INTRODUCTION
The yearly growth rate of bivalve culture in Mexico is close to 1.2 % (CONAPESCA 2013), and has been limited by frequent mortalities caused by infectious diseases of different origin, such as the phenomenon known as summer mortality syndrome. This has been associated with the presence and virulence of pathogenic bacteria, or of herpesvirus OsHV-1 (Samain and McCombie 2008, Cáceres-Martínez and Vázquez-Yeomans 2013, Petton et al. 2015). Most of these diseases are related to factors that favor pathogens growth, such as excessive densities or poor feeding conditions (Trabal-Fernández et al. 2014).
Other factors generally associated to mortality outbreaks are high temperatures, low oxygen availability or excessive energetic expenditures, mainly associated to spawning (Cheney et al. 2000, Li et al. 2007, de Decker and Saulnier 2011). There are several studies on the composition and seasonal variations of the bacterial communities associated to bivalve molluscs (Llanos et al. 2002, Romanenko et al. 2008). Some of these studies stress the role of several species of the genus Vibrio, which may affect larvae, juveniles and adults of several aquatic organisms, including oysters, mussels, clams and scallops (Paillard et al. 2004, Beaz-Hidalgo et al. 2010).
In particular, V. splendidus and V. aestuarianus have been associated to the summer mortalities that affect oyster production worldwide (Gay et al. 2004, Garnier et al. 2007, 2008, Labreuche et al. 2010). These and several other Vibrio species, in particular V. parahaemolyticus, are also important vectors of food-borne diseases for marine shellfish consumers (Daniels et al. 2000, McLaughlin et al. 2005, García-Lázaro et al. 2010, Kokashvili et al. 2015).
This indicates the importance of gaining better knowledge on the composition and seasonal variations of the bacterial communities associated to cultured and wild populations of commercially important bivalves.
The cultivable Vibrio flora harbored in healthy oysters is a bivalve-specific community with densities ranging from 102 to 104 colony forming units (CFU)/mg of tissues, and higher loads may be expected at higher temperatures (Saulnier et al. 2010). However, if bacterial concentrations are too high, they may induce negative stress, with consequent high mortalities and low condition indices (Harekrishna et al. 2014). Additionally there is a general lack of information on the structure and persistence of the bacterial community present in wild and cultivated oysters of commercial interest, such as C. corteziensis and C. sikamea.
This study aimed to determine the concentration and taxonomy of culturable heterotrophic bacteria present in the oysters Crassostrea corteziensis and C. sikamea cultured in Cospita Bay, Sinaloa, NW Mexico, and of the bacterioflora suspended in the surrounding water.
MATERIALS AND METHODS
Cospita Bay lies within coordinates 24º 04’ 30” to 24º 05’ 65” N and 107º 08´ 42’’ to 107º08´06” W. Samples were obtained at quarterly intervals from May 2014 to January 2015. Two sets were from the dry period (M1: dry-hot, May 7th 2014; and M4: dry-cold, January 7th, 2015), and two from the warm-rainy season (M2 and M3, August 29th and October 1st, 2014). Samples of C. corteziensis and C. sikamea (30 of each species) were donated by a local commercial oyster farm. Wild C. corteziensis (30, from roots of different mangroves) and several water samples (mixed in equal parts to give one-L composite sample) were also obtained in sterile containers from areas surrounding each sampling point. Surface salinity, temperature and oxygen concentration were determined in situ in the central area of the oyster farm using a refractometer and an YSI 55 dissolved oxygen meter.
Oyster and water samples were transported in ice boxes (< 7 ºC) to the laboratory, where 100 µL of the original water sample and of its 1:10 dilution were spread plated in triplicate on Zobell medium for total culturable marine bacteria (2216), on cetrimide Pseudomonas medium and on 2.5 % NaCl-added TCBS for Vibrio-like bacteria detection. DIFCO media were used for all isolation and purification work.
Oysters were thoroughly washed and brushed under running tap water, rinsed with sterile distilled water (DW) and each sample of 30 oysters was divided into five groups of six oysters chosen at random. The oysters of each group were shucked and the liquor and soft parts were homogenized for 90 s in 450 mL of 3 % saline solution using a sterile food blender. The resulting suspension was used to prepare four serial dilutions (10-2 to 10-5), which were spread-plated (100 µL) in the same growth media used for water samples. All plates were read after 48 h at 30 ± 2 ºC and the results served to calculate bacteria concentrations in seawater (CFU/mL) and in the oysters’ soft tissues (CFU/g, wet weight).
Colonies with different morphologies, spread-plated on the respective growth medium, were purified with the cross streak technique. Five-six colonies were preserved in 1.5 mL Eppendorf vials with 1 mL of 96 % ethanol, and used later for their identification using molecular techniques. The original purified colonies were considered pure strains, assigned an identification key and preserved in glycerol at -80 ºC in the laboratory’s bacterial cultures collection.
For species identification, the DNA of each ethanol-preserved strain was extracted with the Wizard®Genomic DNA Purification Kit (lot A1120, Promega). After adjusting concentration to 50 ng/µL, the ribosomal 16S gene (16S ARNr) was amplified using end-point polymerase chain reaction (PCR) and universal primers Forward 27f.1 (AGR GTT TGA TCM TGG CTC AG) and Reverse 1492R2 (GGT TAC CTT GTT ACG ACT T). The amplification program was: 16S: 94 ºC/2’ → 35 cycles (94 ºC/1’ → 56 ºC/1’ → 72 ºC/1’) → 72 ºC/5’ → 4 ºC /∝.
The products obtained were sequenced at Macrogen (Republic of Korea), and the sequences obtained were used for species identification with the public databases BLAST (Altschul 1997) and EzTaxon (Chun et al. 2007).
The sequences obtained in FASTA format were aligned with program ClustalW (Thompson et al. 1994). The alignments were exported to version 5.0 of the program MEGA andin order to obtain the molecular phylogenetic tree of each strain (Tamura et al. 2011). Tusing the neighbor-joining test (NJ) (Saitou and Nei 1987) with the p-distance method and 500 bootstrap repetitions was used to that end, considering transitions and transversions. The bacterium Klebsiella pneumonia served as outgroup.
All bacterial concentrations, transformed to the respective R1 rank values (Conover 2012), were normal and homoscedastic (Kolmogorov-Smirnov and Bartlett’s tests). Therefore, the mean concentrations of the bacteria present in the water samples, determined with each of the three growth media in the four sampling dates, were compared using one-way ANOVA tests, separating the different means with Tuckey’s tests. Those of the bacteria present in wild and cultured oysters were compared with two-ways ANOVA and Tuckey’s tests. All statistical tests were performed with SigmaPlot 11.0 software. In all cases, the significance level was α = 0.05 (Zar 1996).
RESULTS
Temperature and salinity values ranged from 32.3 to 23.2 ºC and 35.0 to 23.2 ppt, respectively. In both cases, differences were significant. Oxygen concentrations were significantly higher in the first two sampling dates than in the remaining samplings (8.09 and 7.46, compared to 4.87 and 5.35 mg/L). In all cases differences were not clearly related to climatic events (Table I).
TABLE I MEAN (±SD) TOTAL MARINE AND Vibrio-LIKE BACTERIA CONCENTRATIONS IN 103 COLONY FORMING UNITS/mL (CFU/mL), AND MEAN TEMPERATURE, SALINITY AND DISSOLVED OXYGEN OF THE WATER SAMPLES OBTAINED IN COSPITA BAY ON THE FOUR SAMPLING DATES
| Sampling | Temp. (ºC) | Salinity (ppt) | O2 (mg/L) | CFU/mL | |
| Total* | Vibrio | ||||
| (M1) Dry-warm | 27.5 ± 0.3ab | 35.0 ± 1.0a | 8.09 ± 1.8a | 0.87 ± 0.25a | 0.31 ± 0.18ab |
| (M2) Wet-warm | 32.3 ± 0.2a | 23.2 ± 1.5b | 7.46 ± 2.1a | 3.92 ± 2.87a | 0.13 ± 0.12b |
| (M3) Wet-warm | 28.2 ± 0.5ab | 33.5 ± 0.7a | 4.87 ± 0.9b | 2.36 ± 1.75a | 0.14 ± 0.13ab |
| (M4) Dry-cold | 23.2 ± 0.3b | 33.3 ± 0.9a | 5.35 ± 1.1b | 8.77 ± 0.10a | 0.87 ± 0.35a |
* Non parametric test. Different letters indicate significant differences between data in the same column (p = 0.05, a ≥ ab ≥ b and a > b)
The concentrations of culturable marine bacteria ranged from 875 to 3920 CFU/mL, and there were no differences between sampling dates. Those of Vibrio-like bacteria varied from 127.50 to 875 CFU/mL. These were significantly different, and were determined in M2 and M4 samples, respectively (Table I). The cetrimide medium gave negative results in all water samples.
The concentrations of total marine bacteria tended to be higher in the samples of the rainy than in the dry season, although with overlapping values in dry-cold samples of C. corteziensis and dry-warm samples of C. sikamea, respectively (Table II). The tendency was similar in the case of Vibrio-like bacteria. For C. corteziensis differences between rainy and dry seasons were significant in all cases, while for C. sikamea the lowest and highest values were observed in the samples of the dry-warm and dry-cold season, respectively (Table II).
TABLE II MEAN (± SD) BACTERIAL CONCENTRATIONS (IN 103 CFU/mL) OF Crassostrea corteziensis AND C. sikamea DETERMINED ON THE FOUR SAMPLING DATES M1 TO M4 IN COSPITA BAY
| M1 | M2 | M3 | M4 | |
| Marine bacteria | ||||
| C. corteziensis | 1.86 ± 0.62b | 7.20 ± 6.88ab | 31.88 ± 17.60a | 3.82 ± 0.50b |
| C. sikamea | 0.71 ± 0.36b | 20.60 ± 12.86a | 18.93 ± 14.11a | 9.99 ± 1.00ab |
| Vibrio | ||||
| C. corteziensis | 0.15 ± 0.05c | 4.15 ± 3.27b | 12.35 ± 11.45b | 0.51 ± 0.24c |
| C. sikamea | 0.06 ± 0.03c | 14.88 ± 11.44b | 13.86 ± 9.03ab | 27.33 ± 2.71a |
| Pseudomonas | ||||
| C. corteziensis | 0.06 ± 0.01bc | 0.30 ± 0.15b | 0.07 ± 0.01b | 0.03 ± 0.02c |
| C. sikamea | 0.02 ± 0.01c | 0.38 ± 0.20b | 0.18 ± 0.14abc | 2.28 ± 0.23a |
Different letters indicate significant differences between data in the same row (p = 0.05, a > b)
CFU: colony forming units
Pseudomonas showed the same species-related trends. The lowest and highest mean annual values were recorded in the dry-warm and dry-cold samples in C. sikamea, while in C. corteziensis mean concentrations were higher in the rainy than in the dry season samples (Table II).
In the available samples of the dry period (M1 and M4), bacterial loads were significantly lower in cultured than in wild oysters. In these, marine and Vibrio-like bacteria were significantly higher in the dry-cold, rather than in the dry-warm samples. In cultured oysters, there were no differences between the mean concentrations of Pseudomonas and marine bacteria in samples obtained in the warm or in the cold period while that of Vibrio-like bacteria was significantly higher in the dry-cold samples (Table III).
TABLE III MEAN (± SD) BACTERIAL CONCENTRATIONS (103 CFU/g) OF CULTURED AND WILD Crassostrea corteziensis OF COSPITA BAY IN THE SAMPLES OF THE DRY SEASON M1 (WARM) AND M4 (COLD)
| Cultured | Wild | |
| Marine bacteria | ||
| M1 Dry-warm | 1.86 ± 0.62c | 34.20 ± 18.84b |
| M4 Dry-cold | 3.82 ± 0.50c | 1416.66 ± 381.88a |
| Vibrio | ||
| M1 Dry-warm | 0.08 ± 0.08d | 1.05 ± 0.74ᵇ |
| M4 Dry-cold | 0.51 ± 0.24c | 40.99 ± 0.00a |
| Pseudomonas | ||
| M1 Dry-warm | 0.04 ± 0.02b | 0.57 ± 0.16a |
| M4 Dry-cold | 0.06 ± 0.06b | 2.88 ± 3.72a |
Different letters indicate significant differences (p = 0.05, a > b > c > d)
CFU: colony forming units
Bacteria identification
The number of strains isolated for their morphologies was notably lower in the dry (cold + warm) (M1 + M4 = 95) than in the rainy season (M2 + M3 = 225) samplings. Among these, molecular techniques allowed identification of 84 sequences with 90-100 % similarity to those of taxonomic entities available in public databases (Fig. 1).

Fig. 1 DNA 16s-based phylogenetic tree of the 84 bacterial strains isolated in Cospita Bay oyster and water samples
The families identified in M2 and M3 samplings in both oyster species were Vibrionaceae, Bacillaceae, Brucellaceae, Micrococcaceae, Pseudoalteromonaceae, Rhodobactereceae and Staphylococcaceae, and the most frequent was Vibrionaceae (76 and 71 % of all sequences observed in M2 and M3, respectively), followed by Bacillaceae (8.16 %) and Brucellaceae (25.71 %) in M2 and M3, respectively (Fig. 2).
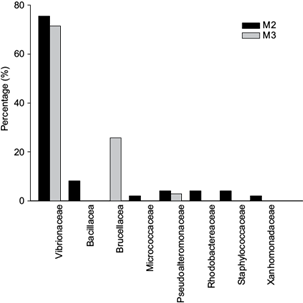
Fig. 2 Bacterial families identified in wild and cultured oysters and in Cospita Bay water samples during the wet-warm season (M2 and M3 samples)
The number of bacteria species observed exclusively in samples of one oyster species was higher for C. sikamea (seven: Vibrio neptunius, V. harveyi, V. diabolicus, V. parahaemolyticus, V. ponticus, V. alfacsensis and Bacillus firmus), than for C. corteziensis (four: Vibrio sinaloensis, Bacillus sp., B. clausii and Micrococcus yunnanensis). Seven species (families Xanthomonadaceae, Rhodobacteraceae, Staphylococcaceae, Vibrionaceae and Bacillaceae) were found only in water samples (Lysobacter spongicola, Donghicola sp., D. eburneus, Bacillus sp., Staphylococcus cohnii, S. warneii and V. hepatarius) (Table IV).
TABLE IV BACTERIAL STRAINS (M) ISOLATED FROM Crassostrea corteziensis, C.sikamea AND COSPITA BAY WATER SAMPLES
| Strain | Identification | C. corteziensis | C. sikamea | water |
| 182 | Vibrio sp. | M2 | ||
| 183 | Vibrio sp. | M2 | ||
| 187 | Vibrio mediterranei | M2 | ||
| 190 | Vibrio sp. | M2 | ||
| 192 | Vibrio mediterranei | M2 | ||
| 195 | Vibrio sinaloensis | M2 | ||
| 196 | Vibrio neptunius | M2 | ||
| 197 | Vibrio harveyi | M2 | ||
| 200 | Vibrio brasiliensis | M2 | ||
| 201 | Vibrio sp. | M2 | ||
| 203 | Vibrio hepatarius | M2 | ||
| 206 | Vibrio rotiferianus | M2 | ||
| 207 | Vibrio rotiferianus | M2 | ||
| 208 | P. flavipulchra | M2 | ||
| 209 | Lysobacter spongiicola | M2 | ||
| 210 | Donghicola sp. | M2 | ||
| 211 | Bacillus firmus | M2 | ||
| 212 | Vibrio owensii | M2 | ||
| 213 | Vibrio hepatarius | M2 | ||
| 214 | Bacillus sp. | M2 | ||
| 218 | Vibrio diabolicus | M2 | ||
| 220 | Vibrio owensii | M2 | ||
| 222 | Donghicola eburneus | M2 | ||
| 225 | Bacillus sp. | M2 | ||
| 226 | Vibrio harveyi | M2 | ||
| 227 | P. flavipulchra | M2 | ||
| 228 | Vibrio brasiliensis | M2 | ||
| 229 | Vibrio sp. | M2 | ||
| 230 | Vibrio communis | M2 | ||
| 231 | Vibrio brasiliensis | M2 | ||
| 241 | Vibrio harveyi | M2 | ||
| 242 | Vibrio parahaemolyticus | M2 | ||
| 245 | Vibrio communis | M2 | ||
| 247 | Staphylococcus cohnii | M2 | ||
| 248 | Vibrio hepatarius | M2 | ||
| 250 | Vibrio owensii | M2 | ||
| 263 | Micrococcus yunnanensis | M2 | ||
| 267 | Bacillusclausii | M2 | ||
| 300 | Vibrio rotiferianus | M2 | ||
| 302 | Vibrio rotiferianus | M2 | ||
| 303 | Vibrio rotiferianus | M2 | ||
| 305 | Vibrio rotiferianus | M2 | ||
| 311 | Vibrio rotiferianus | M2 | ||
| 315 | Vibrio ponticus | M2 | ||
| 318 | Vibrio rotiferianus | M2 | ||
| 322 | Vibrio communis | M2 | ||
| 327 | Vibrio rotiferianus | M2 | ||
| 328 | Vibrio rotiferianus | M2 | ||
| 330 | Staphylococcus warneri | M2 | ||
| 337 | Vibrio rotiferianus | M3 | ||
| 341 | Vibrio alfacsensis | M3 | ||
| 350 | Vibrio rotiferianus | M3 | ||
| 351 | P. flavipulchra | M3 | ||
| 353 | Vibrio mediterranei | M3 | ||
| 359 | Vibrio brasiliensis | M3 | ||
| 360 | Vibrio mediterranei | M3 | ||
| 372 | Vibrio rotiferianus | M3 | ||
| 391 | Vibrio mediterranei | M3 | ||
| 394 | Vibrio mediterranei | M3 | ||
| 401 | Vibrio brasiliensis | M3 | ||
| 407 | Vibrio communis | M3 | ||
| 410 | Vibrio sp. | M3 | ||
| 414 | Vibrio owensii | M3 | ||
| 427 | Vibrio communis | M3 | ||
| 437 | Vibrio xuii | M3 | ||
| 457 | Vibrio natriegens | M3 | ||
| 467 | Vibrio communis | M3 | ||
| 468 | Ochrobactrum cytisi | M3 | ||
| 473 | Ochrobactrum cytisi | M3 | ||
| 474 | Ochrobactrum cytisi | M3 | ||
| 475 | Vibrio owensii | M3 | ||
| 479 | Vibrio rotiferianus | M3 | ||
| 481 | Vibrio diabolicus | M3 | ||
| 494 | Ochrobactrum cytisi | M3 | ||
| 500 | Vibrio natriegens | M3 | ||
| 507 | Ochrobactrum cytisi | M3 | ||
| 508 | Vibrio diabolicus | M3 | ||
| 514 | Vibrio rotiferianus | M3 | ||
| 522 | Ochrobactrum cytisi | M3 | ||
| 533 | Ochrobactrum cytisi | M3 | ||
| 561 | Vibrio communis | M3 | ||
| 570 | Vibrio owensii | M3 | ||
| 574 | Ochrobactrum cytisi | M3 | ||
| 576 | Ochrobactrum cytisi | M3 |
DISCUSSION
Silva-Neta et al. (2015) detected higher bacterial concentrations at the end of the dry season, which corresponds to our M1 sampling, while the general tendency to higher bacteria concentrations in oyster tissues in the rainy season, and the correspondingly lower values observed in C. corteziensis during the dry season, seem to agree with the direct relationship with water temperature described by Deepanjali et al. (2005) and Aagesen and Häse (2014) for V. parahaemolyticus concentrations, or for aerobic heterotrophs and Vibrio-like bacteria determined by Fay et al. (2012).
When they were collected, concentrations of heterotrophic bacteria were higher in wild than in cultured oysters, possibly because of the high levels of organic matter and bacterial concentrations common in their environment (Mahasneh 2001). An additional explanation is the stress caused by tide-dependent exposures (Bishop and Peterson 2006, Farcy et al. 2009), which might have caused an increase in the internal bacterial load. The absence of wild organisms during the rainy period was due to mortalities related to high energy expenditure caused by early summer spawning (Frías-Espericueta et al. 1997) or to stress induced by high temperatures and low salinities. Additionally, summer conditions seem to favor the parasite Perkinsus marinus, whose presence and high incidence was detected in the area object of this study (Cáceres-Martínez et al. 2012, Cáceres-Martínez and Vázquez-Yeomans 2013).
Summer mortalities with similar etiologies have been documented in different geographic areas for several oyster species such as C. hongkongensis (Wang et al. 2016), C. gigas (Li et al. 2007), C. virginica (Soniat et al. 2006, Heilmayer et al. 2008) and C. ariakensis (Kelly et al. 2011). In all these cases, increasing temperatures would seem to be the driving factor, either causing an increase of energy losses or favoring bacterial or parasite establishment and growth.
Although its presence and abundance should be confirmed with long-term regular observations, the dominance of Vibrionaceae in both oyster species in the warm rainy period coincides with the indication that Vibrio spp. are the most abundant bacteria in oysters during summer months (Pujalte et al. 1999, Wang et al. 2016). Among the species of Vibrio detected in C. sikamea, some are known pathogens for invertebrates (V. parahaemolyticus, V. hepatarius, V. brasilensis, V. neptunius and V. owensii [Ruangpan and Kitao 1991, Thompson et al. 2003, Cano-Gómez et al. 2010, Rivera-Posada et al. 2011, Gómez-Gil et al. 2012]), while others, such as V. xuii, V. ponticus, V. alfacsensis and V. harveyi may affect vertebrates (Pujalte et al. 2003, Thompson et al. 2003, Xie et al. 2007). Among these pathogens, V. neptunius was isolated in cultures of diseased Nodipecten nodosus larvae (Thompson et al. 2003), and it may cause high mortalities in mussel larvae (Kesarcodi-Watson et al. 2009) and in oyster hatcheries (Prado et al. 2005).
Known pathogens for molluscs were not detected in C. corteziensis. However, the bacteria V. sinaloensis, Micrococcus yunnanensis, Bacillus clausii and V. natriegens, which were detected only in this oyster, are known pathogens of Lutjanus guttatus, Litopenaeus vannamei and Acanthaster planci (Gómez-Gil et al. 2008, Flores-Miranda et al. 2011, Rivera-Posada et al. 2011).
Of all known pathogenic Vibrio of major medical interest listed by Daniels and Shafaie (2000), only V. parahaemolyticus was present in our oyster samples. Among those isolated from surface waters, the coagulase-negative Staphylococcus warneri and S. cohnii are of mild clinical interest as possible sources of nosocomial infections (Martínez and Máttar 2006, Fariña et al. 2013, Soldera et al. 2013), while Lysobacter spongiicola pertains to a genus of potential importance for its production of several extracellular enzymes (de Bruijn et al. 2015), and because several Lysobacter species have been suggested for biological plant disease control (Hashizume et al. 2004, Li et al. 2008).











 nueva página del texto (beta)
nueva página del texto (beta)


