitations have led to biases in the inten sity of study among habitats and depths: all marine habitats receive less study than terrestrial habitats, scuba observa tions are generally restricted to nearshore habitats at <30 m depth, and remote operated vehicles (ROV) and ship-based submersible expeditions most often explore offshore shelf and deep-sea ecosystems. The study of benthic marine eco system processes often requires specialized gear, including scuba, ROV, submersibles, and a variety of sampling sys tems deployed from oceanographic research vessels. Fur thermore, boat-based activities are temporally restricted by surface conditions, such as storms, wind, or strong tidal cur rents. These limitations have left the natural history of the nearshore mesophotic zone especially poorly studied (Menza et al. 2008, Gori et al. 2017). This zones is defined by the attenuation of light with depth; for the purpose of this paper, we defined the mesophotic zone to include the depths imme diately below the euphotic zone (18-300 m) (Kahng et al. 2017, Baldwin et al. 2018). The depths within the narrow channels of the Salish Sea regularly exceed the euphotic zone (~18 m; Masson and Peña 2009), often reaching 200-300 m within 1 km of shore, but observations in these habitats have been restricted to trawls (Mortensen 1943) and ROV surveys (Britton-Simmons et al. 2012, Pacunski et al. 2013).
In this study, we compiled independent observations from submersible, ROV, and scuba surveys of exceptionally deep red sea urchins and patterns of drift capture. We predicted that red sea urchins could be found deeper than 125 m in the Salish Sea in areas with hard substrate and drift considering the natural history of this species and that drift capture by deep sea urchins would decrease with depth from the mac rophyte zone. Using a combination of crewed submersible and scuba observations, we conducted surveys from 290 m to the surface to test these predictions and understand how drift capture by red sea urchins varies with depth. We paired these findings with observations of deep sea urchins (200 m) in Dume Canyon, California, recorded using an ROV and mea surements of drift capture rates by sea urchins at the upper limit of the mesophotic zone in the Salish Sea to improve understanding of the role of red sea urchins in benthic food webs from the shallow macrophyte to subphotic zones. From these observations we put forth 3 hypothetical scenarios to contrast patterns of drift availability and red sea urchin distribution based largely on bathymetry and water motion (Fig. 1). These scenarios reflect habitats represented by our study sites, yet broadly apply across the habitat continuum and lead to specific testable hypotheses on sea urchin-drift interactions. We present these observations as the basis for expanded research of red sea urchins across their range.
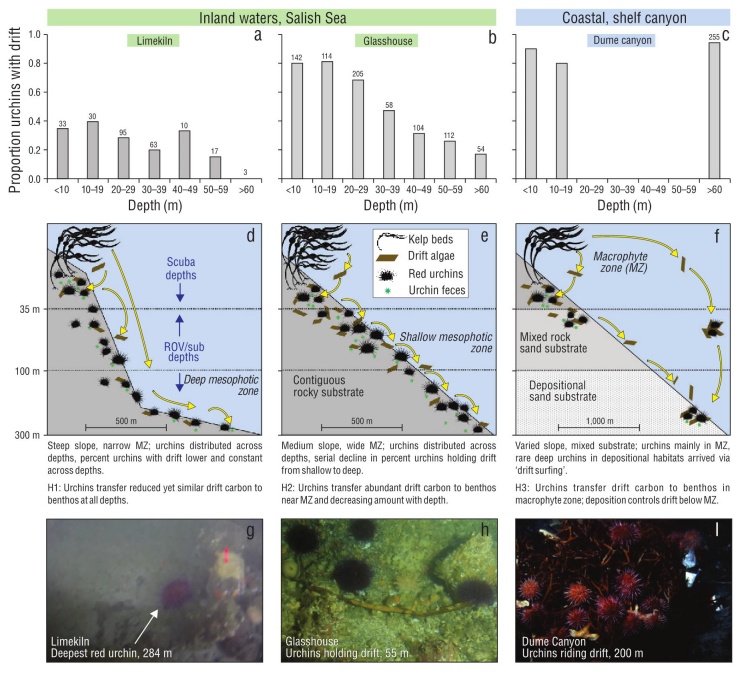
Figure 1 Three hypothesized patterns (H1-H3) of drift algal delivery and capture by red urchins in benthic ecosystems. Observations from macrophyte and mesophotic zones (MZ) suggest different patterns of sea urchin distribution (numbers above bars: total sea urchins observed in depth bin) and drift processing among Salish Sea and open coastal habitats (proportion of sea urchins in each depth with drift; a-c). In the Salish Sea, sea urchins inhabit contiguous rocky reefs to 284 m and actively capture drift algae from the water column, making this resource available to the benthos across depths (d, e, g, h). In contrast, the presence of red sea urchins in mesophotic depths in coastal shelf habitats is likely due to sea urchins ‘surfing’ drift algae from shallow origins to deeper habitats during events like storms, as there is no contiguous rocky habitat to facilitate movement, or suitable settlement substrates, in the deep depositional benthos and nearly all sea urchins are attached to drift (f, i). Shallow bars in Dume Canyon represent expected relationship in kelp forests from literature and therefore do not have sea urchin counts. Scale bar indicates horizontal distance. (g) Snapshot from video of the deepest observed red sea urchin at 284 m with nearby drift kelp at the Limekiln site. (h) Sea urchins and drift from ~50 m depth at Glasshouse (photo: Galloway, Lowe). (i) Sea urchins associated with drift algae in submarine canyon (photo: E. Vetter).
MATERIALS AND METHODS
Prediction and observation of deep sea urchins
Published accounts have reported red sea urchins to 125 m in the central Salish Sea (Mortensen 1943), yet maximum water column depths within the Salish Sea exceed 300 m depth-depths equal to those found on the continental slope many kilometers from the open ocean coast. The steep topog raphy of the fjord-like shorelines and the strong tidal cur rents of the NE Pacific facilitate the delivery of drift to great depths (Britton-Simmons et al. 2012). We predicted that red sea urchins would be present at all depths in which drift could be found amongst hard substrate. To test this predic tion, we conducted 2 submersible dives near San Juan Island, Washington, in Cyclops I, a crewed submersible operated by Oceangate. The expedition, publicized on social media using the hashtag ‘#UrchinSearchin’, was funded by the SeaDoc Society and took place during the week of September 11, 2018. For our first dive, we targeted one of the deepest points in the Salish Sea (~300 m) near Limekiln State Park in Haro Strait, Washington (48º30.888′ N, 123º09.737′ W). We have observed abundant drift algae and sea urchins within diving depths in this area (Lowe et al. 2015). The location also met our depth and habitat requirements, within close prox imity to the Friday Harbor Laboratories, where the expe dition was based. The second submersible dive took place south of Friday Harbor Laboratories at Glasshouse in the San Juan Channel (48º31.250′ N, 122º58.083′ W). The submers ible expedition complemented studies of drift capture by sea urchins at sites within the San Juan Channel, including Point Caution, Point George, and Neck Point (for map see Fig. 1 in Britton-Simmons et al. 2009).
Submersible, scuba, and ROV surveys
During the submersible dives, 2 observers recorded depth, time, location, and number of sea urchins with and without drift to quantify the proportion of sea urchins with drift across depths. Observers recorded data separately and counts were averaged. We conservatively use the term ‘with drift’ to indicate sea urchins that are holding, on top of, or actively feeding on visible drift algae (following Lowe et al. 2015). This method provides conservative estimates of access to drift without disturbing sea urchins, but may miss small pieces of drift algae hidden from view on the oral side of the sea urchin. To confirm observations we recorded video and took photographs from inside the submersible (Fig. 1). We paired submersible-based surveys of the mesophotic ben thos (290.0-30.0 m) with scuba observations (33.5-5.0 m) at the same locations, providing contiguous observations across methods from the mesophotic zone to the surface. The day after the submersible survey, the scuba team returned to the site, descended to 33.5 m and swam at a constant depth along non-overlapping areas. Each diver counted sea urchins with and without drift for 5 min before ascending ~6.1 m and repeating the survey. Five-minute surveys were conducted at each depth zone until reaching a depth of 4.6 m. The steep bathymetry of this region made our zig-zag dive surveys fea sible; the deepest area surveyed during the submersible dives was ~700 m from shore (Fig. 1d) and the scuba dives cov ered less than 200 m of horizontal distance. The number of sea urchins with drift was divided by the total number of sea urchins per depth to calculate the proportion of sea urchins with drift. To contrast patterns from the Salish Sea with outer coast habitats, we analyzed data from ROV surveys con ducted between 200 and 239 m in Dume Submarine Canyon, California, in 1998 (E. Vetter, unpublished data; https://cordellbank.noaa.gov/science/research.html). The number of sea urchins and presence or absence of drift was quantified in stills from ROV video. We calculated drift capture by dividing the number of frames with drift and sea urchins by the total number of frames with sea urchins. We focused on proportion of sea urchins with drift because it was not pos sible to reliably measure area surveyed or density with the submersible or from the existing ROV data. Statistical anal yses of the proportion of sea urchins with drift across depths was not conducted owing to lack of replication due to the logistical restrictions.
Rate of drift capture
We measured the rate of drift capture by red sea urchins at the upper limit of the mesophotic zone in the San Juan Channel to better understand dynamics of drift transport from the macrophyte zone and capture by sea urchins over time. We conducted repeated daily surveys of sea urchins within a permanent transect to estimate drift capture rates (grams of drift per sea urchin per day) across tide phase, season, and sites within season. We delineated 20 m2 (North) and 40 m2 (South) of rocky outcrops at 18 m depth with weighted line. The areas were selected to contain approximately the same number of sea urchins (~80). On the first day of the series of collections, all algal biomass was collected from the sea urchins in the area but was not included in the anal yses of drift capture rate per day because we could not verify the timeframe over which it had been captured. On each of the next 3 consecutive days, the divers counted sea urchins within the delineated area and carefully collected drift captured by sea urchins. Collecting all algae from all sea urchins in the delineated study areas on a daily basis allowed for a calculation of a minimum daily drift capture rate. The collected drift was brought to the lab, identified to lowest taxonomic unit, blotted and weighed (damp weight) to calculate rate of capture in grams per day. The tide phase study occurred over a 2-week period at 2 locations (North and South Point Caution, San Juan Channel; 48º33.722′ N, 123º0.962′ W) during spring and neap tidal cycles in June 2012. This location is 6 km from the Glasshouse and 27 km from the Limekiln submersible dive sites. Our previous work at this site had confirmed little to no daily or seasonal move ment (displacement) of red sea urchins such that collections likely came from the same individual sea urchins (Lowe et al. 2015). The seasonal investigation took place at South Point Caution (PTCS) in April, June, and September of 2012, and January 2013. The spatial investigation took place at PTCS, Neck Point (NPT; 48º34.791′ N, 123º0.992′ W) and Point George (PTG; 48º33.393′ N, 122º59.105′ W) in September 2012. NPT and PTG are within 2.5 km of PTCS on the eastern side of San Juan Channel, Washington.
RESULTS
Submersible and scuba surveys
Minutes into our first submersible dive at Limekiln, we observed a red sea urchin at 284 m, more than doubling the known depth range of this species (Fig. 1g). Visibility was limited, preventing further observations at this depth. We observed sea urchins at intermediate depths on this dive (35-70 m), and on a subsequent dive to 63 m at Glasshouse (Fig. 1h). No attached algae were observed below 20 m at either site.
During these 2 surveys, we quantified the proportion of red sea urchins with drift and the depth of each observation (Fig. 1a). In total, 24% of 1,156 sea urchins observed at both sites had drift (Fig. 1a, b). The deepest sea urchin was not holding drift, but a piece of kelp was observed <1 m away. The total number of sea urchins observed in each depth bin did not show a consistent pattern with depth between the sur face and 60 m, indicating similar sampling effort across depth bins. These numbers do not reflect quantitative densities given the methods of collection. We observed few sea urchins below 60 m owing to logistical limitations of the submersible dives. These shallow and mesophotic paired surveys showed a serial decline in drift capture with depth below the mac roalgal zone at Glasshouse, consistent with our hypothesized pattern, but relatively constant drift capture across depths at Limekiln. These observations contrast data from Dume Sub marine Canyon in Southern California, where topography and currents concentrate drift and drift-associated sea urchins. In this habitat, more than 90% of sea urchins were associated with drift (Fig. 1c).
Rate of drift capture
During the repeated dives to collect drift captured by red sea urchins at the upper limit of the mesophotic zone, we collected on average 15.4 g of drift per sea urchin per day across tide phases, sites, and seasons (Fig. 2). There was high variability among collections resulting in no significant dif ferences among contrasts. Average drift captured during the neap tide in June was 23.38 ± 8.4 g of drift per sea urchin per day compared to 21.68 ± 11.0 g of drift per sea urchin per day during the spring tide phase, with no difference between tides (Fig. 2a; ANOVA: F1,8 = 0.15, P = 0.70) or sites (ANOVA: F1,8 = 3.57, P = 0.09), but a significant inter action (ANOVA: F1,8 = 5.45, P = 0.05). Seasonally, drift cap ture ranged from 2.88 ± 0.79 g of drift per sea urchin per day in April to 22.42 ± 5.44 g of drift per sea urchin per day in June, with no significant effect of season (Fig. 2b; ANOVA: F3,8 = 2.26, P = 0.16). Drift capture did not vary significantly among sites in September (Fig. 2c; ANOVA: F2,6 = 1.08, P = 0.40) and ranged from 4.28 ± 2.00 g of drift per sea urchin per day at PTG to 13.24 ± 10.43 g of drift per sea urchin per day at NPT. The maximum drift capture rate observed was 37.7 g per sea urchin per day during the neap tide series at North Point Caution.
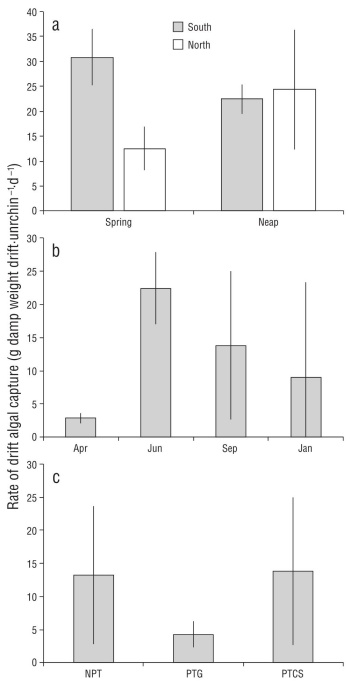
Figure 2 Rates of drift algal capture by red sea urchins at 18 m depth in San Juan Channel, Salish Sea. Means calculated over 3 d (after an initial clearance of all drift from all sea urchins on day zero) in each of the spring and neap tide phases at 2 sites (North and South Point Caution) (a), the 4 seasons at Point Caution South (b), and Neck Point (NPT), Point George (PTG), and Point Caution (PTCS) in September 2012 (c).
DISCUSSION
The submersible expedition successfully generated evi dence to support the prediction that red sea urchins, a kelp-associated species, can be found to 284 m depth within the NE Pacific if preferred substrate and drift are available. Observations below 70 m were limited by conditions and submersible time, but red sea urchins were found at all depths observed, supporting ROV observations that show red sea urchins are common below the macrophyte zone (Britton-Simmons et al. 2012). We observed drift near or captured by sea urchins at all depths from 284 m to the sur face. While limited in spatial and temporal breadth, these observations encourage further study of red sea urchins in deep habitats throughout the sheltered waters of the Salish Sea, British Columbia, and SE Alaska that make up ~90% of red sea urchins’ range in the NE Pacific.
We observed different patterns of drift capture with depth at the 2 sites in the Salish Sea, contrary to our prediction of a serial decline of drift capture with depth throughout the region. The steep ‘wall’ site exhibited uniform drift capture across depth, whereas the sloping site was characterized by a decline of drift capture with depth (Fig. 1a, b). We suspect that these patterns are due to bathymetry and kelp commu nities in the shallows at these 2 sites (Fig. 1, Hypotheses 1 and 2); Limekiln has a steeper slope and narrow shelf dom inated by the canopy-forming kelp Nereocystis luetkeana, whereas Glasshouse has a gradual slope with a wide shelf dominated by prostrate kelps such as Saccharina sp. and Agarum fimbriatum. The steep slope at Limekiln likely con tributed to the uniform drift capture across depths; drift can rapidly descend across the habitats and strong currents can move drift laterally over long distances across this stretch of coastline (e.g., Berglund et al. 2003, Vanderklift and Wernberg 2008). By comparison, the gradual slope at Glass house reduces the sinking rate of drift and may increase the likelihood of drift being captured by shallow sea urchins. These scenarios contrast observations from submarine can yons in which sea urchins are restricted to depositional environments that concentrate drift and are not observed in regions between the kelp forest source and the canyon sink (Fig. 1, Hypothesis 3). The patterns of drift and sea urchin presence in submarine canyons are similar to those observed for green sea urchins, Strongylocentrotus droebachiensis, found near aggregations of drift in the mesophotic zone (Filbee-Dexter and Scheibling 2016). Further work is needed to better understand the spatial and compositional variability of drift algal subsidies (Britton-Simmons et al. 2009) and effects on consumers. Despite the limited spatial scope of our submersible surveys, these results highlight the need for fur ther research to investigate the distribution of red sea urchins throughout the NE Pacific, particularly in areas with suitable habitat below the photic zone.
During our repeated sampling of drift captured by sea urchins, we regularly collected more grams of wet weight of algae per sea urchin per day (Fig. 2) than maximum observed consumption rates in the lab. Opportunistic feeding experi ments with red sea urchins have shown that they will con sume between ~12-22 g wet weight Nereocystis (highest rates) and 2-6 g wet weight Agarum algae per sea urchin per day (Raymond et al. 2014). Across all of our sampling events, sea urchins captured an average of 15.4 g wet weight of drift per sea urchin per day and as much as 37.7 g wet weight of drift per sea urchin per day. We observed seasonal variation consistent with previous studies of drift abun dance (Vadas 1968, Kingsford 1992, Britton-Simmons et al. 2009), although the high variation among consecutive days led to a non-significant effect of season. Using the average sea urchin density within the San Juan Channel of 0.49 sea urchins per square meter (Britton-Simmons et al. 2012), we calculated that sea urchins capture ~7.5 g of algae per square meter per day. When coupled with sea urchin’s poor assimi lation efficiency (Dethier et al. 2019, Yorke et al. 2019) and their broad spatial distribution, these data suggest sea urchins facilitate delivery of a substantial energy subsidy (2.5 kg of algae per square meter per year) to the benthos both directly by providing access of captured drift to other mesograzers (e.g., Duggins 1981) and indirectly through fecal material consumed by benthic invertebrates, which could not have otherwise accessed this carbon source (e.g., Mamelona and Pelletier 2005).
Sea urchin-mediated spatial subsidies to benthic food webs are a potentially important, yet still largely unexplored, ecological difference between open coast and inland waters- especially below the photic zone (Fig. 1). In coastal shelf and deep-sea habitats, large amounts of drift with aggre gations of sea urchins and other grazers have been docu mented for deep depositional environments (Gage and Tyler 1993, Vetter 1994). The presence of both drift and red sea urchins in deep offshore shelf habitats is largely controlled by physical forces that export this interaction offshore and to depth (Fig. 1, Hypothesis 3:c, f, i). The lack of hard sub strate in deep depositional environments or in combination with water motion may lead to unfavorable conditions for red sea urchins (Vetter and Dayton 1999). While red sea urchins from shallow reefs have been observed at 200 m in submarine canyons on the North American west coast, they were almost exclusively found attached to drift (Fig. 1i, Dume Canyon, California; data courtesy of E. Vetter). These sea urchins likely ‘surfed’ on the drift to deep depositional habitats (Fig. 1f), as they are not observed in other nearby habitats outside of the kelp forest. In this scenario, red sea urchins are relatively unimportant to mediating the drift sub sidy, as other invertebrates (including other sea urchin spe cies) quickly colonize and consume the detritus (Vetter 1994, Harrold et al. 1998, Vetter and Dayton 1999). In contrast, red sea urchins inhabit contiguous rocky reefs from the surface to at least 284 m in the Salish Sea (Fig. 1; Mortensen 1943, Britton-Simmons et al. 2012). Red sea urchins capture drift transported by currents with their long spines (George and Carrington 2014) and make it available to non-depositional habitats that may not otherwise receive drift subsidies. This type of animal-mediated spatial subsidy may be unique, as most popular examples document nutrient transfer across ecosystem boundaries by migrating, rather than stationary ‘filter feeding’, animals such as anadromous fish, whales, events, sea urchins captured an average of 15.4 g wet weight of drift per sea urchin per day and as much as 37.7 g wet weight of drift per sea urchin per day. We observed seasonal variation consistent with previous studies of drift abundance (Vadas 1968, Kingsford 1992, Britton-Simmons et al. 2009), although the high variation among consecutive days led to a non-significant effect of season. Using the average sea urchin density within the San Juan Channel of 0.49 sea urchins per square meter (Britton-Simmons et al. 2012), we calculated that sea urchins capture ~7.5 g of algae per square meter per day. When coupled with sea urchin’s poor assimilation efficiency (Dethier et al. 2019, Yorke et al. 2019) and their broad spatial distribution, these data suggest sea urchins facilitate delivery of a substantial energy subsidy (2.5 kg of algae per square meter per year) to the benthos both directly by providing access of captured drift to other mesograzers (e.g., Duggins 1981) and indirectly through fecal material consumed by benthic invertebrates, which could not have otherwise accessed this carbon source (e.g., Mamelona and Pelletier 2005).
Sea urchin-mediated spatial subsidies to benthic food webs are a potentially important, yet still largely unexplored, ecological difference between open coast and inland waters- especially below the photic zone (Fig. 1). In coastal shelf and deep-sea habitats, large amounts of drift with aggre gations of sea urchins and other grazers have been docu mented for deep depositional environments (Gage and Tyler 1993, Vetter 1994). The presence of both drift and red sea urchins in deep offshore shelf habitats is largely controlled by physical forces that export this interaction offshore and to depth (Fig. 1, Hypothesis 3:c, f, i). The lack of hard sub strate in deep depositional environments or in combination with water motion may lead to unfavorable conditions for red sea urchins (Vetter and Dayton 1999). While red sea urchins from shallow reefs have been observed at 200 m in submarine canyons on the North American west coast, they were almost exclusively found attached to drift (Fig. 1i, Dume Canyon, California; data courtesy of E. Vetter). These sea urchins likely ‘surfed’ on the drift to deep depositional habitats (Fig. 1f), as they are not observed in other nearby habitats outside of the kelp forest. In this scenario, red sea urchins are relatively unimportant to mediating the drift sub sidy, as other invertebrates (including other sea urchin spe cies) quickly colonize and consume the detritus (Vetter 1994, Harrold et al. 1998, Vetter and Dayton 1999). In contrast, red sea urchins inhabit contiguous rocky reefs from the surface to at least 284 m in the Salish Sea (Fig. 1; Mortensen 1943, Britton-Simmons et al. 2012). Red sea urchins capture drift transported by currents with their long spines (George and Carrington 2014) and make it available to non-depositional habitats that may not otherwise receive drift subsidies. This type of animal-mediated spatial subsidy may be unique, as most popular examples document nutrient transfer across ecosystem boundaries by migrating, rather than stationary ‘filter feeding’, animals such as anadromous fish, whales, hippos, or zooplankton (Bianchi et al. 2013, Doughty et al. 2016, Stears et al. 2018).
Our observation of exceptionally deep red sea urchins expands our understanding of their natural history and cre ates opportunities for new research in the nearshore meso photic zone, particularly in the area of animal-mediated ecological subsidies. Growing evidence suggests this is an important vector for kelp-derived nutrients in benthic hab itats (Mamelona and Pelletier 2005, Dethier et al. 2019, Yorke et al. 2019), but field observations are still lacking. The source of these deep sea urchins, and whether recruit ment occurs at depth, are unknown; many red sea urchins are likely dislodged from the shallows and sink to depth. These centenarians do not make large-scale vertical move ments in the Salish Sea and may therefore influence benthic processes over decades to centuries (Ebert 2008, Lowe et al. 2015). Climate change is increasingly manifesting in the oceans in the form of marine warming events (Oliver et al. 2018), which diminish kelp forests (Straub et al. 2019). The resulting changes in coastal carbon cycling will affect mesophotic food webs. Our focus here has been on red sea urchins, but temperate and polar latitudes throughout the world host productive macroalgal communities and sea urchins that feed on them (Ling et al. 2015). Tracking the interaction between urchins and algae from within to outside the macrophyte zone is thus a globally relevant process. These deep habitats exist throughout red sea urchins’ range, providing contrast to shallow ‘typical’ habitats and an exciting research opportunity for this model organism of marine ecology.











 nueva página del texto (beta)
nueva página del texto (beta)


