INTRODUCTION
Pollution caused by potentially toxic elements (PTE) in aquatic environments constitutes a special problem because these elements are persistent and bioaccumulate through food chains (Monferrán et al. 2016, Ali and Khan 2018). Even though some of the PTE can be classified as essential (copper [Cu], zinc [Zn]) because they are necessary for organisms, others are non-essential and can be considered harmful even at low concentrations (arsenic [As], cadmium [Cd], chrome [Cr], lead [Pb], mercury [Hg]) because they have no role in biological systems (Lemus et al. 2014, Castaldo et al. 2020). However, the toxicity of each element depends on its concentration and chemical form (Ali et al. 2021).
Pollution of PTE can occur from different sources, including agriculture runoff, industrial effluents, sewage sludge, municipal waste, landfills, mining, and chemical disposal (Abarshi et al. 2017, Liu et al. 2020). Also, these elements are released from natural and geological sources such as volcanic eruptions or weathering of rocks (Ali and Khan 2018, Liu et al. 2020) causing an increase in the pollution of rivers and lakes (Yi and Zhang 2012), where PTE can harm and also bioaccumulate in aquatic organisms, especially in fish tissues (Yang et al. 2021). Fish are generally associated with high nutritional quality and benefits for human health; however, their consumption with high concentrations of PTE represents a risk to public health (Monferrán et al. 2016, da Silva et al. 2021) since it can affect the immune system and produce hepatoxicity, neurological diseases, and reproduction problems (Töre et al. 2021). Due to the accumulation of PTE in fish tissues, the studies regarding this matter have increased worldwide to improve public health (Liu et al. 2020).
In Durango, Mexico, El Tunal River is of vital importance for its inhabitants since it is used to supply hydric resources for agricultural, livestock, and domestic activities. Moreover, this river constitutes a natural drainage system, provided by rainwater, runoff, and groundwater flows that cross the state of Durango. It collects water from the southwest of the western plateau, penetrating through the Sierra Madre Occidental, where it receives the name of El Mezquital River, which later leads into the state of Nayarit with the name of San Pedro River (CONAGUA 2020).
However, Durango is a state with evidence of mining activity since pre-Columbian times. Durango reported extraction of 13 251 kg of gold, 921 560 kg of silver, 6442 t of copper, 3 352 000 tons of iron, 26 179 tons of lead, and 19 502 tons of zinc, according to the recent statistics published by the Mexican Geological Service (SGM 2021). In 2019, mining concessions occupied 9.8 % of the state’s surface and were organized in 23 mining regions, of which three (Durango, Mezquital, and La Parrilla) are located in the area of influence of El Tunal River and report the extraction of Au, Ag, Cu, Fe, Pb, and Zn (SGM 2021). Besides mining activities, other sources of pollution in this river are the discharges of domestic wastewater (28.6 % of the inhabitants of the city of Durango), as well as industrial (8000 m³/day) and agricultural activities, and dumping of significant amounts of trash by people (Vicencio 2004). Also, Durango has reported high values of As in drinking water (0.90-245.10 ug/L), which is originated by natural sources (Osuna-Martínez et al. 2021).
Currently, government research efforts are focused on the analysis of physical and chemical parameters of water at different points along El Tunal River (CONAGUA 2009, SEMARNAT 2013), but there is no available information on how these parameters affect the different aquatic organisms. It is well known that water quality is deteriorated by the presence of toxins and pathogens, decomposition of organic matter, accumulation of sediments, excessive nutrient content, and loss of scenic and recreational value. Nevertheless, it is important to point out that the quantification of contaminants in soil, water, sediments, or air is not sufficient to understand the bioavailability processes, bioaccumulation, or effects on organisms. For this reason, it is vital and urgent to implement a biomonitoring system that allows an evaluation of risks to aquatic organisms present in El Tunal River.
In this regard, a method of measurement is biomonitoring, a process in which living organisms that are sensitive to environmental conditions or accumulate pollutants in their tissues allow for the evaluation of a polluted ecosystem (Lemus et al. 2014). Specifically, fish are considered a good indicator of PTE (Rajeshkumar and Li 2018) since they are at the mid-end of the trophic level and can bioaccumulate pollutants from water and sediments (Mziray and Kimirei 2016, Abarshi et al. 2017).
The common carp (Cyprinus carpio) is a freshwater fish species often used as a bioindicator (Has-Schön et al. 2015, Ali et al. 2021, Fernández-Trujillo et al. 2021). This species is highly variable in shape, proportions, and color. They are omnivorous, feeding on aquatic insects, crustaceans, annelids, mollusks, detritus, weeds and tree seeds, aquatic plants, and algae. Also, this species is a sediment digger and lives on the muddy bottom (CONABIO 2017). They can survive in waters with low oxygen concentrations, which is why they have been introduced and distributed throughout the world to support commercial and recreational fishing (Arlinghaus and Mehner 2003). However, in Mexico C. carpio is considered a high-impact invasive exotic species (CONABIO 2017).
Although there is a high population of carp in El Tunal River, there is no available data for monitoring research on PTE. Therefore, this work aimed to carry out an exploratory study to determine the seasonal variations in the concentration of PTE, including arsenic As, Cd, Cr, Cu, Pb, Hg, and Zn, in muscle and liver of the common carp (C. carpio) that inhabits El Tunal River area, as well as estimating the possible human health risks due to its consumption.
MATERIALS AND METHODS
Sampling site and collecting method
El Tunal River is located in the State of Durango in the northern part of Mexico (24.0277 N-104.653 W), with a length of 346 km and a 18 021 km2 basin (CONAGUA 2020). The collection site is proximal to an agricultural and fishing area known as Francisco Villa Viejo Village (24.068924 N-104.494161 W). Furthermore, this site is located approximately 25 km from a wastewater treatment plant (Fig. 1).
A total of 17 samples of common carp (C. carpio) were collected in March during the dry season (n = 7), and in October during the rainy season (n = 10). An effort was made to collect samples with similar sizes (36.00 ± 2.76 cm, 30.60 ± 2.75 cm, respectively) and weights (726.80 ± 176.46 g, 515.90 ± 123.89 g, respectively). Fish were captured with the help of local fishermen and anesthetized with tricaine metasulfonate through the immersion method. Later, fish were sacrificed using ice to reduce their metabolism and minimize the mobilization of PTE between organs and tissues. Subsequently, they were transported to the Diagnostic Support Laboratory of the Faculty of Veterinary Medicine of Juárez University of the State of Durango. Afterwards, length and mass were measured and a necropsy was carried out for the complete extraction of liver and muscle tissue.
Sample processing
Tissues of liver and muscle were weighed using a precision analytical balance (Sartorious, LE225D), then dried in a forced-air oven (Thermo Scientific model 6964) at a constant temperature of 40 ºC until a constant weight was obtained. Afterward, the dry weight was measured and samples were sent to the laboratory of the Institute of Ecology, Fisheries and Oceanography (EPOMEX) of the University of Campeche for the analysis of inorganic elements. Measurements were performed with a voltammetric analyzer 797 VA Computrace Metrohm Multi-Mode Electrode (Metrohm 2009).
The 797 VA analyzer uses the redissolution technique and provides lower detection limits for metals (10-9-10-12). Thus, it detects metal ions with reasonable accuracy. The redissolution method is based on the concept that the substance to be determined is pre-concentrated at the electrode and then returned to the solution in a reverse process (Metrohm 2009, Cáceres 2014). The total detection limits (ng/kg or ppt) of the analyzer are As (100), Cd (50), Cr (25), Cu (50), Pb (50), Hg (100), and Zn (50). Total concentrations of the elements were compared to the maximum limits allowed by Mexican Official Standard NOM-242-SSA1-2009 (SSA 2011) and by international standards (FAO 1983, CR 2006).
Risk assessment
The concentration of PTE in muscle tissue was used to estimate the target hazard quotient (THQ) index, which estimates non-carcinogenic risk due to high exposure to PTE. It is calculated by the following equation (Töre et al. 2021):
where THQ is the target hazard quotient index; EF is the exposure frequency (365 days/year); ED is the exposure duration considering an average human life of 75 years; FIR is the food intake rate (according to the National Fisheries and Aquaculture Commission [CONAPESCA 2018] the daily consumption of freshwater fish per capita in Mexico is 35.3 g/day); C is the concentration of elements (w/w, mg/kg); RfD is the oral reference dose, which assesses the health risk of fish consumption in mg/kg/day (As = 0.0003, Cd = 0.001, Cr = 0.003, Cu = 0.04, Hg = 0.001, Pb = 0.004, Zn = 0.3); BW is the mean adult body weight of adults (70 kg), and ET is the non-cancerous exposure time (365 day/year × ED) (USEPA 2000).
For the multiple PTE health risks, the following equation was used:
where TTHQ is the total target hazard quotient index. A THQ value < 1 is safe for consumers, and a THQ value > 1 denotes an imminent risk of fish consumption (Maurya et al. 2019, da Silva et al. 2021, Töre et al. 2021).
Statistical analysis
Data obtained on the concentration of PTE in liver and muscle tissues were processed to calculate the mean and standard deviations for PTE concentrations. The statistical analyses were performed with concentrations in wet weight (w/w). A normality test of data was applied through the Shapiro-Wilks test. Statistical comparisons were carried out with the non-parametric Kruskal-Wallis test to establish significant differences in the PTE concentration between seasons and tissue accumulation of the fishes (p < 0.05, Z > 1.96). Additionally, a principal component analysis (PCA) and Spearman’s correlation test (rs) were used to determine the association between elements, seasons, and sources of PTE. All statistical analyses were developed in R studio V4.1.2 (RDCT 2021).
RESULTS AND DISCUSSION
Concentration of potentially toxic elements in fish
The results demonstrated that all PTE analyzed (As, Cd, Cr, Cu, Hg, Pb, Zn) were detected to some extent in all muscle and liver samples (Fig. 2). During the dry season the order in the PTE concentration decrease was Zn > As > Cr > Cu > Pb > Cd > Hg, while in the rainy season it was As > Zn > Cr > Cu > Pb > Cd > Hg, where only the order of As and Zn changed. Furthermore, the Kruskall-Wallis test showed differences between seasons in As (p = 0.005), Cd (p = 0.005), Cr (p = 0.004), Zn (p = 0.001), and Pb (p = 0.005), as shown in figure 2. As and Zn were the most predominant PTE in fish, regardless of the season. On this matter, it is important to analyze the potential sources of PTE to understand their distribution and risk factors. The analysis of PCA (Fig. 3) and Spearman’s correlation (Fig. 4) were used to estimate the potential associations between elements.
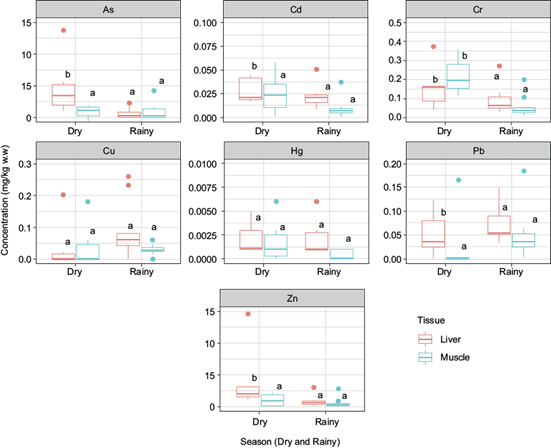
Fig. 2 Box-plot (mean, 25-75 % quartiles, range, and outliers) of potentially toxic elements concentrations in the liver and muscle of Cyprinus carpio during the dry and rainy seasons. Different letters indicate p < 0.05 of tissues between season.
The first PCA component of the dry season describes 39.5 % of the variation, and it is representative of As, Cu, Hg, and Zn (Fig. 3a). Main sources of these elements can be related to agriculture since it is one of the main activities developed near the river, followed by mining (SGM 2021), and dead livestock near the river and municipal wastewater discharges (Vicencio 2004). Also, it is well known that fish are PTE bioaccumulators and are associated with the consumption of organisms near sewage effluents (Mziray and Kimirei 2016, Miao et al. 2021). The contribution of As is higher compared to the rest of the PTE. This could be explained by an increase in groundwater extraction, causing the oxidation and subsequent rise of sulfide minerals such as AsFeS2, due to the presence of oxygen (Martínez-Cruz et al. 2020).
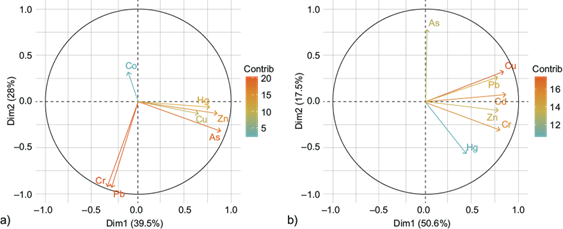
Fig. 3 Principal component analysis of potentially toxic elements in tissues of Cyprinus carpio. (a) Dry season, (b) rainy season.
The second component of the dry season describes 28 % of the total variability and it is composed of Cr, Cd, and Pb. Nevertheless, the Cd contribution is low and not representative of the PCA. Cr and Pb are highly associated in this component (Fig. 4), therefore, they can be related to natural geological sources such as Earth’s crust, rocks, and soils that enter the aquatic system through erosion and leach from the soil, as reported by other studies (Jabeen and Chaudhry 2010, Maurya et al. 2019, da Silva et al. 2021, Nassiri et al. 2021). Also, it has been reported that PTE concentrations tend to increase during the dry season due to the sedimentation of elements as the water volume of the system is reduced (Ali and Khan 2018, Góngora-Gómez et al. 2018). Hence, water tends to stagnate in some stretches of El Tunal River due to a lack of precipitations, which promotes the growth of algae as well as the sedimentation and suspension of elements, reducing the quality of the water mainly by wastewater discharges (Sánchez-Martínez 2012).
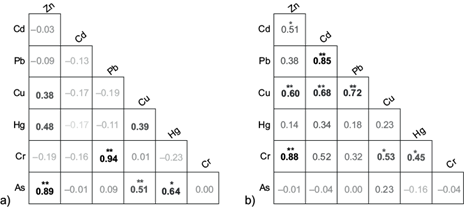
Fig. 4 Spearman’s correlation matrix between elements concentrations in tissue of Cyprinus carpio. (a) Dry season, (b) rainy season. *p < 0.05; **p < 0.01.
On the other hand, the first component of the rainy season describes 50.6 % of the variation, and it is representative of Cu, Cd, Cr, Hg, Pb, and Zn (Fig. 3b), implying a similar origin. This correlation may occur due to a common anthropogenic source, such as mining, agricultural runoffs, soil leaching, mining activity, or inappropriate disposal of wastes (Jabeen and Chaudhry 2010, SGM 2021). During the dry season, agricultural soils are irrigated with municipal wastewater and, depending on the production, application of fertilizers and soil improvers. This might result that during the rainy season there is the possibility of soil leaching of the elements into the river.
The second component of the rainy season describes 17.5 % of the variance and is represented by As, implying its contribution by a natural source (Nassiri et al. 2021, Osuna-Martínez et al. 2021). It has been reported that most of As presence in water bodies is related to geogenic origin such as rock weathering, especially in arid or semi-arid areas (da Silva et al. 2021, Osuna-Martínez et al. 2021). North-central arid states such as Durango, Chihuahua, or Zacatecas have a higher exposure risk to As than arid or humid states (Alarcón-Herrera et al. 2020). Also, the correlations of most PTE indicate that the effect of high dilution of elements might be more significant in the rainy season than the dry season because of the flow velocity of water.
According to the Agency for Toxic Substances and Disease Registry (ATSDR 2022), the order of PTE threatening to human health is As > Pb > Hg > Cd > Zn > Cr > Cu. Species like C. carpio have feeding habits that include phytoplankton, which helps to distribute and cycle As with the possibility to accumulate and transform inorganic As (Fernández-Trujillo et al. 2021). Usually, it is common to find organic As in higher amounts (above 90 %) in the form of arsenobetaine, which is considered non-toxic (da Silva et al. 2021). On the other hand, inorganic As (As III or As IV) is more dangerous and responsible for health risks such as cardiovascular, kidney, and hematological diseases (Osuna-Martínez et al. 2021).
One of the elements with no biological functions in organisms is Pb. It is known for its negative effects on human health, causing, among others, neurotoxicity and hepatotoxicity (Abarshi et al. 2017). Nonetheless, Pb accumulates poorly in fish muscle (Jabeen and Chaudhry 2010), which agrees with the results of this study and previously reported data (Yousafzai et al. 2017). Likewise, Hg is one of the most monitored PTE in fish (Jabeen and Chaudhry 2010). Goldstein et al. (1996) stated that it was common to find higher concentrations of Hg in muscle than in liver tissue when concentrations in the former were below 0.5 mg/kg, which disagrees with our results.
Also, it has been reported that fish from slightly polluted areas present lower concentrations of Hg in muscle rather than in liver (Čelechovská et al. 2007). Thus, the results obtained in this study show that El Tunal River has no evidence of significant concentrations of Hg. Similarly, Cd is lethal to humans, since it produces effects such as chronic lung disease and testicular degeneration (Abarshi et al. 2017). Cd has been highly found in tissues of C. carpio because this species can accumulate and tolerate high concentrations of this element (de Conto et al. 1999, Has-Schön et al. 2015).
Less toxic elements include Zn, which is commonly found in fish organs and is one of the highest concentrated PTE, compared to other elements (Töre et al. 2021, Xu et al. 2021), as shown in table I. It is essential for metabolic processes and fish can easily absorb it (Xu et al. 2021). Besides, during Cd exposure, Zn protects against the toxicity of Cd in fish tissues (de Conto et al. 1999). Even though Cr has an essential role in the diet (Rajeshkumar and Li 2018, Yang et al. 2021), high exposures to this element lead to allergic diseases, liver damage, lung irritation, and, in some cases, cancer (Jabeen and Chaudhry 2010). While it is common to find low concentrations of Cr in fish (Yousafzai et al. 2017), the results of this study show higher concentrations than those reported by other authors (Čelechovská et al. 2007, Yousafzai et al. 2017). Finally, Cu is essential for metabolic functions, but in high concentrations it might represent a risk for liver and kidney (Maurya et al. 2019, Castaldo et al. 2020). However, Papagiannis et al. (2004) indicate that accumulation of Cu is low in freshwater fish, which agrees with the results in this study.
TABLE I COMPARISON OF POTENTIAL TOXIC ELEMENTS CONCENTRATIONS IN LIVER AND TISSUES OF Cyprinus carpio OF DIFFERENT REGIONS.
| Location | Tissue | Unit (mg/kg) | As | Cd | Cr | Cu | Hg | Pb | Zn | Reference |
| Mexico | Liver dry season | d/w w/w | 20.73 ± 20.03 4.83 | 0.79 ± 1.60 0.20 | 7.64 ± 16.99 1.78 | 0.16 ± 0.35 0.03 | 0.01 ± 0.01 0.003 | 1.28 ± 2.60 0.29 | 20.70 ± 20.03 6.51 | Present study |
| Muscle dry season | d/w w/w | 4.33 ± 3.66 1.00 | 0.11 ± 0.10 0.02 | 4.83 ± 4.78 1.12 | 0.17 ± 0.31 0.04 | 0.008 ± 0.008 0.002 | 0.12 ± 0.28 0.02 | 4.33 ± 3.66 1.09 | ||
| Mexico | Liver rainy season | d/w w/w | 7.41 ± 9.50 0.66 | 0.24 ± 0.13 0.02 | 1.01 ± 0.79 0.09 | 1.00 ± 0.96 0.09 | 0.02 ± 0.01 0.002 | 0.84 ± 0.46 0.07 | 9.68 ± 9.26 0.86 | Present study |
| Muscle rainy season | d/w w/w | 9.79 ± 9.50 0.87 | 0.10 ± 0.11 0.01 | 0.63 ± 0.64 0.05 | 0.33 ± 0.18 0.03 | 0.006 ± 0.005 0.001 | 0.53 ± 0.57 0.04 | 7.02 ± 9.28 0.63 | ||
| Spain | Muscle | d/w | 0.17 | <0.0006 | - | - | 0.08 | 0.58 | - | Fernández-Trujillo et al. (2021) |
| Mexico | Muscle | d/w | - | - | - | 0.55 | - | - | 30.43 | Torres et al. (2016) |
| Serbia | Muscle Liver | d/w d/w | 0.66 0.49 | 0.005 0.28 | 0.01 0.01 | 1.30 33.49 | 0.89 1.63 | - | 59.01 325.37 | Subotić et al. (2013) |
| China | Muscle Liver | w/w w/w | - | 0.04 0.03 | 0.08 0.03 | 0.03 0.06 | - | 0.08 0.06 | - | Rajeshkumar and Li (2018) |
| Bosnia | Muscle Liver | w/w w/w | 0.30 0.20 | 0.30 0.70 | - | - | 0.80 0.50 | 1.30 0.80 | - | Has-Schön et al. (2015) |
| Bulgaria | Liver | w/w w/w | 1.3 | 6.8 | - | 53 | - | 5.5 | 154 | Georgieva et al. (2016) |
| Turkey | Muscle | w.w | - | 0.001 | 0.9 | 0.61 | - | - | 9.8 | Varol and Sünbül (2018) |
| China | Muscle | w/w | 0.03 | 0.01 | 0.85 | Hao et al. (2022) | ||||
| Mexico | w/w | - | 0.50 | - | - | - | 0.50 | - | SSA (2011) | |
| Commission regulation | w/w | - | 0.050 | - | - | 0.50 | 0.30 | - | CR (2006) | |
| Food and Agriculture Organization | w/w | - | - | - | 30 | 0.50 | - | 30 | FAO (1983) | |
d/w: dry weight; w/w: wet/weight.
Even though PTE concentrations were found in all samples, they were different in liver and muscle. Higher PTE concentrations were found in liver, similarly to other studies about common carp (Yousafzai et al. 2017, Rajeshkumar and Li 2018), and also when compared to other fish species such as Siganus sutor, Lethrinu harak, and Rastrelliger kanagurta (Mziray and Kimirei 2016). This result was expected since liver is one of the primary metabolic organs that synthesize PTE, which bind to proteins and metallothioneins. On the contrary, the muscle does not synthesize many metallothioneins (Ali and Khan 2018, Xu et al. 2021). Likewise, the liver is considered a contaminant storage since it acts as an active site of chronic effects caused by pollutants in most animals (Mziray and Kimirei 2016); also, it is the main tissue for biomonitoring elements that accumulate through food elements (Albuquerque et al. 2021). When pollutants exceed the accumulation capacity in the liver, the fish begins to store pollutants in other tissues such as muscle (Monferrán et al. 2016, Castaldo et al. 2020).
On the other hand, PTE accumulation in muscle is generally low because it is not a metabolic tissue; however, it is one of the main tissues due to its importance in the food chain and the associated risk to human health (Ali and Khan 2018, Castaldo et al. 2020). Several studies have been published about the bioaccumulation of PTE in the muscle and liver tissues of freshwater carp species globally (Table I). This species is widely distributed worldwide, allowing to compare values with other regions (Fernández-Trujillo et al. 2021).
Likewise, many factors have been reported to affect the bioaccumulation of PTE in fish, including fish characteristics and environmental factors (Ali and Khan 2018). Fish characteristics include length, weight, age, sex, habitat, diet, metabolism, ecological needs, feeding habits, water hydrodynamics, and pollution sources, among others (Yi and Zhang 2012, Mziray and Kimirei 2016, Rajeshkumar and Li 2018, Ali et al. 2021). Meanwhile, environmental factors include the availability of elements that might be directly influenced by physical and chemical factors such as pH, temperature, organic matter, salinity, water hydrodynamics, and pollution sources, among others (Rajeshkumar and Li 2018, Maurya et al. 2019). Another important factor to be considered is seasonal variation, which affects PTE’s concentrations (Liu et al. 2020). Seasonal changes in PTE bioaccumulation in fish have been reported in several studies (Georgieva et al. 2016, Varol and Sünbül 2018, Hao et al. 2022), and a higher PTE accumulation has been reported during the dry season, when the volume of the water diminishes (Ali and Khan 2018).
Health risk assessment of potentially toxic elements
THQ was calculated with muscle tissue, since it is the main edible part of the fish (Mziray and Kimirei 2016, Rajeshkumar and Li 2018) and could have negative effects on human health (Monferrán et al. 2016). THQ in both dry and rainy seasons was significantly lower than 1, indicating a minor risk to public health (Fig. 5). It is important to mention that literature estimates that organic As often accumulates in higher concentrations than inorganic As (Ali and Khan 2018), which represents 10 % of the total analyzed As in fish (da Silva et al. 2021, Töre et al. 2021). The results of THQ and total target hazard quotient (TTHQ) data were estimated considering inorganic As (iAs), based on the latter information. So far, the risk assessment of fish consumption from El Tunal River increases during the dry season. Fish from El Tunal River had a higher THQ value of As and Cr in muscle tissue when compared to other studies (Varol and Sünbül 2018, Yang et al. 2021).
TTHQ is the sum of all PTE, since all these are consumed together (da Silva et al. 2021). TTHQ was found to be 0.40 during the dry season and 0.24 in the rainy season. As and Cr had the major contributions to TTHQ in the dry season, whereas As and Pb were the main contributors in the rainy season. However, all the individual PTE concentrations of carp from El Tunal River were within the established limits and may not be considered a health risk to consumers, especially the population with low daily intake (Liu et al. 2020).
CONCLUSIONS
The results suggest that carp fish can biaccumulate different PTE in liver and muscle with different intensities. The concentrations were seasonally influenced and were mostly related to anthropogenic activities rather than natural origins. The liver tissue of carps contained higher concentrations of PTE during the dry season in comparison to muscle and rainy season. THQ and TTHQ factors were lower than 1, representing a low health risk to fish consumers, regardless of the season and PTE. Regular monitoring is needed, especially for As, Cd, and Pb, as well as studies related to the chemical form of the elements to better understand their toxicity and hazards to human health. This information could be used to regulate the monitoring of PTE pollution along El Tunal River and to reduce any negative impact on fish consumers.











 nueva página del texto (beta)
nueva página del texto (beta)




