Introduction
The production of biofuels from microalgae is promising because microalgae do not compete for food crops or arable land, present high growth rates, and are capable of fixing CO2 through photosynthesis and releasing O2 into the atmosphere. Nitrogen (N) is a key nutrient for the growth and biochemical composition of microalgae, and the accumulation of lipids and carbohydrates increases under N limitation (Liu et al. 2017, Huy et al. 2018).
One of the main problems of large-scale microalgal biomass production is the supply of N and phosphorous (P). N fertilizers require enormous amounts of fossil energy (Metz et al. 2007), the main contributor to the carbon footprint (Hillier et al. 2009). Moreover, the use of fertilizers for culturing microalgae competes with their use in agriculture (Chisti 2013). One of the obstacles to large-scale production of microalgae for energy purposes is still the high cost of production, especially the availability and cost of feedstock (Acién et al. 2012).
The use of organic fertilizers such as animal excreta, sludge from wastewater, and anaerobic digestate with high nutrient contents has been proposed as an economical alternative for microalgae culture (Nayak et al. 2016, Wang et al. 2016a, El Shimi and Moustafa 2017, Liu et al. 2017, Huy et al. 2018). The use of wastewater (Gonçalves et al. 2017, Ho et al. 2019, Rodríguez-Mata et al. 2019) and wastewater enriched with inorganic fertilizers, composed of N, P, and potassium (K), is an economical alternative for large-scale cultivation (Nayak et al. 2016).
Microalgae can remove N and P from municipal wastewater, leaving very low concentrations, while converting these nutrients into biomass (Boelee et al. 2011). The microalgae genera Chlorella and Scenedesmus are the most studied in regards to their culture in wastewaters, particularly the species Chlorella vulgaris and Scenedesmus obliquus (Park et al. 2010, Ji et al. 2013, Xu et al. 2015, Wang et al. 2016b). Scenedesmus obliquus has been used to treat wastewater and to obtain valuable biomass in bubble-column photobioreactors. Scenedesmus obliquus grown in a culture medium supplemented with 40% of piggery wastewater effluent showed biomass and lipid productivities of 15.5 and 0.13 mg·L-1·d-1, respectively, and removals of 96.1 mg total N and 2.48 mg total P were observed (Ji et al. 2013). Removal of 79% of NH4 + and 43% of PO4 3- from a mixture of wastewater (containing 145.2 mg·L-1 NH4 + and 21.1 mg·L-1 PO4 3-) and 7% of landfill leachate was achieved with S. obliquus, which accumulated 12%-16% of lipids in cells (Hernández-García et al. 2019). When Scenedesmus sp. AMDD was grown in treated municipal wastewater in chemostats under different dilution rates or hydraulic retention times, biomass composition was strongly controlled by the rate of wastewater nutrient removal, and total fatty acids were only accumulated when growth rates were very low or when a prolonged nutrient starvation regime was imposed (Dickinson et al. 2013). Microalgae production using secondary treated wastewater decreases process costs by minimizing the use of freshwater and fertilizers while contributing to the water purification process, greatly enhancing process sustainability (Gómez et al. 2013).
The present work compares the effect of 6 unconventional media (3 inorganic and 3 organic), some of which were used and compared for the first time, on the growth and productivity of a microalgal consortium and Scenedesmus sp. and proposes different media for scaling microalgae culture in a sustainable way.
Materials and methods
Pretreatment and characterization of treated water, wastewater, and piggery wastewater
The wastewater (Ww) and the secondary treated wastewater (TWw) were obtained from the “San Juan Ixhuatepec” treatment plant located in Tlalnepantla, Mexico (19º31′13.8′′ N, 99º07′27.8′′ W). Ww and TWw were not filtered or sterilized. Piggery wastewater and piggery wastewater digestate were obtained from Productora Porcina Nopaltepec, located in Nopaltepec, Mexico (19º47′03.5′′ N, 98º43′41.6′′ W). Both piggery wastewater and piggery wastewater digestate were centrifuged at 6,000 rpm for 5 min to remove the solids, and the supernatant was used as substrate. The compositions of the TWw, piggery wastewater, piggery wastewater digestate, and Bayfolan fertilizer used to prepare the culture media are described in Table 1.
Table 1 Composition of the wastewater, treated wastewater, piggery wastewater, and Bayfolan fertilizer used for culture media formulation. Data are presented as mean values and standard errors.
| Parameter | TWw (mg·L-1) | Ww (mg·L-1) | PEM (mg·L-1) | PDM (mg·L-1) | BM (g·L-1) |
| N-NH4 + | 3.10 ± 0.14 | 37.53 ± 0.18 | 2,605.80 ± 67.26 | 1,540.82 ± 43.49 | 44.75 ± 0.30 |
| N-NO3 - | 6.10 ± 0.46 | 0.20 ± 0.06 | 7.82 ± 2.72 | 25.95 ± 4.63 | 14.41 ± 0.73 |
| PO4 3- | 15.81 ± 0.05 | 15.45 ± 0.18 | 851.28 ± 0.61 | 259.30 ± 5.22 | 10.60 ± 0.17 |
| COD | 17.00 ± 4.15 | 239.00 ± 5.50 | 16,877.30 ± 397.61 | 2,368.67 ± 186.23 | 92.36 ± 1.67 |
| TSS | 0.00 ± 0.00 | 198.00 ± 14.00 | 50,000.80 ± 560.32 | 3,000.50 ± 420.25 | 0.00 ± 0.00 |
| pH | 7.80 | 8.42 | 7.13 | 8.22 | 7.01 |
TWw, treated wastewater; Ww, raw wastewater; PEM, tap water with 23.5% of piggery wastewater; PDM, tap water with 39.75% of piggery wastewater digestate; BM, Bayfolan medium formulated with 1 mL of Bayfolan Forte per liter of TWw; COD, chemical oxygen demand; TSS, total suspended solids.
Culture media
The 6 proposed media used in this work for culturing were (1) Bayfolan medium (BM), formulated with 1 mL of Bayfolan Forte per liter of TWw; (2) TWw enriched with 0.3303 g·L-1 (NH4)2HPO4 (PAM); (3) TWw supplemented with 0.1977 g·L-1 NH4HCO3 (BCAM); (4) tap water with 23.5% of piggery wastewater (PEM); (5) tap water with 39.75% of piggery wastewater digestate (PDM); and (6) Ww. N content in BM, PAM, and BCAM was adjusted to 80 mg·L-1 N (NH4 +-N and NO3 --N), according to conventional media BBM and BG 11 with 500 mg·L-1 of sodium nitrate. N sources for BCAM and PAM were chosen based on their availability on the market, and NH4HCO3 and (NH4)2HPO4 served as sources of carbon (C) and P, respectively. For PEM and PDM, tap water treated in a purification plant was used instead of Ww or TWw because when scaling to 2,000 L or higher, water transportation from the closest treatment plant implied logistic issues and higher operational costs. Piggery wastewater and the piggery wastewater digestate were selected because of their high NH4 + content (Hu et al. 2013, Luo et al. 2016) and low cost.
Microorganisms and pre-culture conditions
The microalgae Scenedesmus sp. was obtained from the microalgae collection of the Center for Scientific Research and Higher Education at Ensenada, Baja California, Mexico. The microalgal consortium was obtained from treated water and was mainly composed of Scenedesmus sp. and Chlorella sp. The consortium was obtained by growing the native microflora of the treated water from the plant in a 1-L photobioreactor under the conditions described below. The culture was maintained and reinoculated in treated water. Cultures were carried out in 1-L cylindrical photobioreactors (101 mm diameter × 203 mm height), with working volume of 0.9 L, illuminated on one side by cold-cathode fluorescent lamps (127 μE·m-2·s-1) under a 12:12 (light:dark) photoperiod, with aeration of 0.4 vvm and a temperature of 24 ± 1 ºC. Water losses due to evaporation were amended daily by adding sterile distilled water.
Prior to the experimentation, the consortium and Scenedesmus sp. were cultured in the 6 media. To synchronize the pre-cultures, 3 consecutive cultures were carried out during 13 d each, and the last one was used as the inoculum of the experiments. All the pre-inocula were adjusted to an oxygen demand of 0.8 (600 nm) and all the photobioreactors were inoculated at 10%, with the corresponding pre-inoculum.
Culture conditions
Microalgae were cultivated for 13 d in BM, PAM, BCAM, and Ww. In PEM and PDM media, microalgae were cultivated during 8 d because they grew faster in these media. NH4 +, PO4 3-, and NO3 - contents in the supernatant and productions of biomass, carbohydrates, and proteins were determined every 3 d; pigments, lipids, and the lipid profile were determined at the end of the culture. In addition, the chemical oxygen demand (COD) was determined at the beginning and at the end of the PEM and PDM cultures. NH4 + losses by volatilization (as NH3) in PEM and PDM were quantified by passing the outlet of the gas stream from the photobioreactors through a 0.5 M H2SO4 solution, and dissolved NH3 was determined by a colorimetric method (APHA 1998).
Analytical methods
Determination of biomass, metabolites, and media composition
Biomass was determined by measuring absorbance at 750 and 600 nm with a spectrophotometer (DR3000 UV/Vis Spectrophotometer, HACH; USA). Biomass (dry weight basis) was determined in a moisture analyzer thermobalance using a glass microfiber membrane (Ahlstrom, 4.7 cm diameter, 1.1 μm pore size). Cells were counted using a Neubauer cell chamber. Protein contents were determined following the Lowry method (Lowry et al. 1951); carbohydrates, by the Dubois method (DuBois et al. 1956); and pigments, by the Wellburn method (Ritchie 2006). NH4 +, PO4 3-, and COD were determined following APHA methods (1998) in APHA (1998), and total suspended solids were measured according to standard methods (APHA 2005). NO3 - was analyzed according to the modified method reported by Keeney and Nelson (1982).
Determination of lipids and fatty acid methyl esters
Lipid extraction was performed as described by Ramírez-López et al. (2016). The composition of fatty acid methyl esters (FAMEs) was determined in an Agilent gas chromatograph (Technologies 7890B; Santa Clara, CA, USA) coupled to mass spectrometry (Agilent Technologies 5977A Series GC/MSD System; Santa Clara, CA, USA). A VF-MAXms column (30 m × 0.25 mm, 0.50 μm) was used and Helium was the carrier gas at a flow rate of 2.4 mL·min-1. Injector temperature was maintained at 230 ºC. Oven temperature was adjusted to 140 ºC for 5 min and gradually increased 8 ºC every minute to 250 ºC for 15 min. The temperature of the transfer line (MSD) was maintained at 180 ºC. The temperatures of the source and the quadrupole were 230 and 150 ºC, respectively. Each peak of FAME was identified and quantified with the standard Supelco 37 Component FAME Mix.
Results
Effect of the medium on the production and productivity of biomass
The culture medium that yielded the highest biomass concentration (dry weight) of the microalgal consortium and Scenedesmus sp. was BM with 1.79 ± 0.05 and 1.77 ± 0.10 g·L-1, respectively. These concentrations were significantly higher than those obtained in the other 5 culture media. The media that yielded the highest biomass concentrations after BM were Ww and PEM, and there was a significant difference in biomass concentrations with respect to BCAM, PAM, and PDM (Fig. 1). Therefore, given the significant differences in biomass production and the increasing trend in biomass productivity, the media can be grouped as follows: BM ˃ (Ww and PEM ) ˃ (BCAM, PAM, and PDM), BM being the medium that yielded the highest biomass productivity.
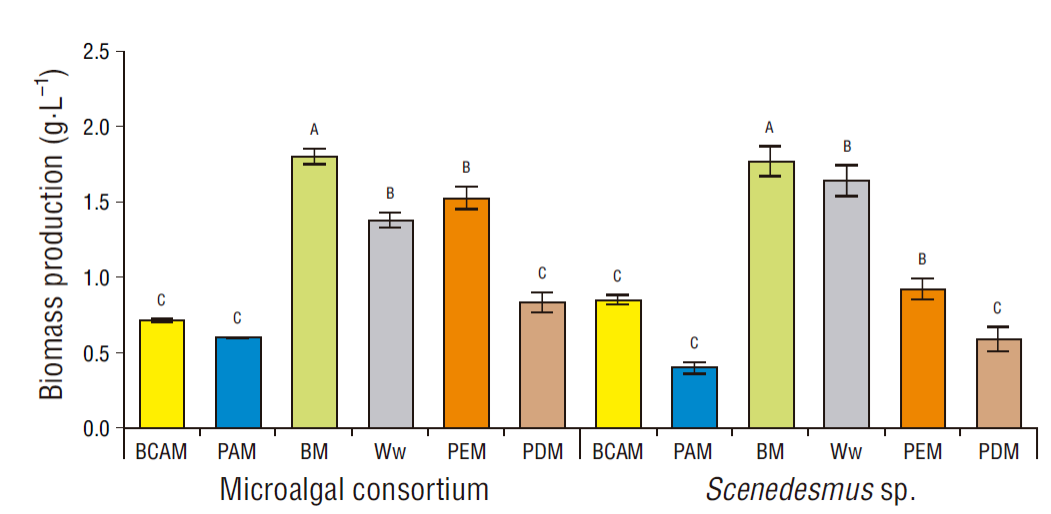
Figure 1 Biomass production with the microalgal consortium and Scenedesmus sp. cultured in different media. BCAM, secondary treated wastewater added with 0.1977 g·L-1 NH4HCO3; PAM, secondary treated wastewater enriched with 0.3303 g·L-1 (NH4)2HPO4; BM, Bayfolan medium; Ww, wastewater; PEM, tap water with 23.5% of piggery wastewater; PDM, tap water with 39.75% of piggery wastewater digestate.
Effect of the medium on lipid productivity and fatty acid methyl esters
The content of lipids in biomass in both the consortium and Scenedesmus sp. was 15%-16% in BCAM, 16%-22% in PAM, 9%-13% in BM, 21%-23% in Ww, 19%-32% PEM, and 30%-52% in PDM (Fig. 2). Lipid productivity in both cases, the consortium and Scenedesmus sp., fluctuated between 34.25 and 36.75 mg·L-1·d-1 in PEM, 32.83 and 38.67 mg·L-1·d-1 in PDM, and 24.87 and 26.41 mg·L-1·d-1 in Ww, all significantly higher than the lipid productivities obtained in BM, BCAM, and PAM (Table 2). In Ww, BCAM, PAM, PEM, and PDM, 90%-100% of NH4 + was removed from day 3 and, consequently, there was N limitation.
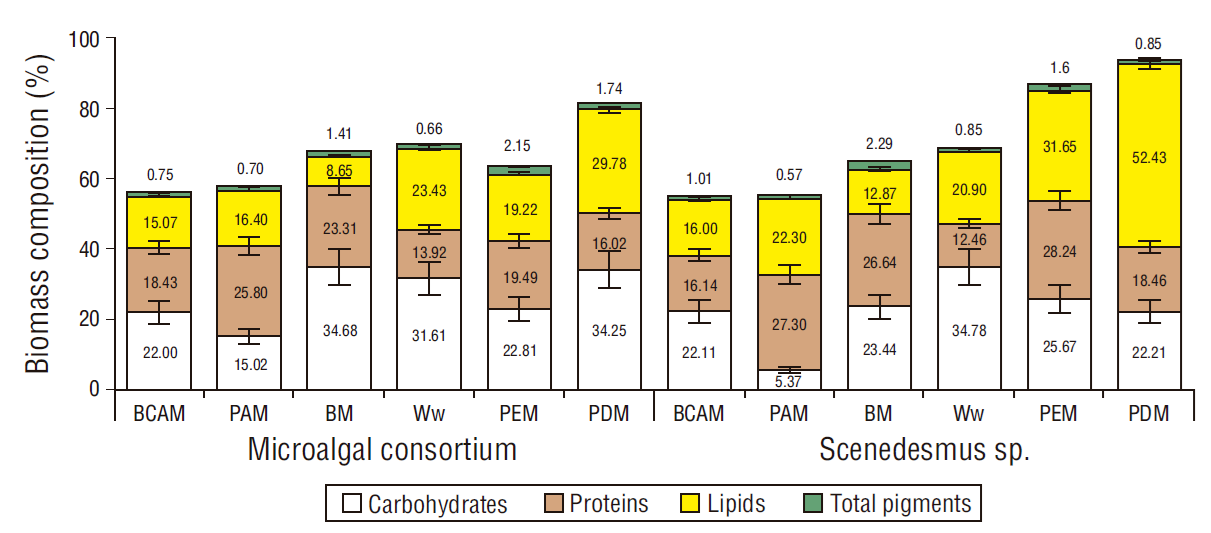
Figure 2 Biochemical composition of the biomass (percent yield) of the microalgal consortium and Scenedesmus sp. cultured in different media. BCAM, secondary treated wastewater added with 0.1977 g·L-1 NH4HCO3; PAM, secondary treated wastewater enriched with 0.3303 g·L-1 (NH4)2HPO4; BM, Bayfolan medium; Ww, raw wastewater; PEM, tap water with 23.5% of piggery wastewater; PDM, tap water with 39.75% of piggery wastewater digestate.
Table 2 Effect of culture media on metabolite productivity of Scenedesmus sp. and the microalgal consortium. Data are presented as mean values and standard errors; the same letter denotes no significant differences (Tukey HDS-test, P ≤ 0.05).
| Microorganism | Medium | Biomass (mg·L-1·d-1) | Carbohydrates (mg·L-1·d-1) | Proteins (mg·L-1·d-1) | Lipids (mg·L-1·d-1) | Pigments (mg·L-1·d-1) |
| Microalgal consortium | BCAM | 54.61 ± 0.77c | 12.13 ± 0.40b | 10.16 ± 0.05b | 8.31 ± 0.71b | 0.42 ± 0.01c |
| PAM | 45.38 ± 0.56c | 6.91 ± 0.14b | 11.86 ± 1.07b | 7.54 ± 2.94b | 0.32 ± 0.03cc | |
| BM | 138.46 ± 3.85a | 48.13 ± 3.38a | 32.34 ± 2.36a | 12.01 ± 1.87b | 1.96 ± 0.09b | |
| Ww | 106.15 ± 2.31b | 33.56 ± 2.26c | 14.78 ± 0.74b | 24.87 ± 6.20a | 0.70 ± 0.08 c | |
| PEM | 191.25 ± 6.25a | 43.63 ± 1.43c | 37.28 ± 5.33a | 36.75 ± 9.90a | 4.12 ± 0.08a | |
| PDM | 102.50 ± 2.50c | 35.53 ± 0.25b | 16.62 ± 1.52 b | 32.83 ± 10.88a | 1.81 ± 0.12b | |
| Scenedesmus sp. | BCAM | 65.38 ± 0.77c | 14.46 ± 0.47a | 10.55 ± 0.03b | 10.46 ± 2.40b | 0.66 ± 0.05c |
| PAM | 30.00 ± 0.28c | 1.65 ± 0.99c | 8.41 ± 2.33b | 6.87 ± 0.62b | 0.18 ± 0.02d | |
| BM | 136.15 ± 7.69a | 31.96 ± 6.01b | 36.32 ± 2.10a | 17.54 ± 5.51b | 3.12 ± 0.15a | |
| Ww | 125.38 ± 5.38b | 43.95 ± 3.32a | 15.74 ± 1.22b | 26.41 ± 5.20a | 1.08 ± 0.01b | |
| PEM | 115.00 ± 6.25b | 29.53 ± 2.08b | 32.68 ± 5.18a | 34.25 ± 3.93a | 1.84 ± 0.16b | |
| PDM | 118.75 ± 7.50b | 16.38 ± 1.02b | 13.62 ± 4.17b | 38.67 ± 1.53a | 0.63 ± 0.13c |
BCAM, treated wastewater added with 0.1977 g·L-1 NH4HCO3; PAM, treated wastewater enriched with 0.3303 g·L-1 (NH4)2HPO4; BM, Bayfolan medium formulated with 1 mL of Bayfolan Forte per liter of treated wastewater; Ww, raw wastewater; PEM, tap water with 23.5% of piggery wastewater; PDM, tap water with 39.75% of piggery wastewater digestate
The fatty acid profiles for the microalgal consortium and Scenedesmus sp. in the different media consisted of palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3) (Table 3), which are suitable for the production of biodiesel (Anand et al. 2018, May-Cua 2019). The culture media PAM, PEM, and PDM exhibited a higher proportion of saturated fatty acids (60%-69% of total FAMEs) than BCAM, BM, and Ww (39%-48% of total FAMEs).
Table 3 Composition of fatty acid profiles of the microalgal consortium and Scenedesmus sp. in different culture media (percentage of total fatty acid methyl esters).
| Fatty Acid | Microalgal consortium (%) | Scenedesmus sp. (%) | |||||||||||
| BCAM | PAM | BM | Ww | PEM | PDM | BCAM | PAM | BM | Ww | PEM | PDM | ||
| C16:0 | 36.76a | 42.26a | 33.39a | 32.30a | 43.57a | 44.23a | 32.12AB | 41.07AB | 34.03B | 32.70AB | 47.76A | 46.00AB | |
| C16:1 | 7.25a | 5.34ab | 5.62ab | 3.27ab | 4.34ab | 4.19ab | 3.97B | 7.28A | 4.55B | 3.00B | 3.13B | 4.46B | |
| C18:0 | 11.60b | 19.95a | 6.65b | 7.15b | 18.93ab | 17.90ab | 9.31BC | 22.07A | 7.05C | 7.12C | 21.33A | 19.16AB | |
| C18:1 | 20.40b | 8.94bc | 14.32bc | 34.77a | 6.50c | 6.58c | 17.63B | 17.65BC | 11.84CD | 35.04A | 5.09D | 5.47D | |
| C18:2 | 9.12ab | 7.49ab | 12.01a | 6.17abc | 4.07bc | 3.78c | 8.64A | 7.16 AB | 11.29A | 5.80AB | 3.18B | 3.54B | |
| C18:3n3 | 14.87a | 16.02a | 28.02a | 16.35a | 22.59a | 23.3a | 28.33A | 10.76B | 31.23A | 16.33AB | 19.52AB | 21.37AB | |
| C16-C18 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Unsaturated | 51.64a | 37.79a | 59.96a | 60.55a | 37.50a | 37.87a | 58.57A | 36.86A | 58.92 A | 60.18A | 30.91A | 34.84A | |
| Saturated | 48.36a | 62.21a | 40.04a | 39.45a | 62.50a | 62.13a | 41.43AB | 63.14AB | 41.08B | 39.82B | 69.09A | 65.16AB | |
BCAM, treated wastewater added with 0.1977 g·L-1 NH4HCO3; PAM, treated wastewater enriched with 0.3303 g·L-1 (NH4)2HPO4; BM, Bayfolan medium formulated with 1 mL of Bayfolan Forte per liter of treated wastewater; Ww, raw wastewater; PEM, tap water with 23.5% of piggery wastewater; PDM, tap water with 39.75% of piggery wastewater digestate.
Mean values that do not share a letter are significantly different. The differences are between the media for each crop (consortium and Scenedesmus sp., separately).
Effect of the medium on the productivity of proteins, carbohydrates, and total pigments
The productivities of proteins in the microalgal consortium and Scenedesmus sp. cultures with BM and PEM were significantly higher with respect to those obtained with BCAM, PAM, Ww, and PDM (Table 2). The protein productivities obtained with the microalgal consortium and Scenedesmus sp. were, respectively, 32.34 ± 2.36 mg·L-1·d-1 and 36.32 ± 2.10 mg·L-1·d-1 in BM and 37.28 ± 5.33 mg·L-1·d-1 and 32.68 ± 5.18 mg·L-1·d-1 in PEM, and while they were significantly lower in BCAM, PAM, Ww, and PDM, the productivity in PAM was 4 times lower than that obtained in PEM and BM.
The highest productivity of carbohydrates from the microalgal consortium was obtained in BM (48.13 ± 3.38 mg·L-1·d-1) and that from Scenedesmus sp. was obtained in Ww (43.95 ± 3.32 mg·L-1·d-1), both significantly higher than those in BCAM, PAM, PEM, and PDM (50% lower than BM) (Table 2). n BM, Ww, and PDM, up to 35% of carbohydrates were obtained in the biomass (Fig. 2).
Regarding the productivities of total pigments, no significant differences were observed between the microalgal consortium and Scenedesmus sp. in the BM and PEM. On the other hand, in BCAM, PAM, Ww, and PDM, the production of total pigments was up to 18 times lower than in BM (Table 2).
Nutrient removal
In BCAM, PAM, and Ww, NH4 + removal was 60% and 95% on the third and sixth day, respectively, whereas in PEM and PDM, 100% of NH4 + had already been removed by day three. In BCAM, NH4 +, NO3 -, and PO4 3- removals were higher than 92%. The initial concentration of PO4 3- was 12-14 mg·L-1 with a removal percentage of 92%, whereas in BM, 93% removal was achieved (Table 4). In BCAM, NO3 - removal was 99%, whereas in PAM it was only 59% with the microalgal consortium and null with Scenedesmus sp. In BM, NH4 + and PO4 3- removals were 90% and 93%-94% with the microalgal consortium and Scenedesmus sp., respectively, whereas the removal of NO3 - with Scenedesmus sp. was 30% lower than that obtained with the consortium (Table 4). Regarding COD, in PEM, 1,043 mg·L-1 of COD were removed with Scenedesmus sp. (86%), whereas in PDM only 26 mg·L-1 of COD (10%) were removed.
Table 4 Initial and final composition and removal efficiency in the 6 culture media with the microalgal consortium and Scenedesmus sp.
| Microorganism | Initial concentration (mg·L-1) | Final concentration (mg·L-1) | Removal (%) | ||||||||||||
| Media | NH4+-N | NO3--N | PO43--P | COD | NH4+-N | NO3--N | PO43--P | COD | NH4+-N | NO3--N | PO43--P | COD | |||
| Microalga consortium | BCAM | 56.37 ± 0.81 | 23.69 ± 1.22 | 3.98 ± 0.49 | - | 1.74 ± 0.01 | 0.05 ± 0.07 | 0.30 ± 0.04 | - | 99.90 | 99.78 | 30.18 | - | ||
| PAM | 57.00 ± 0.87 | 20.24 ± 0.39 | 53.06 ± 0.13 | - | 1.67 ± 0.04 | 8.36 ± 7.89 | 21.21 ± 3.98 | - | 97.07 | 58.70 | 60.02 | - | |||
| BM | 20.66 ± 1.42 | 79.58 ± 5.01 | 40.70 ± 2.24 | - | 4.18 ± 0.11 | 31.30 ± 22.11 | 2.89 ± 0.73 | - | 79.76 | 60.66 | 92.90 | - | |||
| Ww | 40.90 ± 1.44 | 0.00 ± 0.00 | 5.97 ± 0.10 | - | 1.73 ± 0.05 | 0.34 ± 0.30 | 0.64 ± 0.05 | - | 95.80 | * | 89.29 | - | |||
| PEM | 90.61 ± 9.63 | 2.51 ± 1.04 | 14.05 ± 1.36 | 1,165.18 ± 73.20 | 0.00 | 5.27 ± 0.33 | 2.95 ± 0.71 | 169.12 ± 0.60 | 100 | * | 78.98 | 85.49 | |||
| PDM | 78.10 ± 8.44 | 2.78 ± 1.06 | 1.55 ± 0.09 | 79.47 ± 33.78 | 0.00 | 5.80 ± 1.25 | 0.57 ± 0.13 | 58.47 ± 15.27 | 100 | * | 63.11 | 26.43 | |||
| Scenedesmus sp. | BCAM | 56.50 ± 0.36 | 23.80 ± 1.92 | 4.73 ± 0.04 | - | 1.84 ± 0.03 | 0.00 ± 0.00 | 0.37 ± 0.04 | - | 96.86 | 98.39 | 92.27 | - | ||
| PAM | 56.30 ± 0.91 | 21.95 ± 4.90 | 50.13 ± 0.53 | - | 2.75 ± 0.63 | 24.72 ± 2.71 | 13.58 ± 4.43 | - | 95.11 | * | 54.70 | - | |||
| BM | 21.03 ± 0.61 | 78.67 ± 2.58 | 40.06 ± 0.61 | - | 3.81 ± 0.29 | 53.93 ± 18.49 | 2.52 ± 0.81 | - | 89.97 | 31.38 | 93.70 | - | |||
| Ww | 41.54 ± 0.44 | 0.00 | 5.71 ± 0.42 | - | 1.77 ± 0.11 | 0.84 ± 0.87 | 0.62 ± 0.07 | - | 95.80 | * | 89.15 | - | |||
| PEM | 88.08 ± 15.32 | 2.89 ± 3.02 | 17.05 ± 0.50 | 1,218.93 ± 196.99 | 0.00 | 4.26 ± 0.53 | 1.84 ± 0.37 | 176.19 ± 25.91 | 100 | * | 89.23 | 85.55 | |||
| PDM | 77.66 ± 5.02 | 2.80 ± 0.36 | 1.31 ± 0.06 | 104.25 ± 19.99 | 0.00 | 4.51 ± 0.10 | 0.40 ± 0.10 | 77.65 ± 18.06 | 100 | * | 69.04 | 9.64 | |||
COD, chemical oxygen demand; BCAM, treated wastewater added with 0.1977 g·L-1 NH4HCO3; PAM, treated wastewater enriched with 0.3303 g·L-1 (NH4)2HPO4; BM, Bayfolan medium formulated with 1 mL of Bayfolan Forte per liter of treated wastewater; Ww, raw wastewater; PEM, tap water with 23.5% of piggery wastewater; PDM, tap water with 39.75% of piggery wastewater digestate.
*The results show a slight increase in nitrates.
NH4 + losses by stripping
The highest loss of NH4 + by volatilization (as NH3) was recorded in PDM, that is, 33% (8.70 ± 1.78 mg·L-1·d-1 NH4 +) for the consortium and 46% (11.53 ± 0.32 mg·L-1·d-1 NH4 +) for Scenedesmus sp. at pH of 9.93 and 10.03, respectively, with no significant difference between them. In PEM the loss was significantly lower than in PDM, with 2% (2.24 ± 0.58 mg·L-1·d-1 NH4 +) for the consortium and 7% (0.56 ± 0.42 mg·L-1·d-1 NH4 +) for Scenedesmus sp. at an average pH of 9.68.
Discussion
Biomass production in Ww was 15.33% lower with the microalgal consortium compared with that obtained with Scenedesmus sp., whereas in PEM, biomass production with Scenedesmus sp. was 40% lower than that obtained with the microalgal consortium. Lam et al. (2017) cultured C. vulgaris in non-sterilized wastewater (2.70 and 24.19 mg·L-1 of total N and P, respectively), obtaining 40.76 mg·L-1·d-1 (dry weight) of biomass; this productivity was 3 times lower than that obtained in Ww in the present study (initial concentration of 32.20 and 8.84 mg·L-1 of total N and P, respectively). Unlike in the other 5 media, in BM, which was formulated with the commercial fertilizer Bayfolan, the main source of N was NO3 -; moreover, P contents varied significantly between media (Table 4). Since the main goal of the present work was to identify a nonconventional medium that could be used at large scale, the C/P and COD/N/P ratios were not adjusted. However, particular care was given to N concentrations during the preparation of the media. The results obtained from the experimentation with all the media showed that the 2 best options to produce biomass were Ww and PEM, both of which involve residue treatment and are available at low cost.
Nayak et al. (2016) reported the growth of Scenedesmus sp. in a 500-mL Erlenmeyer flask with 200 mL of medium, prepared with 1 g·L-1 of a NPK fertilizer (10:26:26) and 0.10 g·L-1 of urea, obtaining a biomass productivity of 45 mg·L-1·d-1 (peso seco). A 2-fold biomass production and 2.8 times higher productivity were obtained in the present study with Scenedesmus sp. and the consortium in BM. The Bayfolan fertilizer (N:P:K, 11:1:1) is a foliar fertilizer that contains micronutrients, vitamins, and indoleacetic acid (phytohormone). This auxin regulates growth in higher plants, and in several species of microalgae, it increases growth rate, stress tolerance, lipid content, and biomass productivity (Lu and Xu 2015). Muriellopsis sp. and Pseudokirchneriella subcapitata grown in 300-mL bubble-column glass bioreactors containing freshwater with 40%-50% concentrate from a wastewater treatment plant, under controlled conditions (temperature of 25 ºC, aeration of 0.2 vvm, CO2 injected on demand, artificial illumination under a 12:12 h light:dark cycle, 1,850 μE·m-2·s-1), reached biomass productivity values up to 1.13 and 1.02 g·L-1·d-1, respectively (Morales-Amaral et al. 2015). Moreover, Gomez et al. (2013) cultured Muriellopsis sp. in the same bubble-column, using TWw as medium and controlled conditions (temperature of 23 ± 2 ºC, 8.0 ± 0.1 pH, on-demand CO2 injection into the airflow entering the reactors, and artificial illumination 12:12 h light:dark cycle, 800 μE·m-2·s-1), and the maximum biomass productivity they obtained was 0.5 g·L-1·d-1. In the 2 latter reports, productivities were much higher than those obtained in the present study.
Chlorella vulgaris tolerates high NH4 + concentrations. However, when NH4 + exceeds 110-130 mg·L-1, cell growth inhibition occurs (Sanz-Luque et al. 2015). The inhibition of microalgal growth caused by NH4 + becomes severe when the NH4 + content reaches 1,030.36 mg·L-1 (Park et al. 2010). In the present study, the NH4 + concentration in the medium was below 90.61 mg·L-1 (Table 4).
Lipid concentrations in cells increase when the source of nitrogen is limited (Eroglu et al. 2015, May Cua et al. 2019). N is a critical factor for regulating algal cell lipid content; lipid accumulation increases when nitrogen becomes the growth limiting factor (Chen et al. 2011). However, N limitation minimizes algal growth; the 2 conditions of high lipid content and high algal productivity are mutually exclusive.
Nam et al. (2017) cultivated C. vulgaris in residual water of pig excreta with a total N concentration of 307.2 mg·L-1 for 16 d, obtaining a biomass and lipid concentration of 3.96 and 1.072 g·L-1, respectively. In the present work, the production of biomass and lipids was approximately 61% and 72% lower in PEM, respectively, with total N concentration being ~5 times lower (Fig. 1). In the case of Ww, the concentration of biomass and lipids was of 58% and 68% lower, respectively, and the total N concentration was 9 times lower. Regarding BM, the production of biomass and lipids was 54% and 85% lower, respectively, having the same total N concentration as PEM.
The loss of NH4 + can be caused by several factors, such as assimilation by microalgae, denitrification processes by bacteria, and NH4 + stripping at high pH levels in the medium. In the case of P, the removal is due to assimilation by microalgae and precipitation in the form of calcium phosphate at pH levels close to 9 (Walker 2015). In this study, the initial pH of Ww and BM was 8.5 and it increased during the growth of the consortium and Scenedesmus sp., reaching a value of 11.5.
The preference for the type of N source varies from one strain of microalgae to another. NH4 + is the most easily assimilated N source, followed by NO3 - and NO2 -. NH4 + is directly assimilated by the cell, whereas NO2 - and NO3 - are transported to the inner part of the cell and reduced to NH4 + by nitrite and nitrate reductases (Liao et al. 2018). In Ww, PEM, and PDM the concentration of NO3 - was up to twice the initial one (Table 4). The processes of N removal in wastewater and activated sludge, the nitrification of NH4 +, which is oxidized to NO3 -, and the denitrification of NO3 -, which is reduced to N2, have been widely studied (Larsdotter 2006). These processes involve nitrifying and denitrifying bacteria: the former are autotrophic, do not need organic carbon, and consume large amounts of oxygen; the latter are anaerobic heterotrophs or aerobic autotrophs and require a source of carbon. The first step in the nitrification process is the oxidation of NH4 + to NO2 - by bacteria belonging to the genus Nitrosomonas. In the second step, the oxidation of NO2 - to NO3 - occurs by bacteria belonging to the genus Nitrobacter. In denitrification, bacteria reduce NO3 - or NO2 - to N2 gas (Jia and Yuan 2016). In mixed cultures of microalgae and bacteria in wastewater, microalgae can produce organic compounds that bacteria can assimilate; on the other hand, some bacteria produce hormones that promote microalgal growth (Liu et al. 2017). Approximately 60% of NH4 + is oxidized by nitrifying bacteria, while microalgae assimilate 40% (Vargas et al. 2016).
High pH levels can promote NH3 volatilization (removal of NH4-N) and P removal through PO4 3- precipitation with ferric iron, calcium, and magnesium (Park et al. 2011). In PDM, a medium in which greater ammonium loss due to volatilization was observed, pH values were more basic than in PEM; in addition, PEM contained a higher quantity of organic matter that could have minimized NH3 losses. For example, Wu et al. (2017) reported losses of 5% of NH4 + by stripping during 4 d in wastewater without microalgae (initial concentration of 30.26 mg·L-1 NH4 +).
The present study demonstrated that the cultivation of the microalgal consortium and Scenedesmus sp. with piggery wastewater as the medium is suitable to produce biodiesel given the productivity of biomass and lipids and the high content of saturated fatty acids that were obtained. These productivities were reached due to the nutrient supply attributed to piggery wastewater, which in addition is readily available. Furthermore, cultivation with PEM showed efficient nutrient removal within the first 3 d. As a whole, the results of the present study provide insight on low-cost alternatives to culture microalgae, coupled with valorization of waste as an approach to the eventual production of biodiesel.











 texto en
texto en 


