INTRODUCTION
There is evidence of the worldwide occurrence of toxic cyanobacterial blooms and their derived cyanotoxins in freshwater environments (Buratti et al. 2017, Svirčev et al. 2019), which are usually associated with eutrophication in freshwater bodies due to nutrient inputs, climate change, and watersheds alterations (Brooks et al. 2016, Glibert 2020, Chorus et al. 2021, Munoz et al. 2021). Cyanotoxins are chemically diverse metabolites produced by about 40 cyanobacteria genera (Apeldoorn et al. 2007, Aráoz et al. 2010, Svirčev et al. 2019), whose toxigenic adverse effects have been recognized in a wide range of organisms as bacteria, microalgae, zooplankton, fishes, amphibians, birds, mammals and plants (Valdor and Aboal 2007, Tilmanns et al. 2008, Chen et al. 2016, Banerjee et al. 2021, Zhang et al. 2021). Moreover, the bioaccumulation of cyanotoxins (i.e., their presence inside tissues) is well documented in exposed organisms, including fish, which represent a potential route for cyanotoxins transfer to humans via consumption of contaminated food (e.g., fish, shellfish, and crops) (Testai et al. 2016a, Flores et al. 2018, Pham and Utsumi 2018).
The risk for the environment and human health associated with cyanotoxin exposure constitutes a complex scenario where several environmental and social factors could be involved. For example, biological and ecological processes in waterbodies influence the formation of blooms, but social, economic, cultural, conservational, managerial, and regulatory aspects are generally involved when cyanobacterial blooms occur (Poste et al. 2011, Chorus et al. 2021, Mutoti et al. 2022). Among these, from an ecological point of view, there is the trophic transfer of cyanotoxins in aquatic food webs (Ferrão-Filho and Kozlowsky-Suzuki 2011, Lance et al. 2014), from the primary producer cyanobacteria to herbivores that eventually lead to bioaccumulation in higher trophic levels (e.g., predatory fish, piscivorous birds). Also, some potential association of trophic habits and cyanotoxins concentrations have been hypothesized (Flores et al. 2018) with some support (Berry et al. 2011), where phytoplanktivorous organisms showed the highest cyanotoxins bioaccumulation because of the direct uptake of toxins from cyanobacteria. However, only inhabiting a freshwater environment containing dissolved cyanotoxins could be an exposure route for aquatic organisms, as experimentally demonstrated in shrimps and fish (Cazenave et al. 2005, Jiang et al. 2012, Galanti et al. 2013). Biomagnification of cyanotoxins (i.e., the increase of concentrations of toxins in organisms as their trophic level increase) is another aspect of trophic transfer that has only partially been supported (Kozlowsky-Suzuki et al. 2012, Flores et al. 2018).
From the perspective of human health, there are well-documented intoxication events due to cyanotoxins (Humpage and Cunliffe 2021), which promoted the establishment of provisional guideline values (GVs) for human intakes of contaminated water and food by international organizations such as the World Health Organization (WHO) or the European Food Safety Authority (EFSA). Those GVs were adopted in some countries’ legislations. Therefore, tolerable daily intake (TDI) for the whole life has been suggested and recommended by WHO for human consumption of contaminated organisms based on human body weight (bw) for the hepatotoxic microcystins (MCYSTs) of 0.04 µg/kg bw (Fastner and Humpage 2021).
For MCYSTs, Ibelings and Chorus (2007) calculated maximum tolerable intakes to avoid acute exposure by a single consumption of 2.5 µg/kg bw, and 0.4 µg/kg bw for short-term exposure (e.g., two weeks exposure when cyanobacterial bloom occurs). The suggestion of intakes for daily, short-term, and acute scenarios is in accordance with the global documented occurrence of MCYSTs, which is the most studied cyanotoxin worldwide (Flores et al. 2008, Testai et al. 2016a). Other less studied cyanotoxins for which GVs have been suggested included cylindrospermopsins (CYNs),with a TDI of 0.03 µg/kg bw (Humpage and Falconer 2003, Ibelings and Chorus 2007), and the neurotoxic saxitoxins (STXs), for which EFSA suggested an acute reference dose (ARfD) of 0.5 μg STXs equivalents/kg bw (EFSA 2009). These GVs are notably related to the consumption of freshwater fish, which constitute an important protein source around the world (Akpaniteaku et al. 2005) by aquaculture or freshwater fisheries.
Because of freshwater environment modifications by eutrophication, human population increase around waterbodies, and human pressures for food of freshwater origin, the evaluation of bioaccumulation of cyanotoxins in fish communities is a useful tool to assess the potential transfer to humans and intoxication events. Also, from an ecological and conservational point of view, the introduction of species interacting with native species in presence of cyanotoxins could potentially constitute a greater threat to the conservation of native aquatic biodiversity derived from synergistic negative effects of the introduced species (Forneck et al. 2021) and cyanotoxins.
To our knowledge, a comparison of cyanotoxins bioaccumulation in introduced, native, and commercially important freshwater fish species has not been directly addressed, limiting the evaluation of cyanotoxins accumulation by fish of different origins in freshwater bodies. As an example, in Latin America, few studies have focused on more than one species when analyzing cyanotoxins bioaccumulation (Deblois et al. 2008, Berry et al. 2011, 2012, Zamora-Barrios et al. 2019). In this regard, tropical freshwater reservoirs are environments with multiple human uses (e.g., aquaculture of non-native species, artisanal fisheries, water supply, irrigation) that allow the evaluation of ecological and human health risks posed by cyanotoxins. Accordingly, in this research we focused on three fish species from an anthropized reservoir in Central Mexico: Goodea atripinnis, which is endemic (Beltrán-López et al. 2021); Poeciliopsis gracilis, which was originally distributed in southern Mexico in rivers north and west of the Isthmus of Tehuantepec (in both Pacific and Atlantic versants) (Ward et al. 2022), and has been introduced into several basins in Central Mexico, including Pánuco, Balsas (Miller 2009), and upper Lerma (this study), usually accidentally by aquaculture activities of other species (Contreras-MacBeath et al. 2014); finally, Oreochromis niloticus (Nile tilapia), which has been introduced worldwide for aquaculture and local fishing, sometimes resulting in its establishment outside its original culture areas. The introduction of Nile tilapia has been considered a threat to the native aquatic fauna (Forneck et al. 2021, Gracida-Juárez et al. 2022).
Related to previously studied cyanotoxins, STXs bioaccumulations have received little attention in freshwater environments compared to MCYSTs (Flores et al. 2018), and compared to its bioaccumulation in marine organisms (where STXs are mainly originated by dinoflagellates of the genus Alexandrium), somehow masking the potential risk to the environment and human health by this neurotoxin from freshwater bodies (Testai et al. 2016b). Thus, based on the previous ideas, we studied the bioaccumulation of two cyanotoxins in fish from a multi-use reservoir in Central Mexico, with a twofold objective. First, we aimed to determine and compare the patterns of STXs and MCYSTs bioaccumulation in different tissues of native, introduced, and commercially important fish in an anthropized reservoir. Second, we examined the potential human exposure risk to those cyanotoxins due to the consumption of the studied fish species.
MATERIALS AND METHODS
Study site and species
The Santa Catarina Reservoir (20º 48’ 12.21” N, 100º 27’ 12.48” W) is located in the state of Querétaro, Central Mexico (Fig. 1). The reservoir has an area of 210 ha and a maximum capacity of 9.6 million m3. It is the final water collector of the Santa Catarina microbasin, comprising 192 km2 of catchment area (Pineda-López and Hernández-Sandoval 2000), and is part of the Lerma-Santiago basin. The reservoir suffers severe problems of contamination and nutrient input, principally due to the lack of sewage treatment in towns in the catchment area. Also, there is diffuse contamination by runoff from agriculture fields. Therefore, the reservoir has been considered eutrophic (Martínez-Pérez 2019). Two small population centers border the reservoir (Fig. 1). Water use in the Santa Catarina Reservoir includes crop irrigation, aquaculture, artisanal/commercial fishing, occasional cattle water consumption, and recreation. The reservoir is known to contain at least five genera of toxins-producing cyanobacteria, including Cylindrospermopsis, Dolichospermum, Microcystis, Planktothrix, and Raphidiopsis, along with STXs and MCYSTs dissolved in water (Trejo-Sánchez 2021). Fish mortality has been documented in 2013, 2018 (Trejo-Sánchez 2021) and 2021 (personal observation).
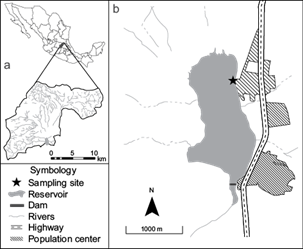
Fig. 1 Map of the Santa Catarina Reservoir (20º 48’ 12.21” N, 100º 27’ 12.48” W), Querétaro, Central Mexico, showing (a) the Santa Catarina microbasin and 1(b) the reservoir surrounded by population centers. The study site is denoted by a star in (b).
To assess STXs and MCYSTs bioaccumulation, we selected three fish species with different feeding habits. G. atripinnis is mainly a phytoplanktivorous species (Berry et al. 2011, Ramírez-Herrejón et al. 2014), P. gracilis is detritivorous (Rodríguez-Cázares 2008) and the Nile tilapia is omnivorous (Gracida-Juárez et al. 2022). In the Santa Catarina Reservoir, Nile tilapia is the main target species of artisanal and commercial fishing. Despite this species being well established and reproductive in the reservoir, there is occasional fry release by local fishermen to maintain the fishery and their economic activity (personal observation).
Field sampling and water parameters
Water and fish samples were collected in November 2021. The sampling site was selected in the north of the Santa Catarina reservoir (Fig. 1), a usual fishing spot for local fishermen close to a population center. In the selected site, we established three sampling points as replicas separated by 15 m. To determine the water parameters potentially associated with cyanobacterial blooms, at each sample point we measured the water temperature, pH, conductivity, and total dissolved solids (TDS) using an oximeter (HANNA HI98125). Also, we measured the concentration of oxygen and percentage of oxygen saturation with the same instrument. Phycocyanin and chlorophyll-a were measured directly at each point using a Turner Flouro Sense (Sunnyvale, CA, USA) fluorometer for each pigment. Additionally, we collected 100 mL of water 1 m apart from the shoreline to determine the concentration of 30 cations and 7 anions at each sampling point. We used a “blank container” exposed to environmental conditions during the collection of water in order to control for any contamination while sampling. Samples were transported inside a cooler to the laboratory and stored at 4 ºC until analysis. Anions analyses were performed by high-resolution liquid chromatography (HPLC) following method 300 of the U.S. Environmental Protection Agency (USEPA 1993), while cations were determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES), following USEPA’s method 200.7 (USEPA 1994), both at the Geosciences Center (CGEO) of the National Autonomous University of Mexico (UNAM).
To access the identity of the cyanobacteria genera inhabiting the reservoir, a plankton net (20 cm diameter, 20 µm mesh size) was cast and dragged several times perpendicular to the border of the reservoir for 1 min. at each sampling point. The concentrated samples (seston) were stored in a 500 mL plastic bottle and transported to the laboratory for cyanobacteria genera determination. For the analyses of cyanotoxins dissolved in water, we collected two samples of 10 and 9 mL of surface water in glass containers at each sample point for MCYSTs and STXs, respectively. For STXs, we immediately added in the field 1 mL of 10X diluent solution according to the sample preparation of the enzyme-linked immunosorbent assay (ELISA) kit employed (see below). Water samples for dissolved cyanotoxins quantification were transported inside a cooler to the laboratory, filtered using a GF/F filter (0.7 µm pore), and then stored frozen until analysis. For the analyses of MCYSTs and STXs in the particulate fraction of water (seston), we collected 500 mL of surface water in a plastic container directly 1 m apart from the shoreline at each sample point. Samples were transported inside a cooler to the laboratory, and 100 mL of water were filtered for the analysis of each cyanotoxin using a pre-weighed and dried GF/F filter (0.7 µm pore) which was frozen until extraction.
Fish species were collected in the sample points using cast and seine nets; fish were immediately euthanized in iced water (around 4 ºC) in the field, transported to the laboratory, and stored at 4 ºC until dissection the next day. For Nile tilapia, we dissected the whole liver and a piece of muscle from the dorsal side (just below the dorsal fin). For G. atripinnis and P. gracilis, we dissected the viscera (excluding eggs or embryos in pregnant females) and kept both the viscera and the whole eviscerated fish separated. We included the liver/viscera for the determination of cyanotoxins content because the accumulation of STXs and MCYSTs in fish is known to occur at high levels in the liver (Castonguay et al. 1997, Berry et al. 2011). All fish tissues were stored frozen until the cyanotoxins extraction. For each cyanotoxin analysis, we included six specimens of Nile tilapia and G. atripinnis, as well as five of P. gracilis.
Cyanotoxins extraction and determination
Prior to extraction, tissues, eviscerated fish, and filters were dried in an oven for 24 h at 50 ºC. We included liver (0.02 to 0.16 g) and muscle (0.18 to 0.26 g) for the extractions of Nile tilapia specimens. For G. atripinnis and P. gracilis we included the whole viscera (0.002 to 0.08 g) and eviscerated fish (0.04 to 0.15 g). When the dry eviscerated fish was heavier than 0.15 g, we cut it into small pieces, homogenized it, and took a sample of 0.15 g.
For STX extractions, we followed the instructions from the Saxitoxin (PSP) ELISA, Microtiter Plate of Abraxis (Warminster, PA) for shellfish with some modifications: the filter + seston or tissue sample was homogenized in 6 mL of 80 % methanol using an IKA T10 basic Ultra-turrax homogenizer, then centrifuged at 5750 rpm for 10 min, and the supernatant was collected. We added 2 mL of 80 % methanol to the residue in the tube, homogenized it for 10 s using a vortex mixer, re-centrifugated it at 5750 rpm for 10 min, and added the supernatant to the first portion. Then we added 80 % methanol to the collected supernatant to obtain a final volume of 10 mL and filtered it using a syringe filter (0.45 µm pore, PET membrane, CHROMAFIL). The extractions of MCYSTs were based on modifications of Wilson et al. (2008) and Berry et al. (2011) as follows: the filter + seston or tissue sample was homogenized in 6 mL of 80 % methanol using an IKA T10 basic Ultra-turrax homogenizer, sonicated at 20 kHz for 10 min, incubated in an orbit shaker for 1 h and then centrifuged at 2500 rpm for 10 min, followed by the collection of the supernatant. Afterwards, we added 6 mL of a solution of 80 % methanol-0.05 % acetic acid to the residue in the tube, homogenized it for 10 s using a vortex mixer, and re-centrifuged it at 2500 rpm for 10 min. The supernatant was added to the first portion, and 80 % methanol-0.05 % acetic acid was used to get a final volume of 15 mL; then, we filtered it using a syringe GF/F filter (0.7 µm pore). Finally, 1 mL of the solution was dried in a water bath at 60 ºC, resuspended in ultra-pure water (Type 1), and sonicated for 15 min at 20 kHz prior to MCYSTs quantification. For all MCYST extraction steps, we used glass tubes to avoid losses by surface adsorption when using tubes of different materials.
STXs and MCYSTs were quantified from filtered water (dissolved fraction) and tissue extracts by ELISA using microtiter plate kits from Abraxis (52255B and 520011SAES). The MCYSTs microtiter plate has a sensitive congener-independent detection, based on the ADDA moiety of MCYSTs and a detection range of 0.05 to 5 μg/L. The STXs microtiter plate has a cross-reactivity of 100 % to the STX variant and varying cross-reactivity to other congeners (i.e., Decarbamoyl STX, NeoSTX, etc.), showing a detection range of 0.02 to 0.4 μg/L. According to the manufacturer, the detection limit of the STXs test is 0.015 μg/L while for MCYSTs it is 0.016 μg/L; also, both microtiter plate kits have been tested for numerous organic and inorganic compounds commonly found in samples and found not to interfere with them. All extracts and filtered water samples were tested twice to obtain a mean for the respective sample.
Concentration of cyanotoxins in tissues and daily intakes calculation
For a set of specimens of the three species collected in November 2021, we calculated a mean percentage of water loss for each fresh tissue type (muscle, liver, viscera, and whole eviscerated fish) by drying them in an oven for 24 h at 50 ºC (Table SI in the supplementary material). The percentages were used to calculate the fresh weight of the dry tissues included in the extractions. We calculated the concentrations of MCYSTs and STXs in fresh fish tissues based on the mean ELISA concentrations of the extracts, the final volume of extractions (10 or 15 mL), and the fresh weight of tissues extracted. Using the cyanotoxins concentration in the fish tissues, we determined the potential STX and MCYST intakes for an adult (70 kg) and a child (30 kg) including a consumption of 100 g following Ibelings et al. (2021b). These potential intakes were compared with international guideline values of WHO and EFSA, the short-term exposure intake GVs of WHO for MCYSTs, and the ARfD values of EFSA for STXs. We included potential cyanotoxin intakes of viscera/liver because of the local people’s habits in some regions of Mexico of eating small fish as a whole (Berry et al. 2011, personal observation), which could constitute an important cyanotoxin exposure route.
RESULTS
Physical and chemical parameters of water in the fish collecting site in the north of the Santa Catarina reservoir are summarized in table I. The photosynthetic pigment phycocyanin showed a higher concentration than chlorophyll-a (Table I). Fourteen cations were detected in water, with Ca+2 > Na +> K+ showing the highest concentrations, while anions Cl- and SO4 -2 showed the highest concentrations. However, the quantification limit varied among them (Table SII). The water surface at the study site showed green streaks usually present in blooms of buoyant cyanobacteria (Fig. 2a) associated with species of three toxin-producer genera that were observed in the dragged and concentrated samples: Microcystis, Dolichospermum, and Planktothrix (Fig. 2b-d respectively).
TABLE I MEAN OF THE PHYSICAL AND CHEMICAL PARAMETERS (± Sindex-archivosTANDARD DEVIATION) OF THE WATER FROM THE SANTA CATARINA RESERVOIR, NOVEMBER 2021.
| Factor | Mean (± standard deviation) | Range |
| Temperature (ºC) | 16.33 ± 0.58 | 16-17 |
| pH | 7.67 ± 0.55 | 7.3-8.3 |
| Conductivity (µS/cm) | 386.67 ± 55.08 | 350-450 |
| TDS (mg/L) | 190 ± 26.46 | 170-220 |
| Oxygen Saturation (%) | 90.63 ± 4.67 | 87-95.9 |
| Oxygen (mg/L) | 7.26 ± 0.42 | 6.94-7.74 |
| Dissolved phosphorus (µg/L) | 524.6 ± 11.40 | 511.5-532.7 |
| Dissolved nitrate (µg/L) | 270 ± 37.40 | 240-310 |
| Phycocyanin (µg/L) | 183 ± 27.71 | 151-199 |
| Chlorophyll-a (µg/L) | 119.33 ± 13.28 | 104 - 127 |
TDS: total dissolved solids.
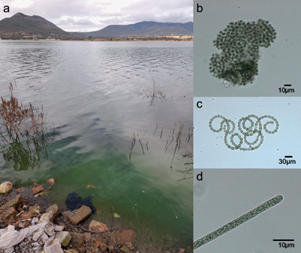
Fig. 2 Study site at Santa Catarina Reservoir (a) showing the typical streaks of cyanobacterial blooms and the cyanobacteria genera found in the reservoir: (b) Microcystis, (c) Dolichospermum, and (d) Planktothrix. Novembrer 2021.
Both cyanotoxins, STXs and MCYSTs, were found in water samples. We found dissolved STXs in one of the three sampled points, with a concentration of 0.026 µg/L, while dissolved MCYSTs were not detected at any sampling point. However, in the sestonic fraction of water both cyanotoxins were found at all three sampling points, showing mean concentrations (± standard deviation) of 0.027 ± 0.004 µg/L for STXs and 0.013 ± 0.005 µg/L for MCYSTs.
We detected STXs and MCYSTs in all tissues of the three analyzed species. In general, MCYST concentrations were higher than those of STXs by one or two orders of magnitude (Fig. 3). For STXs, liver or viscera showed higher concentrations than muscle in all species and specimens (Table SIII). The lowest concentrations in muscle were detected in Nile tilapia, while the highest were found in P. gracilis (Fig. 3a). For MCYSTs, some tissue extracts (three muscle/three viscera for G. atripinnis, two muscle/two liver for Nile Tilapia, and one viscera for P. gracilis) showed concentrations above the upper limit of detection of ELISA (> 5 µg/L). From the extracts within the detection limits of ELISA, the average of the calculated concentrations was higher for liver or viscera than for muscle (in Nile tilapia and P. gracilis, respectively), or similar (G. atripinnis) (Fig. 3b). However, in G. atripinnis one specimen showed a concentration in muscle higher than the concentration in viscera (Table SIII). In muscle, the lowest concentrations of MCYSTs were detected in Nile tilapia, while the highest in G. atripinnis (Fig. 3b).
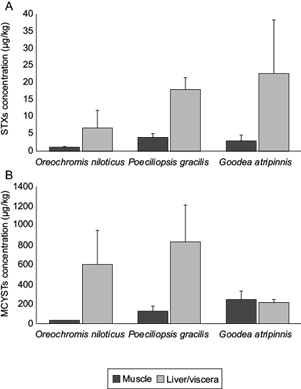
Fig. 3 Means of (a) saxitoxins (STXs) and (b) microcystins (MCYSTs) accumulation in muscle and liver/viscera of the studied species in the Santa Catarina Reservoir, Querétaro, central Mexico. November 2021. Bars indicate standard deviation.
The patterns of potential daily cyanotoxins intakes and the risk for human health based on comparisons to international guideline values varied between the two analyzed cyanotoxins. For STXs, the calculations of daily intakes for adults or children never exceeded the ARfD of 0.5 µg/kg bw for any tissue or species (Table II). For MCYSTs, only the consumption for adults of Nile tilapia liver and P. gracilis viscera exceeded the daily intake guideline value of 0.4 µg/kg bw for short-term exposure; while for children, only the consumption of muscle from Nile tilapia did not exceed the daily intake for short-term exposure (Table II). This suggests a higher potential risk for children compared to adults. The highest potential daily intake of MCYSTs was for P. gracilis viscera for children, which exceeded almost sevenfold the short-term intake.
TABLE II POTENTIAL DAILY INTAKES (DI) OF SAXITOXINS (STXs) AND MICROCYSTINS (MCYSTs) BY BODY WEIGHT (BW) FOR ADULTS AND CHILDREN DUE TO THE CONSUMPTION OF THREE FISH SPECIES FROM THE SANTA CATARINA RESERVOIR*.
| Species | Tissue | DI adult µg/kg bw | DI child µg/kg bw |
| STXs | |||
| Goodea atripinnis | M | 0.004 ± 0.002 | 0.010 ± 0.005 |
| V | 0.032 ± 0.022 | 0.076 ± 0.052 | |
| Oreochromis niloticus | M | 0.001 ± 0.0001 | 0.003 ± 0.0003 |
| L | 0.009 ± 0.007 | 0.021 ± 0.017 | |
| Poeciliopsis gracilis | M | 0.006 ± 0.001 | 0.013 ± 0.003 |
| V | 0.026 ± 0.004 | 0.059 ± 0.011 | |
| MCYSTs | |||
| Goodea atripinnis | M | 0.35 ± 0.13 | 0.81 ± 0.3 |
| V | 0.30 ± 0.06 | 0.71 ± 0.13 | |
| Oreochromis niloticus | M | 0.04 ± 0.01 | 0.09 ± 0.02 |
| L | 0.82 ± 0.61 | 1.92 ± 1.42 | |
| Poeciliopsis gracilis | M | 0.17 ± 0.08 | 0.41 ± 0.18 |
| V | 1.20 ± 0.53 | 2.79 ± 1.24 | |
*Upper limits of cyanotoxins consumption according to guideline values for comparison: STXs ARfD OF 0.5 µg/kg bw (EFSA 2009), MCYSTs TDI of 0.04 µg/kg bw (WHO; (Fastner and Humpage 2021), MCYSTs tolerable intakes for short-term exposure of 0.4 µg/kg bw (Ibelings and Chorus 2007) and tolerable intakes to avoid an acute exposure by a single consumption of 2.5 µg/kg bw (Ibelings and Chorus 2007). M: muscle, V: viscera, L: liver.
DISCUSSION
The Santa Catarina Reservoir showed some physical and chemical water parameters generally associated with cyanobacterial blooms. The potential toxicity of water was confirmed by the presence of the analyzed cyanotoxins in dissolved (only STXs) and particulate fractions of water (both STXs and MCYSTs). Moreover, we detected the presence of STXs and MCYSTs in all tissues and species studied, some containing a high amount of MCYSTs by kilogram of fresh tissue, which in turn exceeded the recommendations of MCYSTs daily intake for a two-week exposure time (Ibelings and Chorus 2007). These findings are remarkable because the commercial fishery in the reservoir (besides other water uses like irrigation and recreation) and the consumption of these fish could represent a route to human exposure to cyanotoxins, particularly to hepatotoxic MCYSTs. A similar scenario of cyanotoxins presence and bioaccumulation has been reported in other waterbodies of Mexico and Latin American countries (see Berry et al 2011, Zamora-Barrios et al. 2019, Oliveira et al. 2013, Lopes et al. 2020), highlighting the need to detect cyanotoxins in water and tissues for the assessment of bioaccumulation and potential human exposure.
Water parameters and cyanotoxins concentrations
Several environmental factors have been studied in relation to cyanobacteria and cyanotoxins occurrence in freshwater bodies, including nutrients, light availability, temperature, thermal stratification, mixing, hydrodynamics, waterbody morphometry, and pH (Ibelings et al. 2021a). Although we did not analyze the hydrodynamics of the reservoir, some water parameter values (high phycocyanin and chlorophyll-a concentrations, high pH, TDS) have been associated with the formation of cyanobacterial blooms or have been seen to positively influence it, namely dissolved phosphorus and nitrogen concentrations.
Chlorophyll-a is commonly used as a measure to estimate total phytoplankton biomass and is particularly useful during cyanobacterial blooms when the phytoplankton mainly consists of one or few cyanobacteria taxa (Padisák et al. 2021). Also, phycocyanin in freshwater is mainly produced by cyanobacteria and has been used as an indicator specifically for cyanobacterial biomass (Gregor et al. 2007, Bastien et al. 2011). Phycocyanin measurements in the Santa Catarina Reservoir were higher than chlorophyll-a, suggesting a dominance of cyanobacteria in the phytoplankton, which is typical of cyanobacterial blooms and in accordance with the observed cyanobacteria genera in the reservoir (Microcystis, Dolichospermum, and Planktothrix). Also, pH values in the study site were alkaline, which is a common condition during cyanobacterial blooms. The uptake of hydrogen carbonate by intense photosynthesis of the increased cyanobacteria biomass shifts the equilibrium between carbonate and hydrogen carbonate to render the water alkaline (Ibelings et al. 2021a). Trejo-Sánchez (2021) also found an alkaline pH in the Santa Catarina Reservoir during cyanobacterial blooms in April, August, and October 2019.
Nitrogen and phosphorus are considered key nutrients that enhance or-when lacking-limit the phytoplankton biomass and, therefore, cyanobacterial blooms (Ibelings et al. 2021a). For this reason, they have been used to assess and control the risk of cyanobacteria increased densities by managing their loads from the catchment area (Chorus and Zessner 2021). Ibelings et al. (2021a) pointed out that if dissolved phosphorus and nitrogen concentrations are above 5-10 μg/L and 100 μg/L, respectively, phytoplankton cells would be saturated, and these nutrients are not limiting the phytoplankton needs. This is the Santa Catarina Reservoir situation, where phosphorus (or even more phosphate, Table SII) and nitrate are about 100-50 and 2.7 times higher than the values suggested for a limitation condition, respectively. These results, together with the high TDS values found, indicate the eutrophic state of the reservoir, also suggested in 2019 by Trejo-Sánchez (2021).
Of the three cyanobacteria genera known to produce cyanotoxins found in this study during November 2021, Dolichospermum and Planktothrix produce STXs, while, together with Microcystis, all three are known to produce MCYSTs (Burrati et al. 2017, Fastner and Humpage 2021, Testai 2021), and they are responsible for dissolved (only STXs), particulate, and bioaccumulated cyanotoxins in the Santa Catarina Reservoir. In addition, Trejo-Sánchez (2021) found the genera Cylindrospermopsis and Raphidiopsis during the blooms of 2019 in the Santa Catarina Reservoir, which also are toxin producers. We did not detect dissolved MCYSTs in water, contrasting with the study of Trejo-Sánchez (2021), which found them in concentrations ranging from 0.056 to 0.081 µg/L. Dissolved MCYSTs have been found in other Mexican water bodies including Lake Texcoco (0.20-2.4 µg/L; Zamora-Barrios et al. 2017), Valle de Bravo Reservoir (0.5-5.56 µg/L; Alillo-Sánchez et al. 2014), Lake Pátzcuaro (0.16-0.19 µg/L; Berry et al. 2011), and Lake Zumpango (0.1-1.4 µg/L; Zamora-Barrios et al. 2019). In the particulate fraction of water, the mean MCYSTs concentration found was notably lower (0.013 µg/L) than other reported concentrations in Mexican water bodies (Vasconcelos et al. 2010, Berry et al. 2011, Zamora-Barrios et al. 2019). For example, Vasconcelos et al. (2010) reported concentrations of 4.9 to 78.0 µg/L from four waterbodies, Berry et al. (2011) concentrations of 0.02-0.36 µg/L from Lake Pátzcuaro, and Zamora-Barrios et al. (2019) concentrations of 0.94-34.13 µg/L from Lake Zumpango. The dissolved STXs concentration was lower than that reported by Trejo-Sánchez (2021) of 0.04 µg/L in the Santa Catarina Reservoir in October 2019. For sestonic STXs, the concentration in our study site (0.027 µg/L) was higher than the only other reported for a Mexican waterbody of 0.005 µg/L from Lake Catemaco (Berry and Lind 2010). Both sestonic STXs and MCYSTs concentrations found in the Santa Catarina Reservoir suggest a route to cyanotoxins exposure to fish that actively consume plankton or by trophic transfer from other phytoplanktivorous organisms.
Fish cyanotoxins bioaccumulation and potential human exposure
Despite the fact that we did not detect dissolved MCYSTs in the water of the Santa Catarina Reservoir and that the concentration of sonic MCYSTs was low when compared to other studies from Mexico, we found clear evidence of bioaccumulation of both STXs and MCYSTs in the tissues of the three fish species studied. For MCYSTs, with the exception of one specimen of G. atripinnis, the bioaccumulation in the three species followed the general pattern for fish of a higher MCYSTs accumulation in viscera or liver than in muscle (Flores et al. 2018, Ibelings et al. 2021b). Remarkably, we found the same pattern for STXs in the three species, and, besides the study of Galvao et al. (2009) for Nile tilapia, to our knowledge, these are the only studies worldwide reporting higher STXs accumulation in fish liver/viscera than in muscle, since other studies that found STXs accumulation in fish focused only in muscle (e.g., Clemente et al. 2010, Calado et al. 2017, 2019). The generality of this pattern for STXs bioaccumulation among other fish tissues could be addressed in future studies.
Among the three studied species, the commercially important but also introduced Nile tilapia showed the lowest mean STXs and MCYSTs accumulation in muscle, and, in the case of STXs, also in liver/viscera; while between the native G. atripinnis and the introduced P. gracilis the differences in cyanotoxins contents were less clear (with exception of fewer MCYSTs contained in G. atripinnis viscera). The toxigenic adverse effects in fish by MCYSTs and STXs have been widely documented, and they include physiological (e.g., oxidative stress), reproductive (e.g., ovaries and testis lesions), and behavioral (e.g., mobility reduction) alterations (Banerjee et al. 2021). The lowest STXs and MCYSTs in Nile tilapia could suggest a potential lower toxigenic effect in this species when compared to the other two studied species and to the detriment of the native G. atripinnis. The threat to the native aquatic fauna due to the introduction of Nile tilapia has been previously suggested (Forneck et al. 2021). To what extent there are differences in toxigenic effects of cyanotoxins associated with differential bioaccumulation between native and introduced species from freshwater eutrophic environments is a promising field for research and for native species conservation. Also, when compared among the studied species and contrary to the pattern found and suggested by Berry et al. (2011) of a relation between trophic habits and cyanotoxins bioaccumulation (where the phytoplanktivorous Goodea sp. showed the highest MCYSTs accumulation compared to the zooplanktivorous Chirostoma sp. and the omnivorous carp, Cyprinus carpio, in Lake Pátzcuaro), the lack of clear differences between the mainly phytoplanktivorous (G. atripinnis) and the detritivorous (P. gracilis) studied species in the Santa Catarina Reservoir prevented us to support the hypothesis of a trophic habit-cyanotoxins accumulation relation.
The MCYSTs bioaccumulation found in fish from the Santa Catarina reservoir was generally higher than that reported for the same and other species in Mexico and for Nile tilapia muscle in other Latin American countries. Because of its importance in aquaculture, Nile tilapia is one of the most studied species for MCYSTs bioaccumulation. In Mexico, Zamora-Barrios et al. (2019) determined MCYSTs in Nile tilapia from Lake Zumpango finding the highest concentrations in muscle of ~2 and ~9.5 µg/kg for the liver, which were considerably lower than the 28.43 and 605.99 µg/kg for muscle and liver, respectively, found in the Santa Catarina Reservoir. Other studies reporting MCYSTs in Nile tilapia from Latin America are from Brazil reservoirs, which found lower accumulations in muscle than those found in the Santa Catarina Reservoir of 11.7 µg/kg (Deblois et al. 2008), 0.37 µg/kg (Vasconcelos et al. 2013), 2.7 µg/kg (Hauser-Davis et al. 2015), and 0.03 µg/kg (Mendes et al. 2016), except for the high accumulation reported in Lopes et al. (2020), which range from ~350 to 1040 µg/kg from eight Brazilian waterbodies.
In Lake Pátzcuaro, Berry et al. (2011) studied muscle and viscera MCYSTs accumulation for Goodea sp., finding 157 and 867 µg/kg, respectively. In our study site we found higher values in muscle (243.86 µg/kg) and lower in viscera (212.47 µg/kg) for G. atripinnis. The results here constitute the first time P. gracilis is studied in regard to cyanotoxins accumulations; however, its MCYSTs accumulation (122.12 µg/kg for muscle and 837.50 µg/kg for viscera) is remarkable because it is higher than the accumulation of other species analyzed from Mexico including Cyprinus carpio (4.99 µg/kg for muscle, 93.6 µg/kg for liver), Chirostoma sp. (18.50 µg/kg for the whole fish) from Lake Pátzcuaro (Berry et al. 2011) and Chirostoma jordani (~7 µg/kg for the whole fish) from Lake Zumpango.
Studies for STXs bioaccumulation in freshwater fish are scarce (Testai et al. 2016b). Berry et al. (2012) reported STXs accumulation for muscle in nine species from Lake Catemaco, south of Mexico, ranging from 0.03 to 0.71 µg/kg, which were lower than the STXs reported here for the muscle of the three species (1.07 to 4.09 µg/kg). Other studies reporting higher STXs accumulations than in the three species analyzed here are from Brazil, including muscle of Nile tilapia with 19.5 µg/kg (Galvao et al. 2009) and the highest muscle STXs equivalents accumulations reported for Geophagus brasiliensis with 19.7 µg/kg (Clemente et al. 2010), 48 µg/kg (Calado et al. 2017) and ~187 µg/kg (Calado et al. 2019).
The differences between the accumulation of MCYSTs and STXs in fish from the Santa Catarina Reservoir were highlighted in the calculations of potential daily intakes of each cyanotoxin and, therefore, in the risk for human health. For STXs, the Food and Agriculture Organization (FAO) and WHO previously established a limit of 800 µg/kg for mollusk flesh based on an ARfD of 0.7 µg/kg bw (FAO/WHO 2018). Based on a scientific panel that worked on contaminants in the food web, EFSA (2009) determined a more conservative ARfD of 0.5 µg/kg bw for food matrices in general. Even in the most conservative scenario for STXs, the consumption of all fish species and tissues during the study did not represent a potential problem for human health, since all STXs daily intakes were lower than the ARfDs recommended. In fact, the highest potential daily intake of STXs was for G. atripinnis viscera of 0.15 µg/kg bw, which comprised only 30% of the EFSA ARfD.
The scenarios of potential risk for human health are different for MCYSTs, where the calculations of daily intakes for consumption of all species and tissues analyzed from the reservoir exceed the TDI established by WHO of 0.04 µg/kg bw (Fastner and Humpage 2021). Additionally, for children, most of the potential intakes also exceed the daily intake for short-term exposure of 0.4 µg/kg bw (Ibelings and Chorus 2007) (not for the muscle of Nile tilapia). Besides, the daily intakes of viscera of P. gracilis for children exceed the reference doses to avoid acute exposure of 2.5 µg/kg bw proposed by Ibelings and Chorus (2007). Since only the muscle is eaten in the Nile tilapia, which is the target of the fishery in the Santa Catarina Reservoir, there is no risk of human intoxication for viscera consumption. However, smaller species like G. atripinnis are sometimes consumed as a whole, mainly by local people (Berry et al. 2011, personal observation), and, together with P. gracilis, their consumption should be avoided because of the high MCYSTs content. Notably, the USEPA (2006) proposed values for acute and chronic tolerable daily intake guidelines of MCYSTs of 0.006 and 0.003 µg/kg bw, which are seven-fold lower than those of WHO. Under a scenario considering the guidelines of USEPA (2006), the consumption of all species and tissues from the Santa Catarina Reservoir, both for adults and children, could potentially represent a health risk, highlighting the need for more compressive studies on health conditions and risk of specific local populations exposed chronically to MCYSTs.
Regarding the Mexican legislation related to the consumption of cyanotoxins, there is no regulation for the content of any cyanotoxin in freshwater organisms used for human consumption in the country. Regulation exists only for MCYSTs concentrations in water for human consumption with a limit of 1 µg/L on the Official Mexican Standard PROY-NOM-127-SSA1-2017 (SEMARNAT 2019), which is similar to the limit established by WHO (Fastner and Humpage 2021). However, the water from Santa Catarina Reservoir is not used for human consumption. Finally, the potential risk of harmful effects by MCYSTs and STXs on other organisms consuming contaminated fish from the Santa Catarina Reservoir (e.g., wild birds, domestic cats) is also of consideration and, to our knowledge, is not usually determined.
CONCLUSIONS
Our findings demonstrated the accumulation of two harmful cyanotoxins in three fish species from an anthropized reservoir in Central Mexico, with a higher MCYSTs accumulation than STXs. Despite all three studied species being shown to accumulate both cyanotoxins, the risk for humans is apparently lower for the consumption of muscle of Nile tilapia, the target of the local fishery in the Santa Catarina Reservoir, than for the other species studied (whose consumption should be avoided). Fish from aquaculture and freshwater fisheries are important sources of protein in Mexico and tropical countries, and there is a need to evaluate and determine the cyanotoxins accumulation in other water bodies in order to reduce the problems associated with cyanotoxins exposure. In this sense, Nile tilapia in tropical regions and locally G. atripinnis (particularly because of its wide distribution in Central Mexico and high MCYSTs accumulation) are candidate species as bioindicators of cyanotoxins accumulation and useful for determining and monitoring the potential human exposure risk in water bodies.











 nueva página del texto (beta)
nueva página del texto (beta)


