Introduction
Soil contamination by heavy metals involves certain risks due to their toxicity and persistence in the environment and such characteristics are related to the chemical forms of the metals (Tchounwou, Yedjou, Patlolla and Sutton, 2012). The concern about the levels of heavy metals in soil is also attributed to their retention, mobility, and bioavailability (Petruzzelli, Pedron and Rosellini, 2020). The availability of some metals must be determined because they are beneficial at low concentrations but can be harmful at higher concentrations (Jordao et al., 2006). In addition to the high concentrations of Cd that occur naturally, Cd inputs from the use of agricultural fertilizers, pesticides, and lime, others such as industrial and domestic wastes are the main sources of Cd contamination in soils. Under these conditions, Cd can be absorbed by plants or cause groundwater contamination. Cadmium is not essential in soil organisms and this presence in the environment at levels above the back-ground could pose some threat to their well-being (Adriano, Wenzel, Vangronsyeld and Bolan, 2004), entering the food chain and causing toxic effects on human health, manifesting itself, especially, in bones and kidneys (Pérez and Azcona, 2012).
Treatment in-situ of contaminated soils with the use of soil amendments can cause changes in heavy metal speciation, which immobilize the metals from soil solution (Singh and Oste, 2001; Chen, Ma, Singh, Cao and Melamed, 2003). The efficient metal-removing ability of this technique improves the contaminated soil quality by reducing the potential toxicity of metals to plants and human beings (Li et al., 2019). The use of soil amendments has been an alternative for areas with economically important and contaminated perennial crops (Antonious, 2016). Various soil amendments have been evaluated between these gypsums, agricultural lime, hydroxyapatite, and other soluble sources of P, as well as oxides, aluminosilicates, and biosolids of Fe and Mn with reduced concentrations of metals (Hamon, McLaughlin and Cozens, 2002; Basta and McGrowen, 2004). These amendments stabilize heavy metals through chemical bonds that lead to the formation of stable compounds with reduced mobility or toxicity (Kabata-Pendias, 2001).
Organic amendments interact with metals through coagulation, chelation, and peptization (Kabata-Pendias, 2001), but it depends on the organic ligand, soil pH, and the mobilization of the pollutant. Immobilization of heavy metal ions and complexes on inorganic amendments occurs as a result of surface complexation, ion exchange, hydrophobic interaction, and electrostatic interaction (Wahba and Zaghloul, 2007).
Heavy metal availability in the soil can be determined by several extractants, but for Cd, an officially recommended extractant does not exist (Caridad-Cancela, Paz and Abreu, 2005). The most used Cd extractants in soil are DTPA (diethylenetriamine pentaacetic acid) and Mehlich-3, but controversial results of their efficiency have arisen especially in soils fertilized with organic residues (Mantovani, Cruz, Ferreira and Alves, 2004). The DTPA extractant has been widely used for the determination of heavy metal availability in soil due to its efficient chelating agent action (Karak and Bhattacharyya, 2010). Uptake of Cd by plants depends on the concentration of Cd in the ionic form present in the soil solution and the concentration at the exchangeable sites of the cation exchange complex (Smith and Paterson, 1995). The concentration of Cd2+ ions presented in soil solution is reduced with the use of DTPA (Abreu and Abreu, 2001).
The Melhlich-3 extractant has been also used to predict metal uptake by plants due to its acid characteristics, the successful application to different metals, and the strong capacities of metal complexation by fluoride ions and EDTA (Silva, 2009). The methodologies of metal extraction most widely used for soils employ a single extractant whose content for one element correlates with plant availability and can be used to predict plant uptake or the likelihood of deficiency or toxicity symptoms occurring in plants or animals (Ure, 1995). Besides the methodology using single extractants, sequential extractions of soil using chemical reagents have been used to identify operationally-defined fractions of metals in soil (McGrath, 1995; Kiekens, 1995). This technique allows understanding of the metal distribution over different forms and various reagents have been proposed to extract fractions representing exchangeable, carbonate, reducible, organic, and residual forms (Kiekens, 1995).
Sequential extraction procedures allow the determination of the metal as it is bound to soil solid particles, its mobility, and transformations when receiving chemical, physical or organic treatments. In this context, the use of this technique is useful for evaluating metal behavior in the soil-plant systems (Melo, Nascimento, Santos and Silva, 2008), although it presents certain limitations such as the effects of temperature and time of drying samples, sample-extractant contact time, the volume of the agitation flask, agitation time, agitation method, temperature of the environment, lack of metal selectivity of some extractants and metal redistribution among the different extracted forms (Tessier, Campbell and Bisson, 1979).
As mentioned early, the use of soil amendments reduces heavy metal availability to plants. This can be achieved because the addition of inorganic or organic soil amendments affects the dynamic equilibrium of the metal in the soil solution/solid phase system (Ribeiro, Siqueira, Curi and Simão, 2001). Predictions of the dynamics and toxicity of Cd can be based on the predominant chemical forms (Haider et al., 2021; Rassaei, Hoodaji and Abtahi, 2020). However, there is a great difficult to predict the effectiveness and the behavior of soil amendments due to the soil complexity; in this sense, chemical fractionation of contaminated soil/soil amendment mixtures gives important information about the available forms of Cd associated with the different chemical bonds (Rassaei et al., 2020).
The present study aimed to evaluate the variation in pH, Cd availability, and its forms of association in two previously contaminated tropical soils, corrected with mineral and organic soil amendments in twelve months experiment. The approach involved the use of DTPA and Mehlich-3 extractants for the extraction of Cd available forms as well as a sequential extraction procedure to investigate the chemical associations of Cd in the various geochemical fractions of contaminated soil/soil amendment mixtures.
Materials and Methods
Location
This research was developed at room temperature, in the greenhouse and laboratory of the Department of Soils of the Federal University of Vicosa, Minas Gerais (Brazil). Located at coordinates 20° 45’ 37″ S, 42° 52’ 04″ W, with an altitude of 648 meters above sea level.
Soil and soil amendment collection, handling, and characterization
A Red-Yellow Latosol and a Red-Latosol (Brazilian Soil Classification System, SiBCS) were sampled in southeaster Brazil (Minas Gerais State) at a depth of 20-60 cm after removing the vegetation. The sample soils were air-dried and sieved to separate the particles smaller than 2 mm for the physical and chemical analyses. The soil characteristics are listed in Table 1.
Table 1: Selected physicochemical properties of the studied soils.
| Characteristic | Red-Yellow Latosol | Red-Latosol |
| pH in H2O (1:2.5) | 4.92 | 5.73 |
| OM† (%) | 2.97 | 1.03 |
| Available P‡ (mg dm-3) | 0.6 | 1.0 |
| Available K‡ (mg dm-3) | 7 | 29 |
| Available S (mg dm-3) | 60 | 22 |
| Exchangeable Ca (cmolc dm-3) | n.d | n.d |
| Exchangeable Mg (cmolc dm-3) | 0.02 | 0.03 |
| Exchangeable Al3+ (cmolc dm-3) | 1.1 | n.d |
| H + Al§ (cmolc dm-3) | 9.2 | 3.6 |
| Sum of the bases (cmolc dm-3) | 0.04 | 0.10 |
| CECe¶(cmolc dm-3) | 1.14 | 0.2 |
| CECt ¶(cmolc dm-3) | 9.24 | 3.7 |
| Soil bulk density¶ (kg dm-3) | 1.07 | 1.36 |
| Particle density¶ (kg dm-3) | 2.15 | 2.48 |
| Sand (%, w/w) | 18 | 82 |
| Silt (%, w/w) | 3 | 4 |
| Clay (%, w/w) | 79 | 14 |
| Texture | Clayish | Sandy loam |
† Organic matter (OM) was determined by the Walkley-Black method (Gaudette, Flight, Toner and Folger, 1974), ‡ Available P and K were extracted with the Mehlick-1 extractant (Teixeira, Donagemma, Fontana and Teixeira, 1997), § H + Al extracted with 0.5 M Ca (OAc)2 solution at pH 7.0 (Defelipo and Ribeiro, 1997). n.d = not detected, ¶ Effective and total CEC, particle size distribution, particle density and soil bulk density were evaluated by the method of Embrapa (Teixeira et al., 1997).
The mineral and organic soil amendments used in this work included commercial samples of cattle manure vermicompost, lime, phosphate rock (Araxá), and zeolite collected from local suppliers. The other soil amendments were sugarcane filter cake, collected from a sugar mill patio in Minas Gerais State, Brazil, and palm kernel pie, from a palm plant in Bahia State, Brazil. The soil amendments were ground and sieved to separate the particles smaller than 1 mm to be used in the experiments. Subsamples of the organic materials were used for physico-chemical characterization (Table 2). Some characteristics of the mineral soil amendments were as follows: dolomitic lime (CaO = 36%; MgO = 14.4%); phosphate rock (P2O5 total = 24%; P2O5 citric acid soluble = 3%); zeolite (Heulandite group zeolite). The neutralizing power, reactivity, and relative power of total neutralization of lime were 97.3%, 68.5%, and 66.6%, respectively.
Table 2: Characteristics of the organic soil amendments.
| Soil amendment | Apparent density | pH in H2O | Ca | Mg | K | P | N | Humic acids | Fulvic acids | Humin | Ctotal | C/N ratio |
| kg dm-3 | 1:2.5 | - - - - - - - - - - - - - - - - - - - - - Percentage† - - - - - - - - - - - - - - - - - - - - - - - | ||||||||||
| Vermicompost | 1.0 | 5.28 | 0.85 | 0.15 | 0.06 | 0.35 | 1.13 | 0.76 | 0.51 | 6.14 | 5.11 | 4.52 |
| Sugarcane filter cake | 0.4 | 6.51 | 1.40 | 0.12 | 0.15 | 0.71 | 1.38 | 0.59 | 0.77 | 22.42 | 19.35 | 14.02 |
| Palm kernel pie | 0.7 | 5.47 | 0.20 | 0.20 | 0.28 | 0.39 | 2.26 | 1.11 | 1.31 | 25.60 | 38.24 | 16.92 |
† Nutrients, humic and fulvic acids, humin and total carbon were determined following the procedures of Mendonça and Matos (2005).
Soil contamination with cadmium
Soil samples were contaminated with 4.5 mg kg-1 of Cd as Cd(NO3)2 in order to obtain an equivalent dose of 1.5 times the guideline value of investigation for agricultural areas, according to CONAMA (2009). The guideline values represent the concentration of chemical substances that supply orientation about the condition of soil quality and underground water. They are used as instruments for the prevention and control of the contamination and administration of polluted areas under investigation. Values of investigation are the concentrations of substances in soil or in underground water above which they present a potential risk to human health, considered the scenery of generic exhibition. For soil, the value of investigation was calculated using the procedure of health risk assessment for human exposure to chemicals in the scenery of agricultural (3.0 mg kg-1), residential (8.0 mg kg-1), and industrial areas (20.0 mg kg-1).
Soil incubation
The contaminated soil samples were incubated in a greenhouse at room temperature for one month and distilled water was added to plastic bags, keeping the water content close to 80% of the field capacity (3.3 kPa) replacing the weight of water loss. The incubated soil samples were subjected to daily movements for their homogenization.
Cadmium availability in contaminated soil/soil amendment mixtures
A randomized complete block design with 3 replications was used. An amount of 0.4 kg of the contaminated soil/soil amendment mixtures at a ratio of 40:1 (w/w) was placed in a polypropylene bag. In the experiments, eighteen plastic bags were used for each soil since six soil amendments were mixed, in a very clayish soil (Red-Yellow Latosol) and a sandy loam soil (Red-Latosol) with three repetitions. The plastic bags containing the mixtures were opened for two days at the end of each month for twelve months. The soil moisture was kept close to 80% of the field capacity. Daily, the experimental units were homogenized with manual movements.
Cadmium availability was determined in the mixtures along the twelve months of the experiment by using the DTPA and Mehlich-3 extractants, following the procedure adapted from the available literature (Caridad-Cancela, Abreu and Paz, 2002).
a) DTPA: 0.005 mol L-1 DTPA (diethylenetriamine pentaacetic acid), 0.1 mol L-1 TEA (triethanolamine) and 0.01 mol L-1 CaCl2 at pH 7.3. Soil volumes of 10 cm3 and 20 mL of DTPA solution were used for the extraction. The suspensions were placed in polyethylene flasks covered with a plastic stopper and shaken by horizontal-circular movements at 240 oscillations per minute for two hours. Then, the suspensions were filtered and Cd concentration was measured.
b) Mehlich-3: 0.2 mol L-1 CH3COOH, 0.25 mol L-1 NH4NO3, 0.015 mol L-1 NH4F, 0.013 mol L-1 HNO3, 0.001 mol L-1 EDTA adjusted to pH 2.5. Soil volumes of 5 cm3 with 50 mL of Mehlich-3 extractant were taken. The suspensions were placed in polyethylene flasks covered with plastic stoppers and shaken by horizontal-circular movements at 240 oscillations per minute, for 5 minutes. After filtration Cd content was determined.
Sequential extraction procedure
At the end of the twelve months of the experiment, the soil/soil amendment mixtures of each experimental unit were submitted to a sequential extraction procedure of Cd, following the procedure adapted from Tessier et al. (1979).
The six fractions examined are described as follows:
Exchangeable: extraction of Cd with 1 mol L-1 MgCl2 at pH 7.0 for the soluble forms and adsorbed on the surface of colloids by electrostatic forces.
Carbonate: extraction of Cd from the previous residue with 1 mol L-1 NaOAC adjusted to pH 5.9 with HOAc for Cd carbonate.
Reducible: extraction of Cd from the remaining residue with 0.04 mol L-1 NH2OH.HCl in 25% (v/v) HOAc adjusted to pH 2.0 with HNO3 for specifically adsorbed on iron oxides and manganese oxides.
Organic: extraction of Cd from the residue obtained from the reducible fraction with 0.02 mol L-1 HNO3, 30% (v/v) H2O2 pH 2.0, and 3.2 mol L-1 NH4OAc for organically complexed metal.
Residual: extraction of Cd from the remaining residue of the organic fraction with 60% (w/w) HNO3, 37.5% (w/w) HCl, and 40% (w/w) HF for forms of Cd within the crystal structure of minerals.
Total content of Cd was determined by the sum of several fractions.
Instrumentation and some relevant information
Cadmium concentrations in the extracts were determined with a Varian atomic absorption spectrophotometer (model Spectra 220 FS) by direct aspiration of the solutions into an air-acetylene flame at 228.8 nm. All glassware and materials for the metal analysis were cleaned (20% solution of HNO3 mixture and HCl in 1:1 ratio, followed by rinsing with distilled water and finally with ultra-pure water) and analytical grade reagents used (all from Merck) were of the highest purity available.
Statistical analysis
All monthly data of available Cd contents were compared within each soil amendment and Cd contents were submitted to analysis of variance and Duncan’s post hoc mean test (P < 0.05). The statistical package used was Statistica 8.0 (Statsoft, 2008). For sequential extraction data, descriptive statistics were used. Correlation coefficients among Cd extracted by DTPA and Mehlich-3 extractants, with the exchangeable and carbonate fractions and soil pH in the last month of the experiment were examined by the t-test.
Results and Discussion
Cadmium availability
The importance of mineral and organic soil amendments for the recovery of polluted agricultural areas with heavy metals has been recognized many years ago (Lwin, Seo, Kim, Owens and Kim, 2018) and our investigations indicated that most of the soil amendments used in this work reduce Cd availability in tropical soils.
In this batch experiment, most Cd was extracted by the Mehlich-3 extractant as compared with DTPA. It was observed in all combinations of contaminated soil/soil amendment experiments (Figure 1). The largest effectiveness of Cd removal with the Mehlich-3 extractant is due to the much more aggressive action of acid extractants, which result in the release of non-available metal forms to plants, especially in alkaline soil samples (Oliveira, Salviano, Moraes and Duda, 2008). However, a larger capacity of metal extraction with Mehlich-3 as compared with DTPA, except for soil with pH in the neutral or alkaline range, has also been reported (Caridad-Cancela et al., 2005). This finding was related to the higher effectiveness of DTPA for metal extraction under neutral or alkaline conditions (Caridad-Cancela et al., 2002). In our study, the smallest Cd extraction by DTPA can also be explained by the relatively high metal content in the solution that probably exceeds the complexing ligand capacity of the extractor (Norwell, 1984; O’Connor, 1988).
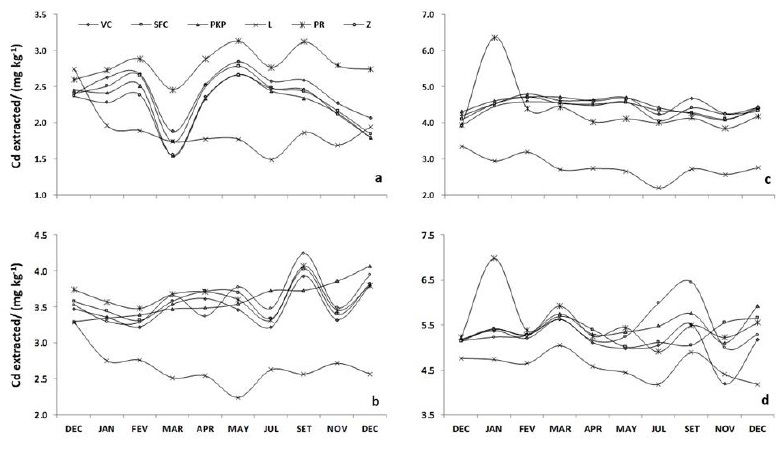
Figure 1: Extraction of Cd from Red-Yellow Latosol and Red-Latosol with DTPA extractant (a,c) and with Mehlick-3extractant (b,d) amended with vermicompost (VC), sugarcane filter cake (SFC), palm kernel pie (PKP), lime (L), phosphate rock (PR) and zeolite (Z) in a long-term experiment.
Cadmium availability was larger for the Red-Latosol than the Red-Yellow Latosol at the same doses applied along the twelve months of the experiment. The cadmium concentration extracted from the Red-Yellow Latosol varied from 2.19 to 6.35 mg kg-1, while the values for the Red-Latosol were between 1.49 and 3.13 mg kg-1 (Figure 1). The higher Cd adsorption in the Red-Yellow Latosol could be attributed to the high cation exchange capacity caused by the higher organic matter and clay content of this soil (Goncalves et al., 2016).
Lime addition resulted in smaller Cd release to soil solution as compared with the other soil amendments. Lime also produced a reduction trend in the available Cd content during the twelve months of the experiment, except for Cd extraction from the Red-Latosol in the months of March and September (Figure 1d).
Extraction of Cd from amended soils with DTPA and Mehlich-3 extractants resulted in different values of Cd concentrations at the beginning of the experiment, certainly due to the different adsorption capacities of soil amendments used. For instance, the release of Cd extracted with DTPA from the Red-Yellow Latosol amended with lime was larger than of zeolite, as shown in Figure 1a. By contrast, the release of Cd extracted with the Mehlich-3 extractant from the Red-Latosol amended with lime was small when compared with most of the other soil amendments (Figure 1c).
In the long-term batch experiments with the Red-Yellow Latosol, the existence of three distinct groups of soil amendments regarding Cd extraction with DTPA can be recognized (Figure 1a). The first group includes phosphate rock with a lower effect for Cd immobilization.
The second group had similar effects on the release of Cd during the twelve months of the experiment and includes vermicompost, sugarcane filter cake, palm kernel pie, and zeolite. They presented a significant reduction in Cd availability in the third month, then gradually immobilized Cd in the fifth month and reached the last month with smaller concentrations than the initial ones. The reduction of Cd availability in the mid-term after the application of soil amendments can be explained in terms of solubility and mobility of the metal. Also, the immobilizing effect of organic matter on the availability of metals depended on the source of organic matter (Singh and Oste, 2001). Lime was included in the third group because immobilized more Cd in almost the whole year as compared with the other soil amendments.
For the extraction of Cd with the Mehlich-3 extractant, two groups can be observed. One group includes lime while the other soil amendments belong to the second group (Figures 1b and 1c). As in the case of Cd extraction by DTPA, lime had the largest immobilization efficiency, leading to the decrease of Cd availability along the period of time examined. The other soil amendments presented similar behavior among them from the beginning to the end of the experiment.
In contrast to the results obtained for the amended Red-Yellow Latosol, the values of Cd extracted with DTPA and Mehlich-3 extractants from the amended Red-Latosol were similar among the treatments at the beginning of the experiments, except for the lime amended soil (Figure 1).
As in the case of the Red-Yellow Latosol, lime was the most efficient soil amendment in Cd immobilization in the amended Red-Latosol (Figure 1). The effect of lime in the decrease of Cd availability was larger when using the DTPA extractant than the Mehlich-3 extractant, probably due to the acid neutralizing capacity of lime for the acidic extractant. As mentioned above, the Mehlich-3 extractant seems to have a limited capacity to retain Cd in neutral and alkaline soils (Caridad-Cancela et al. 2005).
Effects of soil amendments on the final ph of the contaminated soil/soil smendment mixtures
The pH values reached at the end of the experiments are shown in Figure 2. The use of lime as soil amendment resulted in a pH value larger than 8 for the Red-Latosol, while no more than 6.32 was observed for the other materials. Organic amendments had only small effects on the final pH values of the contaminated soil/soil amendment mixtures as compared with the initial pH of the soils, except for palm kernel pie in which the pH increased in the Red-Latosol. Mineral amendments caused enhancement of the pH and the most pronounced effect occurred with the use of the lime as expected. Among inorganic amendments, special attention has been given to lime because it is commonly used as a soil acidity corrective in agricultural soil and applied to reduce the levels of contaminants in acidic soil (Singh and Oste, 2001; Accioly, Siqueira, Curi and Moreira, 2004).
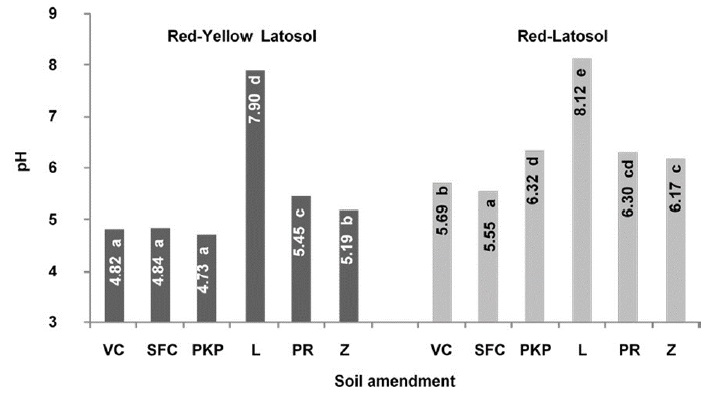
Figure 2: pH values of the contaminated soil/soil amendment mixtures obtained after a year-long incubation period. Vermicompost (VC), sugarcane filter cake (SFC), palm kernel pie (PKP), lime (L), phosphate rock (PR) and zeolite (Z). Mean of three replicates. Mean values followed by at least a same letter in the bars for each variable are not different at the 5% level, according to the Duncan’s test.
Sequential extraction of cadmium
Figure 3 shows the Cd concentrations extracted by the selective chemical extractants from the several geochemical fractions of contaminated soil/soil amendment mixtures incubated for a year. The application of lime to the soils resulted in relatively low exchangeable Cd contents. This is an indication that the use of this soil acidity corrective produced larger Cd immobilization as compared with the other soil amendments. On the other hand, the exchangeable Cd content had primary importance for the other soil amendments and ranged from 66% to 80% of the total Cd concentration.
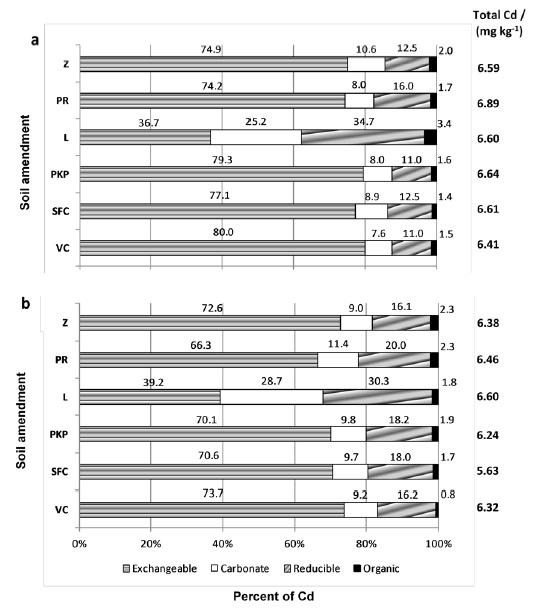
Figure 3: Cadmium partitioning in amended Red-Yellow Latosol (a) and amended Red-Latosol (b) with vermicompost (VC), sugarcane filter cake (SFC), palm kernel pie (PKP), lime (L), phosphate rock (PR) and zeolite (Z). Cadmium was not found in the residual fraction.
In the lime-amended soil, cadmium appeared to be concentrated in less available forms such as carbonate, reducible, and at a smaller proportion in the organic matter (Figure 3). A similar effect of lime in the enhancement of other geochemical forms than the exchangeable form has been also for a dystrophic Red-Yellow Latosol from Minas Gerais State (Ribeiro et al., 2001). Lime application to a clayish soil transferred 40% of exchangeable Cd for the organic matter (Melo et al., 2008).
Exchangeable Cd can be transferred to less available forms in lime-amended soil due to the pH increment of the natural soil (Figure 3). The enhanced pH is responsible for Cd adsorption as well as the formation of Cd hydroxide and/or carbonate, as suggested by other authors (Costa et al., 2009).
The use of organic amendments in the soils was unable to reduce exchangeable Cd contents, one reason for this could be the application of insufficient amounts of the organic amendments. An increase of Cd content in the exchangeable fraction with the use of low vermicompost doses has been reported (Ribeiro et al., 2001). However, a reduction in the soluble and exchangeable Cd and an increase in the organic fraction were observed with the application of poultry manure at 60-240 Mg ha-1 to soil (Liu, Chen, Cai, Liang and Huang, 2009).
Cadmium was not found in the residual fraction at the experimental conditions used in this work. The application of 6.8 mg kg-1 of Cd to the soils resulted in Cd distributed entirely in the other fractions. The residual fraction deals with minerals, which contain Cd within their crystalline structure and are not expected to release the metal under conditions normally found in nature. Only extreme conditions such as high temperature and high pressure could lead to Cd retention by the residual fraction.
Correlations between Cd extracted by DTPA and Mehlich-3 extractants with available and potential available forms and soil pH
There was a positive correlation between Cd extracted by DTPA and Melhich-3 extractants, with a correlation coefficient of 0.90** (Table 3). A similar value (0.98**) for soils from the rio Grande do Norte State (Brazil) has also been reported (Oliveira et al., 2008). However, no significant correlation was observed between the natural levels of Cd extracted by these extractants from the soils of Spain (Caridad-Cancela et al., 2005). There was no positive correlation between Cd extracted by DTPA and Melhich-3 extractants with the exchangeable fraction when both soils were taken into account, but it was found a significant correlation (0.96**) between this fraction and the Mehlich-3 extractant for the Red-Yellow Latosol. These extractants presented positive correlations with the exchangeable fraction for the Red-Latosol.
Table 3: Correlations among Cd extracted by DTPA and Mehlich-3 extractants with the exchangeable fraction, carbonate fraction and soil pH at the end of the experiments.
| Characteristic | RYL + RL† | RYL | RL | |||
| DTPA | Mehlich-3 | DTPA | Mehlich-3 | DTPA | Mehlich-3 | |
| DTPA | - | 0.90** | - | 0.42** | - | 0.72** |
| Exchangeable | -0.11ns | 0.14ns | 0.11ns | 0.96** | 0.72** | 0.60* |
| Carbonate | -0.22ns | -0.39* | -0.17ns | -0.96** | -0.94** | -0.73** |
| Exc. + Carb. | 0.33ns | -0.07ns | 0.07ns | 0.94** | 0.21ns | 0.24ns |
| pH | 0.17ns | -0.07ns | 0.05ns | -0.96** | -0.87** | -0.64** |
† RYL + RL, Red-Yellow Latosol + Red-Latosol. *Significant at 5% of probability (t test); ** Significant at 1% of probability (t test); ns = non-significant.
The DTPA and Mehlich-3 extractants are commonly used for the evaluation of heavy metal availability in the soil to plant and higher correlations were expected for the two soils examined. A lack of correlation between Cd extracted by DTPA and the exchangeable fraction obtained by using the methodology of Tessier et al. (1979) has also been reported by other workers (Ribeiro et al., 2001).
There was no correlation between Cd extracted by DTPA and the sum of the available fraction and the potentially available fraction (exchangeable + carbonate), but a positive correlation with the Mehlich-3 extractant in the Red-Yellow Latosol was observed.
Concentrations of cadmium extractable by DTPA and Mehlich-3 extractants showed negative correlations with the carbonate fraction. Although some of the coefficients were not significant, these results indicated an inverse relationship between carbonate fraction and the available forms.
Inverse relationships between Cd extracted by DTPA and Melhich-3 extractants with soil pH were also found and it might be explained by the effect of the enhancement of the pH in the reduction of Cd availability in soils.
Conclusions
Most mineral and organic soil amendments reduced cadmium (Cd) availability in contaminated soils. Phosphate rock had the lower effect for Cd immobilization while lime was the most efficient soil amendment. Cadmium availability was larger for the sandy loam soil (Red-Latosol) than the very clayish soil (Red-Yellow Latosol) at the same doses applied along the twelve months of the experiment and this can be attributed to the largest organic matter and clay contents of the latter. Cadmium appeared to be concentrated in less available forms such as carbonate, reducible, and at a smaller proportion in the organic matter in the lime-amended soil. Since Cd immobilization is greatly dependent upon soil pH, the enhancement of the pH resulted in the formation of Cd hydroxide and carbonate.
The application of lime as a soil amendment seems to be an interesting option for the recovery of polluted agricultural areas with Cd. In addition to the easy application to land and the general availability of the material at a low-cost in the market, lime can provide a significant reduction of Cd availability in polluted soils.
There was a positive correlation between Cd extracted by DTPA and Melhich-3 extractants. The Mehlich-3 extractant had a high correlation with the available fraction examined by the sequential extraction procedure used in this study.
The organic amendments had only small effects on the final pH values of the contaminated soil/soil amendment mixtures as compared with the initial pH of the soils, except for palm kernel pie in which the pH increased in the Red-Latosol. The mineral amendments caused enhancement of the pH and the most pronounced effect occurred with the use of the lime as expected.











 nueva página del texto (beta)
nueva página del texto (beta)



