INTRODUCTION
Human-mediated introduction of species to new geo graphic locations is an unprecedented and unique form of global change (Ricciardi 2007). For the majority of these introductions, impacts have not been quantified, challenging our understanding of the consequences of invasion and com plicating management decisions (Blackburn et al. 2014, Jeschke et al. 2014). However, some generalizations have emerged over several decades of synthesis, including that introduced species performing a novel function within the invaded locale often produce the greatest impacts (Parker et al. 1999, Simberloff 2011). This is because the distinc tiveness of the invader’s traits leads to novel resource use or changes in habitat structure that fundamentally change the invaded system (reviewed by Kumschick et al. 2015). For example, plants that colonize intertidal mud or sandflats are a category of invasive species that are considered “trans formers,” i.e., the ~10% of invading plant taxa that have clear ecosystem impacts through wholesale changes to the character, condition, form or nature of ecosystems over sub stantial areas (Richardson et al. 2000).
The Hawai‘ian islands are among the world’s marine invasion hotspots, with a high proportion of harmful invaders (Molnar et al. 2008), including a sandflat “transformer”, the red mangrove, Rhizophora mangle. This mangrove species was introduced from Florida to Hawai‘i in 1902 to help stabilize shorelines around the island of Moloka‘i . Since their introduction, mangroves have spread rapidly, colo nizing vast areas across the archipelago (Chimner et al. 2006) and causing habitat loss for native birds including the Hawai‘ian duck (Anas wyvilliana), Hawai‘ian coot (Fulica alai), Hawai‘ian stilt (Himantopus mexicanus knudseni), and common moorhen (Gallinula chloropus sandvicensis), which find the mangroves unsuitable for foraging and nesting (Allen 1998). Instead, Hawai‘ian mangroves provide habitat for invasive species such as the cattle egret (Bubulcus ibis), which feeds on the chicks of native birds (Allen 1998). In general, Hawai‘ian mangroves appear to be underutilized ecologically (Walsh 1967); for example, Hawai‘ian sedi ment invertebrates do not consume mangrove detritus as in the mangrove’s native range, likely because they have not evolved to consume the tannin-rich leaves, which are unpal atable and/or toxic to some species (Demopoulos et al. 2007). However, these mangroves have created novel hab itat through increased detritus deposition (exceptionally high in Hawai‘i) (Allen 1998, Cox and Allen 1999) and accu mulation of finer sediments and organic matter, shifting the community to subsurface deposit feeders not found on nearby sandflats (Demopoulos and Smith 2010). Further, introduced shrimp, fish, and crabs have all been identified within this new habitat (Nakahara 2007), and the mangrove’s prop roots support at least 6 species of introduced barnacles and sponges (Demopoulos and Smith 2010). These man grove prop roots may also provide a substratum on which the red alga Gracilaria salicornia can attach and grow or may trap drifting mats (authors’ personal observations). Gracilaria salicornia was introduced to the state sometime before 1946 and collected from the island of Hawai‘i for use in aquaculture on O‘ahu and subsequently on Moloka‘I (Smith et al. 2002, Smith et al. 2004). This alga can form mats up to 2 m across (Larned 1998), affecting the food web through its relative unpalatability and habitat degradation for reef fishes and corals (Smith et al. 2004, Williams and Smith 2007). Red mangroves were introduced to the island of O‘ahu circa 1922 (Chimner et al. 2006) and the shores of Kāne‘ohe Bay support some of the largest mangrove stands (Devaney et al. 1982).
The red mangrove stands interrupt shallow sand flats that are home to the endemic Hawai‘ian shrimp goby Psilogobius mainlandi, which co-occurs in burrows with the native snapping shrimp Alpheus rapax (Langston and Spalding 2017). Other mutualistic shrimp/goby pairs are abundant throughout the Pacific, but P. mainlandi is the only shrimp-associated goby that occurs in Hawai‘i (Karplus and Thompson 2011). In this obligate mutualistic relation ship (Karplus and Thompson 2011, Lyons 2013), the shrimp excavates a burrow and, because of its poor eyesight (Zeng and Jaafar 2012), relies on the goby to act as a lookout. The fish alerts the shrimp to potential predators with a flick of its tail, triggering both to retreat into the burrow (Hoover 2016). Small, cryptobenthic fishes, including gobies, comprise up to 60% of the consumed biomass in and around coral reefs, making these fishes a cornerstone of ecosystem functioning. Therefore, we expect factors affecting the abundance and dis tribution of the native shrimp/goby pairs in Hawai‘i to likely have ripple effects through multiple trophic levels. However, to our knowledge the effects of red mangrove invasion on these species has not been examined.
We propose that mangroves invading Hawai‘ian shore lines reduce habitat for the sandflat dwelling native shrimp and goby pairs. In places where G. salicornia has invaded, it may also contribute cover that interferes with shrimp/goby burrowing; this alga can grow either attached to substrata, in dense mats without anchorage points near the shore, or as loose thalli that dislodge and become trapped in depressions (Nelson et al. 2009). The potential for mangroves to trap and promote growth of G. salicornia is a concern for nearby coral reefs as well, as spread of this alga can lead to smothering and killing of coral (Smith et al. 2004).
We conducted a field survey to estimate density of shrimp/goby burrows, percent cover of benthic debris, and composition of this debris along mangrove-invaded shores of a small island in Kāne‘ohe Bay, O‘ahu. Specifically, we hypothesized that shrimp/goby burrows are less abundant under mangroves than on nearby sandflats, and that burrow densities increase with distance from the mangrove edge. We predicted that burrow density would be negatively correlated with benthic cover, including G. salicornia and accumulated leaves. We also conducted a benthic debris removal experi ment to evaluate whether numbers of shrimp/goby burrows quickly increase after debris removal; if so, this would sug gest that accumulation of debris (perhaps including invasive G. salicornia) is a primary mechanism for reduced shrimp/ goby burrowing along the mangrove edge.
MATERIALS AND METHODS
Survey of burrow density and benthic cover with distance from the mangroves
We chose 3 study sites where mangroves were abundant next to sandy shrimp/goby habitat along the shores of Moku o Lo‘e, at the Hawai‘i Institute of Marine Biology (Fig. 1). Sites 1 and 2 were located in shallow lagoons with little to no coral nearby, while site 3 was located near a deeper lagoon with scattered coral heads in close proximity. A 25-m tran sect tape was placed along the mangroves and the number of shrimp/goby burrows was counted every 5 m within a 0.25-m2 quadrat that extended into the mangroves from the prop root edge. The quadrat could be opened in order to place it around prop roots when encountered. These burrow counts were repeated along 2 parallel transects on the adjacent sand flats at 1.5 m and 5.0 m from the mangrove edge. Within each quadrat, we first used the point-intercept method at 9 points to estimate percent benthic cover (following Karl et al. (2016), then gently lifted the debris to count burrows under neath. The types of cover evaluated were leaves, coral rubble, G. salicornia, no benthic debris (bare sand), or, rarely, “other cover”. We also counted the prop roots within each quadrat under the mangroves. As our presence sometimes led to retreat of shrimp/goby pairs into their burrows, we counted all the burrows whether or not we saw the shrimp and gobies.
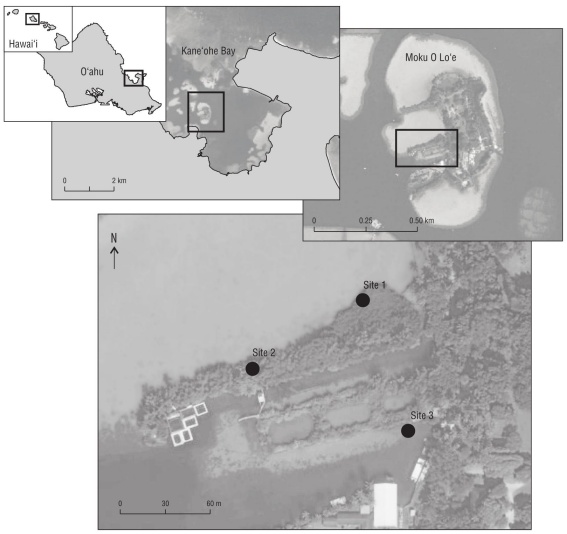
Figure 1 Map of study locations at the Hawai‘i Institute of Marine Biology on Moku O Lo‘e, Kāne‘ohe Bay, O‘ahu, Hawai‘i, USA. Site 1: 21º26′01.2″ N, 157º47′20.9″ W; site 2: 21º25′59.7″ N, 157º47′23.4″ W; and site 3: 21º25′58.4″ N, 157º47′20.1″ W.
Analyses were conducted in R v.3.6.3 (R Core Team 2020). Generalized linear models were used to evaluate the effects of site and distance from mangrove edge on measured variables. Burrow density was analyzed using a Poisson dis tribution, while percent cover metrics were logit-transformed to achieve homogeneity of variances and analyzed with a Gaussian distribution. Tukey tests were used to evaluate sig nificant main effects using the multcomp package (Hothorn et al. 2008). We also evaluated the relationship between total percent cover of benthic debris (logit transformation) and burrow density (log10 +1) using a linear regression. The relationship of burrow density to both G. salicornia and leaf cover were evaluated separately using linear regressions with logit transformations of cover data.
Debris removal experiment
In order to determine if cover of benthic debris is a factor that affects the distribution of shrimp/goby burrows, we manipulated debris beneath the mangrove edge. After initially evaluating burrow density and percent cover of all material, we removed the loose or attached debris (primarily leaves and algae) within a 0.25-m quadrat at 3 of the plots along the transects (0, 10, and 20 m) at all 3 sites. Additional material that accumulated in the plots was cleared every other day for a 2-week period. After 2 weeks of maintaining cleared plots, burrow density was re-assessed. We evaluated the effects of time (before and after debris removal) and site as fixed fac tors and their interaction using a generalized linear model with a Poisson distribution.
RESULTS
Distance from mangroves is associated with lower cover of debris and higher burrow density
We found ~4-5× lower densities of shrimp/goby bur rows under the mangrove edge compared to either dis tance away on the adjacent sandflat (Fig. 2, Table 1; Tukey tests P < 0.0001 for both sandflat distances). There were 11.6 burrows·m-2 under the mangrove edge, compared to the 52.7 and 64.7 burrows·m-2 at 1.5 and 5.0 m from the man groves, respectively (Fig. 2); these sandflat burrow densities were somewhat different from each other (Tukey test, P = 0.047). There was also a difference in burrow densities by site, with site 1 having greater densities than site 3 (Tukey test, P = 0.0238) and site 2 having intermediate densities. The pattern of increasing burrow density with distance from the mangrove was consistent across sites (no interaction, Table 1).
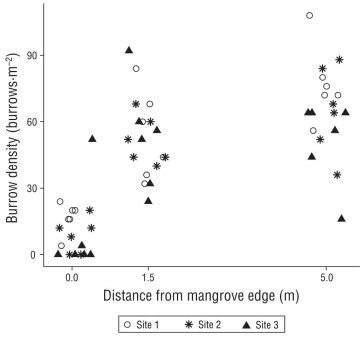
Figure 2 Burrow counts per square meter with distance (0, 1.5 and 5.0 m) from the mangrove edge at the 3 study sites. Points were jittered at each discrete distance to improve visibility.
Table 1 Results of generalized linear models, with model used and transformations (if needed) indicated in parentheses.
| (a) Burrow density with distance from mangrove and site (generalized linear model with Poisson distribution) | ||||
| d.f. | Residual d.f. | Deviance | P | |
| Distance | 2 | 51 | 197.94 | <0.0001 |
| Site | 2 | 49 | 7.14 | 0.0282 |
| Distance × Site | 4 | 45 | 5.74 | 0.2175 |
| (b) Total percent cover (log+1) with distance from mangrove and site (generalized linear model with Poisson distribution) | ||||
| d.f. | Residual d.f. | F | P | |
| Distance | 2 | 51 | 24.81 | <0.0001 |
| Site | 2 | 49 | 10.61 | 0.0002 |
| Distance × Site | 4 | 45 | 1.04 | 0.3994 |
| (c) Gracilaria salicornia percent cover (logit) with distance from mangrove and site (generalized linear model with Gaussian distribution)> | ||||
| d.f. | Residual d.f. | F | P | |
| Distance | 2 | 51 | 9.10 | 0.0005 |
| Site | 2 | 49 | 0.25 | 0.7797 |
| Distance × Site | 4 | 45 | 2.50 | 0.0556 |
| (d)Leaf percent cover (logit) with distance from mangrove and site (generalized linear model with Gaussian distribution) | ||||
| d.f. | Residual d.f. | F | P | |
| Distance | 2 | 51 | 9.10 | 0.0005 |
| Site | 2 | 49 | 0.25 | 0.7797 |
| Distance × Site | 4 | 45 | 2.50 | 0.0556 |
| (e) Effect of debris removal at 3 sites on burrow density (generalized linear model with Poisson distribution) | ||||
| d.f. | Residual d.f. | Deviance | P | |
| Time | 1 | 16 | 49.24 | <0.0001 |
| Site | 2 | 14 | 32.26 | <0.0001 |
| Time × Site | 2 | 12 | 7.09 | 0.0289 |
Benthic debris cover consisted primarily of fallen leaves, G. salicornia, or rubble (coral skeletons or rock). Of all ben thic debris cover observations, 48% were fallen mangrove leaves; therefore, benthic debris cover under the mangroves and in nearby sandflats was largely associated with man groves per se. In addition, G. salicornia composed 28.7% of the total debris cover, while coral rubble and “other” cover made up 21.3% and 0.1% of the debris cover obser vations, respectively. Prop root density was variable and ranged from a mean of 12.7 roots·m-2 (SD = 8.5) at site 2 to 16.7 roots·m-2 (SD = 10.3) at site 1 to 27.3 roots·m-2 (SD = 19.2) at Site 3.
Total percent cover of benthic debris, which could exceed 100% if cover types were layered, was ~4× higher under the mangrove edge compared to that on sandflats at 1.5 m and 5.0 m away (Fig. 3; Tukey tests, P < 0.0001 for both distances). Benthic debris cover reached 66% under the mangrove edge (75% total cover with layering) and was composed of leaves, algae, and other debris. The adjacent sandflats exhibited significantly less cover of debris, with 19% and 13% at 1.5 m and 5.0 m from the mangrove edge, respectively (and no difference between 1.5 m and 5.0 m; Tukey test, P = 0.312). There was also a significant differ ence in total cover of benthic debris by site (Table 1); site 3 had greater total debris cover than sites 1 and 2 (Tukey test, P < 0.02 for both), which were not different from each other (Tukey test, P = 0.9899). There was no interaction between distance from the mangroves and site (Table 1).
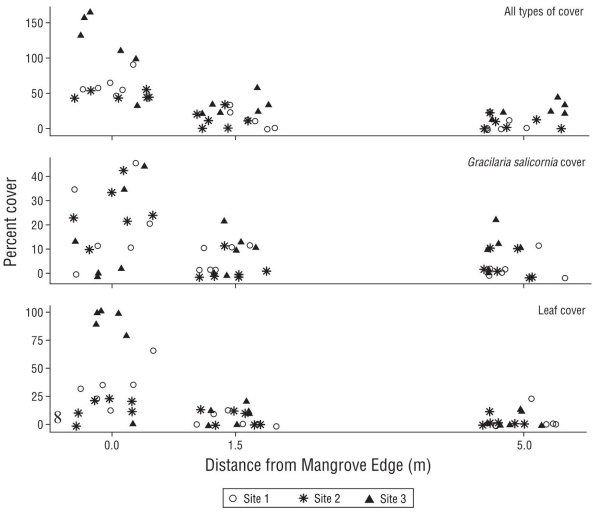
Figure 3 Total cover of benthic debris, as well as the subsets of Gracilaria salicornia cover and leaf cover, with distance (0, 1.5 and 5.0 m) from the mangrove edge at the 3 study sites. Points were jittered at each discrete distance to improve visibility.
Because G. salicornia is invasive in Hawai‘i, and the only alga we encountered under or near the mangroves, we com pared algal cover separately. We found signficantly (~4×) less algal cover at both distances from the mangroves than under the mangrove edge (Fig. 3, Table 1; Tukey tests, P < 0.003 for both), with the 2 sandflat locations not different from each other (Tukey test, P = 0.9586). Algal cover was 20% under the mangrove edge and 5.5% and 4.9% at 1.5 and 5.0 m away, respectively. There was no difference in cover of the alga by site and no interaction between distance from mangrove and site (Table 1).
On average, the cover of fallen leaves dropped mark edly with distance from the mangrove edge (32.7% vs 6.2% and 3.1% at 1.5 and 5.0 m away, respectively); however, this was largely due to very high cover of leaves at site 3 (Fig. 3; Table 1, significant interaction between distance and site). Leaf cover was approximately the same at the 2 sandflat dis tances at all sites (Fig. 3).
Evaluating burrow density and total percent cover in all surveyed quadrats, we found a significant negative rela tionship between burrow density and total benthic debris cover (Fig. 4; adjusted R2 = 0.53, P < 0.0001). Burrow density also decreased with increasing G. salicornia cover (Fig. 3; adjusted R2 = 0.22, P < 0.0002) and leaf cover (Fig. 3; adjusted R2 = 0.58, P < 0.0001) when those cover classes were evaluated separately.
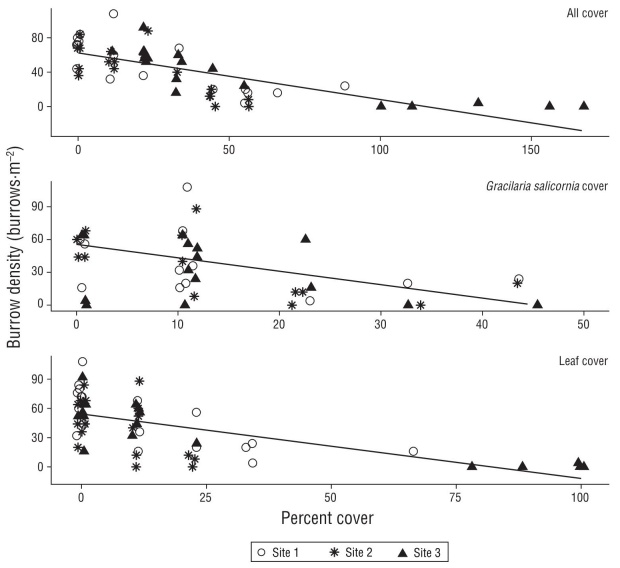
Figure 4 Relationship between the total cover of debris (all debris cover types can lead to >100% due to layering), Gracilaria salicornia cover, or leaf cover and burrow density across the 3 sites. Points were jittered at each discrete distance to improve visibility. Lines indicate significant linear regressions.
Removal of benthic debris resulted in increased shrimp/ goby burrow density
Removal of loose and attached debris (primarily leaves and algae), maintained over 2 weeks, substantially increased the number of shrimp/goby burrows under the mangrove edge. The average burrow density across sites was 8.44 per square meter before removal and 39.6 per square meter after removal. However, burrow density increased more dra matically at site 2 (8-fold) than at the other sites (3-fold), explaining the interaction between time (before and after debris removal) and site (Fig. 5, Table 1).
DISCUSSION
We hypothesized that invasive red mangroves (R. mangle) in Hawai‘i create inhospitable conditions for sandflat-dwelling obligate pairs of an endemic goby (P. mainlandi) and native shrimp (A. rapax). Our field survey at 3 sites along an island in Kāne‘ohe Bay, O‘ahu, documented that shrimp/ goby burrow density was at least 4× lower under the man grove edge compared to nearby sandflats, which had 4× less benthic debris. Further, our experiment testing the effects of debris removal under mangroves resulted in large increases in burrow densities after just 2 weeks, indicating that this debris reduces habitat suitability for the native shrimp and goby pairs.
The benthic debris along the mangroves was largely com posed of fallen and trapped leaves, which accounted for 58% of the variation in shrimp/goby burrowing density across sites and distances from the mangrove. Further, as our ini tial observations suggested, the mangrove roots trap drift G. salicornia and also provide surfaces onto which the alga attaches. While a small amount (~5% cover) of this invasive red alga was found on the sandflats at both 1.5 and 5.0 m from the mangrove edge, much more (average of 20% cover) was present under the mangrove edge. We sometimes found accumulation of leaves and algae together, the combination of which created a thick mat on the benthos. The mangrove prop roots themselves also may have inhibited burrowing, as lower burrow densities were found at site 3, where mangrove root counts ranked highest (although variability was quite high). As the accumulation of leaves was also significantly greater at site 3, we suspect that the cover of leaves was a greater deterrent to burrowing than the prop roots; however, more prop roots may trap more leaves, making the 2 potential controlling factors difficult to disentangle.
Our survey and the rapid increase in burrowing densi ties following experimental removal of debris beneath man grove roots provide clear evidence that material covering the sandflats has negative effects on shrimp/goby burrowing. Other studies have found these same shrimp/goby pairs to be deterred by cover over the benthos; sandflat invasion of the alga Halimeda kanaloana is associated with much lower densities of these mutualist burrow-dwellers (Fukunaga 2008, Langston and Spalding 2017). In our experiment, nearby shrimp were apparently able to quickly perceive and colonize newly available habitat upon debris removal, despite their poor eyesight (Zeng and Jaafar 2012). Beyond that, we can only speculate as to the role that debris plays in shrimp/goby presence; as the goby uses visual cues to detect potential predators and warn the shrimp, surface debris may impede this communication system and reduce the likelihood of the shrimp venturing away from the burrow for foraging (Nelson 2005).
Since we found a small but significant increase in the number of shrimp/goby burrows as we moved from 1.5 m to 5.0 m from the mangrove edge, without a significant decrease in debris covering the sediment over the same distance, there may be effects of the mangroves that extend beyond their physical presence and associated debris coverage. These mangroves are known to change the chemical properties of sediment, with fallen leaves leaching tannins and leading to organic carbon accumulation and decomposition that reduces dissolved oxygen and increases hydrogen sulfide concen trations (Demopoulos and Smith 2010). It may be that such physicochemical changes somewhat reduce suitability for shrimp/goby pairs near (at least within 1.5 m of) the man grove edge. Food quality may also be reduced near the man groves; a previous study found that red mangrove detritus contributes little to the diet of native detritivores in Hawai‘i, unlike in the mangrove’s native range, where organisms have evolved the ability to digest these materials (Demopoulos et al. 2007).
Our finding that invasive G. salicornia is associated with mangroves at our study site is troubling considering that this species can be harmful to coral reefs through overgrowth and drawdown of oxygen, negatively affecting coral reef organ isms (Smith et al. 2004, Martinez et al. 2012). Hawai‘ian coral reefs support many fishes and other organisms that con tribute to the local economy, with a value over $3.5 billion US dollars (Bishop et al. 2011). Kāne‘ohe Bay corals have been recognized for high resiliency to bleaching and thus are being used as models for other regions to aid in under standing of this phenomenon (Jury and Toonen 2019). This G. salicornia does not invade new regions easily; rather, it has been observed to primarily spread very locally through frag mentation after it was intentionally introduced to several loca tions in the islands for aquaculture studies (Smith et al. 2002). Nonetheless, care should be taken to minimize further spread.
As with most invaders, red mangroves have both positive and negative effects, which must be weighed by managers in the context of ecological, social, and financial perspectives/ constraints. Positive effects include protection of coral reefs by trapping land‐derived sediments (D’iorio 2003) and pro vision of habitat to some native (and to non-native) fishes (MacKenzie and Kryss 2013, Goecke and Carstenn 2017), which many experience reduced risk of predation through prop root structural complexity (Nagelkerken 2009). Man groves in Hawai‘i also convert and store substantial amounts of atmospheric carbon in live tissues and detritus and accrete sediments along shores, both of which can serve to mitigate climate change impacts (Soper et al. 2019). In contrast, nega tive effects include displacing endangered native bird habitat (Allen 1998) and altering nearshore invertebrate commu nity composition and food web structure while facilitating numerous non-native invertebrate species (Demopoulos et al. 2007, Demopoulos and Smith 2010). Invasion of fish ponds, which are highly valued cultural resources to native Hawai‘ians (Bremer et al. 2018), results in mangroves growing directly into and degrading the containment walls (Chimner et al. 2006). To this list of negative effects, our research adds that invasive red mangroves in Hawai‘i dis place habitat utilized by 2 native (one endemic) sandflat spe cies through increased benthic debris cover, including cover of an invasive alga.
In conclusion, although this project was limited in spatial scope, we posit that red mangroves are likely to have similar negative effects on this mutualistic shrimp and goby inter action elsewhere within their widespread distribution in the Hawai‘ian archipelago. A high degree of detritus accumula tion documented in Hawai‘ian red mangrove stands relative to other regions (Cox and Allen 1999) reinforces our expec tation that similar negative effects could occur in other loca tions in the islands. Although our study focused only along the edge of mangrove stands, these mangroves can cover hectares of sandflat, presumably displacing shrimp/goby pairs throughout their footprint. Further, where G. salicornia has been introduced, it may add to the negative effects of mangroves on shrimp/goby burrowing through prop root trapping and attachment to the roots. While acknowledging potential benefits of invasive mangroves for shoreline pro tection and habitat for some fishes in Hawai‘i, current efforts to remove mangroves to restore natural and cultural values would also help reverse negative impacts to the endemic goby and native shrimp pairs studied here.











 nueva página del texto (beta)
nueva página del texto (beta)



