Introduction
Water is indispensable to human development; water uses include the production of food, the generation of energy, and the provision of sanitation, among others. However, factors such as population growth, the proportion of the population living in urban settings or cities, and climate change are increasing hydric stress worldwide. A decrease in water availability has been observed in regions like western Africa, southwestern Australia, the Yellow River basin in China, and the Pacific Northwestern of the United States of America (USA) (UNESCO, 2020). It is estimated that 4 billion people worldwide face severe water scarcity for a month per year (Mekonnen and Hoekstra, 2016). Also, the quality of freshwater resources is decreasing due to discharges of untreated municipal or industrial wastewater, the use of fertilizers and pesticides for agriculture, and intensive livestock farming (UNESCO, 2020; Mateo-Sagasta, Marjani and Turral, 2018). Some of the contaminants frequently found in freshwater are organic matter, parasites, heavy metals, fertilizers, pesticides, and emerging pollutants.
Coagulation and flocculation are technologies used worldwide for wastewater treatment due to their overall efficiency, robustness, and cost-efficiency (Jiang, 2015). The most used coagulants are aluminum and iron salts (aluminum sulfate, aluminum chloride, sodium aluminate, ferric sulfate, ferrous sulfate, and ferric chloride). However, the use of metal coagulants has shortcomings such as high carbon footprint, health effects due to residual aluminum in treated water, high cost of disposal of sludge containing aluminum (considered as hazardous waste in some regions), and environmental impacts associated with mining (Saleem and Bachmann, 2019; Kyung, Kim, Park and Lee, 2013).
In this context, many natural materials have been tested for their use in coagulation and flocculation (Saleem and Bachmann, 2019; Saritha, Karnena and Dwarapureddi, 2019). Chitosan is a natural polysaccharide with favorable characteristics for being used as a coagulant or flocculant (Renault, Sancey, Badot and Crini, 2009). Chitosan is a partially deacetylated form of chitin, one of the most abundant biopolymers. Chitin is found in the shells of crustaceans and mollusks, exoskeletons of some arthropods, and cell walls of some fungi (Miretzky and Cirelli, 2009). Chitosan is a linear hydrophilic copolymer that contains glucosamine and acetylglucosamine units. In acid environments, chitosan becomes a soluble cationic polymer, and below pH 5 protonation of amino groups on glucosamine occurs (Renault et al., 2009).
Table 1 presents different types of wastewater effluents treated with chitosan. Although relatively high removal percentages of pollutants have been achieved with this coagulant, in many cases, the pH at which coagulation is carried out is below pH 6. Simultaneous use of inorganic and natural coagulants can substantially lower the dose of metallic salt coagulants, such as aluminum sulfate. Chitosan is produced from renewable sources, the raw material (chitin) to produce chitosan is abundant in the natural environment and frequently is a waste from the seafood industry, non-toxic, biodegradable, and has been studied to treat a wide range of effluents.
Table 1: Performance of natural coagulants used to treat different types of effluents (TSS: total suspended solids, COD: chemical oxygen demand, NTU: nephelometric color units, BOD: biological oxygen demand).
| Effluent | Coagulant | Results |
| Synthetic water (Saritha, Karnena and Dwarapureddi, 2019) | Chitin from HiMedia chemicals (300 mg L-1) | 67.73% turbidity removal at pH 6 |
| River water (Ruelas-Leyva et al., 2017) | Chitosan from Sigma-Aldrich (500 mg L-1) | 83% turbidity removal at pH 6.72 |
| River water with manganese (Ruelas-Leyva et al., 2017) | Chitosan from Sigma-Aldrich (15 mg L-1) | 25% Mn removal at pH 6.7 |
| Agricultural/urban wastewater (Ruelas-Leyva et al., 2017) | Chitosan from Sigma-Aldrich (10 mg L-1) | 75% turbidity removal at pH 7.57 |
| Grey wastewater from Tamil Nadu, India (Thirugnanasambandham, Sivakumar, Maran and Kandasamy, 2014) | Chitosan from Sigma chemicals, Mumbai (300 mg L-1) | 95% turbidity removal, 91% BOD removal and 72% COD at pH 4 |
| Rice mill wastewater (Thirugnanasambandham, Sivakumar and Prakash, 2013) | Chitosan from HiMedia Laboratories (600 mg L-1) | 95% TSS and 98% COD at pH 4.5 |
This research studied the use of chitosan as a coagulant aid to reduce the amount of aluminum sulfate used in wastewater treatment. The coagulation/flocculation operating conditions investigated were the order in which coagulants are added, the dosage of coagulant, pH, and slow mixing velocity on the removal of total suspended solids (TSS) and turbidity.
Materials and Methods
Experimentation was carried out in four stages, preparation, and characterization of chitosan from shrimp exoskeleton waste, determination of the optimum dose for chitosan and aluminum sulfate, and the evaluation of the combined use of chitosan and aluminum sulfate in coagulation/flocculation. Preparation and characterization of chitosan were conducted in the Laboratory of Sustainable Technologies at the Autonomous Metropolitan University (AMU), and coagulation/flocculation experiments were carried out with raw wastewater from the wastewater treatment plant of the AMU.
Preparation and characterization of chitosan
Chitosan was prepared through demineralization, deproteination, and deacetylation of shrimp exoskeleton powder (Hernández-Cocoletzi, Águila, Flores, Viveros and Ramos, 2009). Shrimp waste was collected from a local seafood restaurant in Mexico City. Heads, tails, and organic matter were removed from shrimp waste. Shrimp exoskeleton waste was cleaned with tap water and dried in a stove at a temperature from 55 °C to 101 °C.
Exoskeletons were pulverized with a blender with stainless steel blades and sieved. Powder that passed through a sieve american size 100 (149 µm) was demineralized. Exoskeleton powder was placed in a beaker with hydrochloric acid 0.59 N at a mass: volume ratio of 1:11, at a temperature from 29 °C to 35 °C for 3 h. This product was washed with deionized water. Deproteinization was carried out by placing the powder on a beaker with a 1% sodium hydroxide solution at a mass: volume ratio of 1:11, at a temperature from 24 °C to 32 °C for 24 h. Next, the product was washed with deionized water. Deacetylation was carried out by placing the powder on a beaker with a 50% sodium hydroxide solution at a mass: volume ratio of 1:4, at a temperature from 52 °C to 74 °C for 2 h, and then at a temperature from 80 °C to 105 °C for 2 h. Finally, the powder was washed with deionized water, dried to constant weight in a stove, and stored.
Chitosan was analyzed with Fourier Transform Infrared (FtIR) spectroscopy (Brucker ATR Alpha II) to identify functional groups present in the chitosan prepared.
Wastewater characterization
Wastewater was characterized before and after coagulation/flocculation. The evaluated parameters include pH (APHA, 2012a), total suspended solids (TSS) (APHA, 2012b), and turbidity (T) (APHA, 2012c). Chemical oxygen demand (COD) (APHA, 2012d) was determined for selected samples.
Determination of the optimum dose and pH for chitosan and aluminum sulfate
The coagulation/flocculation experiments were carried out in the jar test equipment (Phipps & Bird, model PB-700) with 2 L capacity jars. The performance of the studied coagulants was evaluated by comparing TSS and turbidity before and after the treatment.
Stock solutions of 50 000 mg L-1 of aluminum sulfate (Al2(SO4)3 ·18 H2O) and 10 000 mg L-1 of chitosan were prepared. A sample of 100 mL of raw wastewater was placed in a laboratory beaker. Next, the stock solution was added in doses of 0.1 mL of the stock solutions until the formation of flocs was observed; this was registered as the qualitative dose. Then, raw wastewater was poured into each of the six jars of the jar test equipment. The pH was adjusted in five jars from 5 to 9 units, and the pH was not adjusted in one jar. Adjustment of pH was carried out with 1 M sodium hydroxide (NaOH) or 1 M hydrochloric acid (HCl) solutions accordingly. The qualitative dose of the coagulant was added to each jar at a rapid mixing velocity of 250 rpm for 2 min. The flocculant was added, and the mixing velocity was set at 40 rpm for 15 min for the second flocculation stage. Finally, sedimentation was allowed for 20 min. The jar with the highest percentage of removal of TSS was considered the optimum pH.
The optimum dose for the coagulant was determined by adding 500 mL of raw wastewater in each jar of the jar test apparatus, adjusting the pH to the optimum value, and adding a dose from 25 to 150% of the qualitative dose of the coagulant.
Effect of the operation conditions
The effect of the order of addition of coagulants, the dose of the coagulants, pH, and slow mixing velocity for flocculation were studied. The order in which coagulants were added was aluminum sulfate followed by chitosan, and chitosan followed by aluminum sulfate. The coagulant dosages studied were 125% (high), 100% (medium), and 75% (low) of the optimum dose. pH values investigated were the optimum pH and natural pH of the wastewater. Finally, slow mixing velocities (during the first stage of flocculation) studied were 70 rpm (high), 60 rpm (medium), and 44 rpm (low). In all the experiments rapid mixing velocity was set at 250 rpm for 2 min, the slow mixing velocity for the first stage of flocculation was kept for 15 min, the velocity for the second stage of flocculation was set at 40 rpm for 15 min, and sedimentation was allowed for 15 min. Experiments were conducted with two replicates.
The effect of operating conditions on the percentage of removal of TSS was evaluated with an analysis of variance (ANOVA) with the software Statgraphics 18® (Statgraphics Technologies, 2017).
Results and Discussion
Preparation and characterization of chitosan
The preparation method of chitosan produced a white powder, 100 g of shrimp exoskeletons with a size particle less than 149 µm yielded 20 g of chitosan (20% yield). From FtIR spectroscopy (Figure 1) the band between 3400 cm-1 and 3300 cm-1 was attributed to N-H stretching vibrations (primary amines), the band between 3550 cm-1 and 3200 cm-1 to O-H stretching, the band between 1650 cm-1 and 1580 cm-1 to N-H bending (primary amines), functional groups characteristic of chitosan (Li, Yang and Yang, 2015). This FtIR spectrum is consistent with the results obtained by Hernández-Cocoletzi et al. (2008).
Wastewater characterization
Average values and standard deviation for pH, TSS, turbidity, and chemical oxygen demand of wastewater used for coagulation/flocculation experiments in this research are presented in Table 2.
Effect of the operation conditions
The optimum dose and pH were found at 100 mg L-1 and 5 units for aluminum sulfate (Figure 2a) and 180 mg L-1 and 4 units for chitosan (Figure 2b). pH value for aluminum sulfate concurs with those reported by Du et al. (2021). Due to the protonation of amine groups below pH 5 chitosan in acidic solutions behaves like a cationic coagulant, enhancing the efficiency of coagulation (Desbrières and Guibal, 2018). Chitosan doses between 135 and 270 mg L-1 achieved TSS removal percentages higher than 90%, comparable to those obtained with aluminum sulfate at similar doses. In comparison, Chung, Wu and Chen (2013) reported 91 to 94% of turbidity removal with chitosan from kaolinite solutions with 40 - 300 NTU initial turbidity at initial pH of 4.
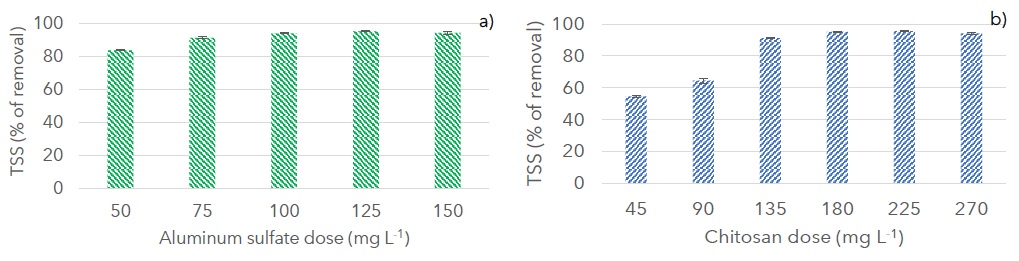
Figure 2: Removal of total suspended solids (TSS) in percentage for a) aluminum sulfate at pH 5 and b) chitosan at pH 4
Figure 3 shows the removal of TSS with aluminum sulfate (100 mg L-1) and chitosan (180 mg L-1) using the optimum dose for each coagulant. The performance of chitosan as a coagulant depends strongly on pH, while aluminum sulfate in this pH range is relatively unaffected. TSS removal with chitosan dropped from 96% at pH 4 to 25% at the pH of wastewater (8.21±0.24). The suggested removal mechanism for chitosan is charge neutralization. Chitosan is a partially deacetylated form of chitin, amines protonate below pH 5, creating favorable conditions for charge neutralization with negatively charged particles in the wastewater (Desbrières and Guibal, 2018; Ali, Laghari, Ansari and Khuhawar, 2013). In comparison, experiments with reagent grade chitosan, presented in Table 1, achieved 95% turbidity removal from gray wastewater with an initial pH of 4 (Thirugnanasambandham, Sivakumar, Maran and Kandasamy, 2014), and 95% TSS removal from rice mill wastewater with an initial pH of 4.5 (Thirugnanasambandham, Sivakumar and Prakash, 2013).
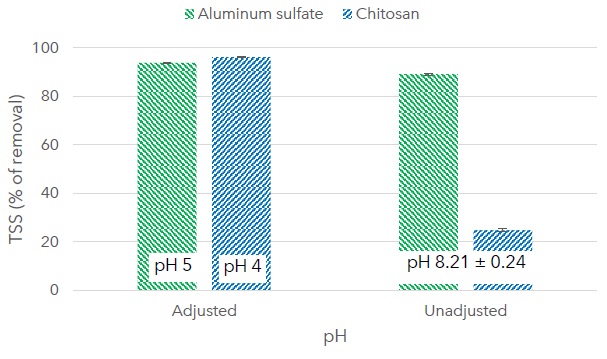
Figure 3: Removal of total suspended solids (TSS) in percentage for aluminum sulfate (100 mg L-1, and adjusted pH 5 units) and chitosan (180 mg L-1, and adjusted pH 4 units). The pH of wastewater was 8.21 ± 0.24 units (unadjusted).
The factors and levels used to evaluate the effect of the operating conditions of coagulation/flocculation are presented in Table 3. Although the optimum pH for coagulation/flocculation with chitosan was found at 4 units, it was decided to carry out experiments at a pH value of 5 units since the addition of aluminum sulfate caused a drop in pH. The observed drop in pH with aluminum sulfate is due to the production of H+ due to the hydrolyzation of aluminum ions Al3+, as follows.
Table 3: Factors and levels used in the evaluation of the operation conditions of coagulation/flocculation.
| Factor | Level | ||
| High | Medium | Low | |
| Aluminum sulfate dose (mg L-1) | 125 | 100 | 75 |
| Chitosan dose (mg L-1) | 225 | 180 | 135 |
| Slow mixing velocity for the first stage of flocculation (rpm) | 77 | 60 | 44 |
| pH | 5 | Unadjusted (pH of wastewater) | |
| Order of addition of coagulants/flocculants | Aluminum sulfate followed by chitosan | Chitosan followed by aluminum sulfate | |
The effect of coagulant dose and pH on the removal of TSS is presented in Figure 4 for the addition of aluminum sulfate followed by chitosan, and in Figure 5 for the addition of chitosan followed by aluminum sulfate. Turbidity removal showed a similar performance.
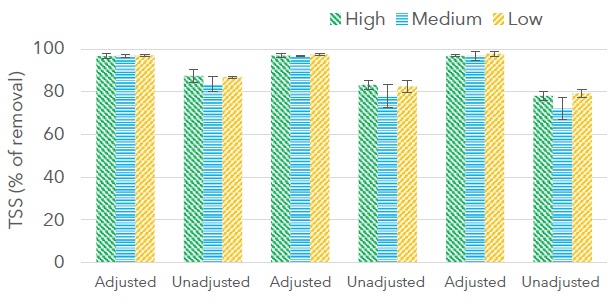
Figure 4: Effect of coagulant dose and pH on removal of total suspended solids for the addition of aluminum sulfate followed by chitosan.

Figure 5: Effect of coagulant dose and pH on removal of total suspended solids for the addition of chitosan followed by aluminum sulfate.
The error bars on the figures represent the standard deviation of duplicate experiments. Removal of TSS presented higher values with the addition of aluminum sulfate followed by chitosan (72.1% - 97.8%) than with the addition of chitosan followed by aluminum sulfate (37.5% - 97.1%). Since chitosan presents higher removal efficiencies in wastewater with acid pH, the drop in pH due to the addition of aluminum sulfate to wastewater had a positive effect on TSS removal with chitosan. Removal of TSS for the addition of aluminum sulfate followed for chitosan was higher than 96.6% when the pH was adjusted (pH 5) and between 72.1% and 86.7% at the pH of wastewater (pH 8.21±0.24). Removal of TSS for the addition of chitosan followed by aluminum sulfate was higher than 90% for experiments at pH 5 and high and medium flocculation velocity, and from 37.5% to 86.5% for experiments carried out at the pH of the wastewater. In comparison, coagulation of a palm oil mill effluent with 3 g L-1 aluminum sulfate and 0.4 g L-1 of chitosan at a pH of 4.51 achieved 99. 69% TSS removal (Jagaba, Abubakar, Lawal, Latiff and Umaru, 2018), and coagulation of a kaolinite solution with a mixture of water-soluble chitosan and aluminum sulfate at a 1:1 mass ratio achieved 90% turbidity removal (Chung et al., 2013). On the other hand, removal of TSS with chitosan was reported at 90% in wastewater (Hassan et al., 2022), >90% in rice mill wastewater with an initial pH of 4.5 (Thirugnanasambandham et al., 2013), and 90% in wastewater from the fish processing industry with an initial pH of 6 (García et al., 2016). It can be concluded that removal efficiencies of TSS with chitosan depend on the type and pH of the treated wastewater, however, high removal efficiencies can be achieved with this material and the addition of chitosan to the coagulation/flocculation process may reduce the use of aluminum sulfate.
Finally, Figure 6 shows that removal of COD (for selected samples) was between 37.1% and 43.8% except for two samples (addition of aluminum sulfate followed by chitosan/high velocity of flocculation/adjusted pH/high dose of coagulants and addition of chitosan followed by aluminum sulfate/low velocity of flocculation/unadjusted pH/high dose of coagulants). Remanent COD could be due to soluble organic compounds that are unaffected by conventional coagulation/flocculation processes.
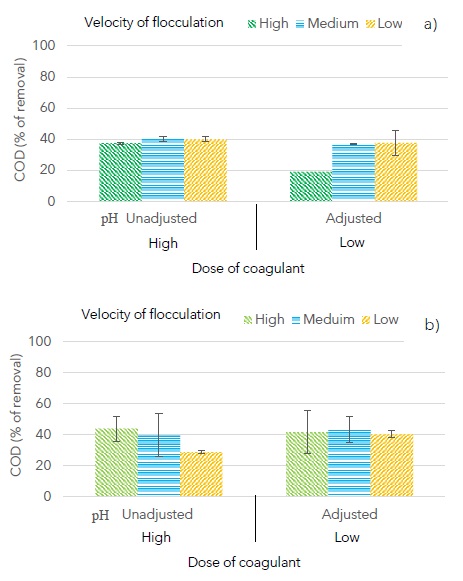
Figure 6: Effect of the order of coagulants addition, coagulant/flocculant dose and pH on removal of chemical oxygen demand, a) addition of aluminum sulfate followed by chitosan, b) addition of chitosan followed by aluminum sulfate.
The ANOVA confirmed that the order of coagulant/flocculant application and adjustment of pH were statistically significant (P-value < 0.05) for the removal of TSS. Also, the interactions of the order of coagulant addition-slow mixing velocity, of the order of coagulant addition-adjustment of pH, and the dose of coagulant-adjustment of pH were statistically significant (P-value < 0.05).
In addition to showing a good performance as a coagulant aid, the economic viability and environmental footprint of chitosan must be investigated. Currently, the commercial cost of chitosan is approximately $133 US dollars per 100 grams (Chitolytic, 2023). However, this cost lowers as the amount of chitosan increases. In comparison, the cost of aluminum sulfate is $1.78 US dollars per kilogram (AquaProducts, 2023). Studies have shown that the production cost of chitosan can be significantly lowered. Riofrio, Alcivar and Baykara (2021) estimated a production cost of $8.39 US dollars per kg with a selling price of $58 US dollars per kg in Ecuador, and Gómez-Ríos, Barrera and Ríos (2017) a production cost of $10.5 -12 US dollars in Colombia. In the future, the production cost of chitosan could decrease as demand increases for this material. Finally, the current cost of aluminum sulfate does not include the environmental and health impacts of this chemical.
On the other hand, qualitative analysis of the environmental performance of chitosan is scarce. A life cycle analysis carried out with data from two real producers of chitosan found that the use of waste reduces the environmental impact of this material (Muñoz, Rodríguez, Gillet and Moerschbacher, 2018). One producer is based in Europe and uses crab exoskeletons for medical-grade chitosan, while the other is based in India and uses shrimp exoskeletons for general-purpose chitosan. In Europe, exoskeletons are treated in a compost facility, and in India are used for animal feed. The highest environmental impacts of the production of chitosan in Europe are climate change, freshwater ecotoxicity, land use, and water use. The use of coal for electricity generation plays a key role in the environmental impact of European chitosan. The highest environmental impacts of the production of chitosan in India are acidification and land use. The negative impact on land use is due to the substitution of exoskeletons by barley and soybean for animal feed. The origin of waste to produce chitosan, the location, and use of the chitosan produced greatly influence the environmental performance of this material.
The production cost and environmental impact of chitosan depends on several factors, nevertheless performance of chitosan as a coagulant aid, cost, and environmental analysis support further investigation of this material.
Conclusions
This research investigated the preparation and use of chitosan as a coagulant aid to reduce the amount of aluminum sulfate used in wastewater treatment.
Chitosan was successfully obtained from shrimp exoskeleton waste, but the yield of the process was relatively low (20%). It is recommended to optimize this methodology to reduce the cost of chitosan.
The coagulation/flocculation experiments confirmed that chitosan can achieve high removal of TSS on its own, but the process is highly dependent on the pH of wastewater and requires a relatively high dose of chitosan (180 mg L-1). Removal of TSS dropped from 96% at a pH of 4 units to 25% at a pH of 8.21 units.
It was also found that the order of addition of coagulants and pH of wastewater were statistically significant (P-value < 0.05) for the removal of TSS in the coagulation/flocculation experiments carried out with the combination of aluminum sulfate and chitosan. Higher removal of TSS was achieved when aluminum sulfate was added followed by chitosan at a wastewater´s pH of 5 units.
This research showed that the dose of aluminum sulfate can be reduced using chitosan as a coagulant aid without affecting removal efficiency for TSS. However, it is necessary to reduce the production cost of chitosan and to study its environmental performance.











 nueva página del texto (beta)
nueva página del texto (beta)




