Introduction
Biosolids are sludge generated during the treatment of wastewater and stabilized physically, chemically, or biologically, and are therefore susceptible to reuse (Sharma et al., 2017). It is estimated that the United States of America and Europe produce approximately 7.2 and 10 million tons per year, respectively (Lloret et al., 2016), while Mexico generates 232 thousand tons per year (Gutiérrez-Avedoy et al., 2012). The State of Guanajuato, in Mexico, produces 31 400 t per year, from which 2380 t are estimated to be produced in the municipality of Celaya, Guanajuato (Universidad Nacional Autónoma de México [UNAM], 2014).
The application of biosolids to soils is a common practice to maintain or improve microbial and enzymatic activity and soil quality, especially in soils that are low or deficient in organic matter content (Rigby et al., 2016). Biosolids are abundant in organic matter content, and micro and macronutrients, making it an economic and sustainable substitute instead of inorganic fertilizers (Alvarenga et al., 2016). In addition, soils amended with biosolids improve their physical properties such as porosity, apparent density, aggregate stability, and water retention capacity, having a positive impact on the yield of various crops (Roig et al., 2012). However, the use of biosolids should be periodically evaluated and monitored for the presence of contaminants and nutrient releases (C and N) that may limit their safe disposal to the environment or their use in agricultural systems (Clarke & Cummins, 2015).
On the other hand, over the years, the biological and biochemical properties of soil have gained importance in assessing the sustainability of ecosystems (Singh et al., 2015). Soil microorganisms play an important role in organic matter mineralization, nutrient recycling, and energy flow processes in biogeochemical cycles (Ferreira et al., 2016). Similarly, soil enzymes are of importance in soil productivity and fertility because of their constant production, accumulation, inactivation, or degradation in the soil (Burns et al., 2013). Most biological processes in the soil depend on enzymatic activity, the release of nutrients in forms available to plants and microorganisms, and the breakdown of organic matter (Sharma et al., 2017).
In the same context, urease hydrolyzes urea and amides, producing CO2 and NH3. Urease is important because of its relationship to the nitrogen cycle (Celis et al., 2011; Tabatabai, 1994). According to Azeem et al. (2016)), increased urease activity in soil is associated with increased microbial biomass content, which coincides with Nannipieri et al. (1979), who suggested that urease activity (UA) increases with microbial biomass, since this is an intracellular constituent. However, Adetunji et al. (2017) established that a higher UA is associated with higher organic matter content in the soil rather than increase in microbial biomass.
Currently, there are no studies on the effect of the addition of biosolids to agricultural soils in the Bajío region of Mexico pertaining C and N mineralization and microbial activity. The objective of this study was to evaluate the mineralization of C and N, the microbial biomass carbon (MB-C), and UA as a result of the short-term application of biosolids at different doses.
Materials y Methods
The study was carried out on agricultural land located south of Celaya, Guanajuato, with geographic coordinates 20° 30’ 25’’ N and 100° 52’ N.37’’ W. The soil was sampled in April 2017 and a representative area of 100 m x 100 m was selected. Soil samples were taken using the zigzag method by dividing the area into three equal zones, with 12 sampling points in each zone. At each point a sample of approximately 0.5 kg was collected to form composite samples of 6 kg per zone. The samples were dried, homogenized and sieved using a mesh with a 2-mm hole and then stored at 4 °C until analysis.
The biosolid was sampled in July 2017 at a municipal wastewater treatment plant in the Bajío region of Mexico, located at the geographic coordinates 100° 51’ 64’’ W and 20° 30’ 78’’ N. The biosolids were produced by aerobic digestion processes and dehydrated by means of pressed band filtration systems. The biosolids were taken six times over a four-hour period on the conveyor belt at the outlet of the filter press. Approximately 50 kg in total of the sample on a wet basis (85%) was collected. After that, the sample was taken to the laboratory, dried in the air, sieved through a 2 mm diameter, and stored at 4 °C for further analysis. The analysis of their properties did not exceed the storage time of one week. The biosolids were class "C", which could be used for agricultural and soil improvement purposes. The content of parasites, pathogens and heavy metals in the biosolids was under the permissible limits for their use according to current Mexican regulations (Diario Oficial de la Federación [DOF], 2003).
The results obtained, both in the characterization of the soil and biosolid and in the incubation experiments for the mineralization dynamics, are expressed on a dry basis.
Physicochemical characterization of soil and biosolid
The pH and electric conductivity (EC) were measured in an aqueous extract (1:5 w/v) with the help of a digital pH and conductivity meter (Rhoades, 1996; Thomas, 1996). Soil texture was determined by the Bouyoucos method (Gee & Bauder 1986). Total organic carbon (TOC) was analyzed by oxidation with potassium dichromate according to the Walkley & Black (1934) method and modified by downscaling and colorimetric determination (Alef & Nannipieri, 1995). Total nitrogen (TN) was analyzed by the micro-Kjeldhal system (Bremner, 1996). Inorganic nitrogen (NH4 +, NO3 -, and NO2 -) was extracted from the samples with a solution of K2SO4 0.5 M (1:6 w/v) and colorimetrically determined (Fernández-Luqueño et al., 2016; Keeney & Nelson, 1982). In addition, the C/N ratio was obtained.
Experimental design to evaluate the dynamics of C and N mineralization
The effect of the application of different doses of biosolids on C and N mineralization and microbial activity in laboratory experiments for 56 days was determined. For experimentation, 50 g of soil were weighed into 100 ml glass jars (microcosm). A droplet of deionized water was added to the soil surface with a pipette up to 60% water retention capacity (WHC). The samples were then pre-incubated at 25 °C for 7 days in the dark. After pre-incubation, 50 g of soil were modified with different application rates of biosolids. The application rate of biosolids based on the soil NH4 + content was 0 mg kg-1 (T0 treatment = 0 Mg ha-1), 100 mg kg-1 (T1 treatment, 30 Mg ha-1 of biosolids), and 200 mg kg-1 (T2 treatment, 60 Mg ha-1 of biosolids). In addition, 50 g of biosolids without soil (T3 treatment) were weighed to evaluate the mineralization of organic matter in the biosolid without the influence of soil processes. Each treatment was performed in triplicate.
At the start of incubation, the moisture content was adjusted to 60% of the WHC for each microcosm and placed in 1 L jars. In addition, two vials of 20 ml of 1 M NaOH solution and 2% H3BO3 solution were added to each jar to trap the CO2 emitted and NH3 volatilized, respectively. The 1 L jars with the microcosms and vials were hermetically sealed and incubated at 25 °C in the dark for 56 days. To maintain aerobic conditions, all the jars were opened for 10 min every 15 days and the vials containing the 1 M NaOH solution were removed to prevent atmospheric CO2 from being trapped (López-Valdez et al., 2010).
After 0, 1, 3, 7, 14, 28, and 56 days, three 1 L jars were randomly selected for analysis (Fernández-Luqueño et al., 2017). In addition, all the treatments and subsamples of soil were analyzed for MB-C, UA, and inorganic nitrogen content (NH4 +, NO2 -, and NO3 -) over time.
Determination of physicochemical characteristics in mineralization dynamics
The CO2 trapped in the NaOH solution vials was determined by volumetric titration with a solution of 1 M HCl and phenolphthalein as an indicator. Prior to titration, 1 ml of 0.5 M BaCl2 solution was added to precipitate the carbonates. In parallel, three blanks were analyzed in the same way as the treatments for each day of sampling (Rojas-Oropeza et al., 2010). The percentage of mineralized C was determined by calculating the organic C emitted to CO2 in relation to the organic C added to the biosolids.
The volatilized NH3 was determined with the titration of H3BO3 at 2% with solution of H2SO4 0.1 M (Beltrán-Hernández et al., 2007). The concentrations of NH4 +, NO2 -, and NO3 - were obtained by extraction from the soil matrix with 0.5 M K2SO4 solution and measured colorimetrically (Fernández-Luqueño et al., 2016; Keeney & Nelson, 1982).
The nitrogen available in the biosolids was calculated using the equation reported by Andreoli et al. (2001):
Where forg= percentage of mineralized biosolids in the studied soil; fvol= percentage of volatilized NH3 during the incubation period;
Determination of microbial activity in mineralization dynamics
The quantification of MB-C by the substrate-induced respiration method (SIR) was determined. One jar of 100 ml containing 20 g of soil with glucose as substrate at a concentration of 1750 mg kg-1 (dry base) and one vial of 25 ml containing 0.1 M NaOH solution were placed in a 1 L jar and incubated for 4 h at 25 °C. The NaOH solution was titrated with 0.1 M HCl. The MB-C concentration was determined using the equation established by Anderson & Domsch (1978):
Where C mic = Microbial biomass carbon (µg g-1); B= volume used in the titration of the HCl solution for the controls (ml); S= volume used in the titration of the HCl solution for the samples (ml); K= HCl solution concentration (mmol ml-1); SW= sample weight (g); 30= microbial respiration constant (mg Cmic h ml-1 CO2); 22=CO2 absorption factor (1 mmoL HCl - 22 mg CO2); 1000=Conversion factor (µg Cmic at g Cmic); 1.8294= CO2 density at 0 °C and 101.32 Pa (mg ml-1); and 4= incubation time (h).
For the determination of UA, 1 g of dry soil was placed in a 100 ml vial and mixed with 2.5 ml of 80 mM urea as substrate. Then, it was shaken and incubated for 2 h at 37 °C. After that, 50 ml of a 1 M KCl solution was added and shaken for 30 min and then filtered out. From the filtrate, 1 ml was taken, and 9 ml of sterile distilled water was added to dilute the extract in a 1:10 ratio. For color development, 5 ml of 5.6% sodium salicylate solution dissolved in 0.1 M NaOH solution and 2 ml of 0.1% sodium dichloroisocyanurate solution were mixed, and the absorbance was measured at 690 nm after 30 min. Each sample was compared to a white one, which was prepared in the same way as the sample, with the only difference that distilled water was added instead of urea. UA was expressed with respect to the NH4 + content in the corresponding treatment (Kandeler & Gerber, 1988).
Statistical analyses
The experimental units were the microcosms, and the experimental design was completely random. Statistical analysis of the data was performed using Minitab Statistical Software. To determine the statistical significance of the differences between the averages of treatments, an ANOVA was performed followed by Tukey's test (p ≤ 0.05) on the results of the experiment.
Results
The physicochemical characterization of the soil and biosolids used in the experiments are shown in Table 1. The soil presented a pH value of 6.83, classifying it as neutral. The soil presented an EC of 0.67 dS m-1. Soils with EC and pH values greater than 4 dS m-1 and 8.5, respectively, reflect salinity problems (DOF, 2000). Thus, the fertility of this soil, which presented lower values than those reported, was not affected by a high content of salts in the soil solution. Furthermore, the mineralization of the biosolids added to the soil was not affected by the presence of salts in the soil. The soil presented 70% clay, 22% sand and the rest of silt, classifying it as a fine textured soil (Shirazi & Boersma, 1984). Fine textured soils can lead to the physical/chemical protection of organic matter or reduce substrate availability, making it inaccessible to microorganisms and enzymes (Cassity‐Duffey et al., 2020). In addition, a decrease in N mineralization has been reported in fine textured soils (Sorensen & Jensen, 1995). The soil had a content of TOC of 55.15 g kg-1, so it is classified as a medium fertility soil, as established in Mexican regulations (DOF, 2000). On the other hand, the TN content (2.10 g kg-1) was found within the parameters that have been reported for agricultural soils by other studies (Zheng et al., 2013). The inorganic N content (sum of NH4 +, NO3 -, NO2 -) in the soil was 144.59 mg kg-1. The obtained value of inorganic N in the soil is classified as very high for fertility according to Mexican regulations (DOF, 2000). Loss of N has been observed in agricultural soils regardless of the inorganic N content it has (Chen et al., 2014). The C/N ratio controls the development of the soil microbiota as well as the process of mineralization of organic matter (Probert et al., 2005). The C/N ratio in the soil was 26.26, which represents a soil with excess carbon and energy (soils with a C/N ratio > 11.5). Therefore, an organic amendment is required that does not release all its energy or organic matter in the short term, that is, an amendment with a lower C/N ratio than that of the soil. Thus, the release of mineral N and the TOC content in the soil can be controlled to reach C/N ratio values of 8.5 to 11.5, which are considered ideal in agricultural soil (Abbasi et al., 2015).
Table 1 Physicochemical characteristics of the soil and biosolids used in the experiment.
| Soil | Biosolid | |
| pH | 6.83 ± 0.10 | 7.37 ± 0.06 |
| EC (dS m-1) | 0.67 ± 0.03 | 3.84 ± 0.02 |
| Texture | Clay | - |
| TOC (g kg-1) | 55.15 ± 1.76 | 78.21 ± 2.37 |
| TN (g kg-1) | 2.10 ± 0.77 | 6.88 ± 0.05 |
| C/N | 26.26 ± 2.51 | 11.37 ± 0.84 |
| NH4+ (mg kg-1) | 120.57 ± 11.99 | 859.31 ± 13.53 |
| NO3- (mg kg-1) | 23.13 ± 4.07 | 129.68 ± 1.72 |
| NO2- (mg kg-1) | 0.89 ± 0.24 | 18.52 ± 4.01 |
Note. The values represent the mean, ± represents the standard deviation (n = 3). The results are obtained on a dry basis from the soil and biosolid as appropriate.
Source: Author’s own elaboration.
The biosolids presented a pH of 7.37, which is considered neutral. The EC value was 3.84 dS m-1, which is higher than that presented by the soil. The TOC and TN content were 78.21 g kg-1 and 6.88 g kg-1. The C/N ratio was 11.37, so it was expected that, when added to the soil, the N content would increase. Inorganic N content (sum of NH4 +, NO3 -, NO2 -) of the biosolids was 1.008 g kg-1, which represents an important contribution of mineral N that could be reflected in the volatilization of N as NH3. These reported values for the different parameters in biosolids coincide with those reported in other studies (de Melo W et al., 2018; Medina-Herrera et al., 2020).
The pH of the soil and biosolids samples was 6.83 and 7.29, this parameter was not significantly affected by adding the biosolids to the soil at the beginning and end of the experiment (p ≤ 0.05). However, a downward trend was observed in pH in T1, T2, and T3 treatments, with values of 6.72, 6.52, and 7.15 at the end of incubation. With respect to the EC, the amended treatments with biosolids (T1 and T2) showed a significant increase by 2.40 and 3.10 times (EC = 1.64 and 2.12 dS m-1) from the initial soil value. The T0 and T3 treatments showed no significant changes (p ≤ 0.05) in the EC with respect to the beginning of the experiment.
The CO2 emission presented significant differences (p ≤ 0.05) among treatments (Figure 1). The CO2 derived from the mineralization of the biosolids is the CO2 emission in the T1 and T2, that is, the biosolid treatment minus the one produced in the soil without biosolids (T0), which after 56 days was 1014.21 mg kg-1 and 1654.73 mg kg-1, respectively. At the end of the incubation, an average of 10.12% of the organic C of the biosolid was mineralized when added to the agricultural soil, while the treatment with only biosolid (T3) mineralized 4.67% of its organic C.
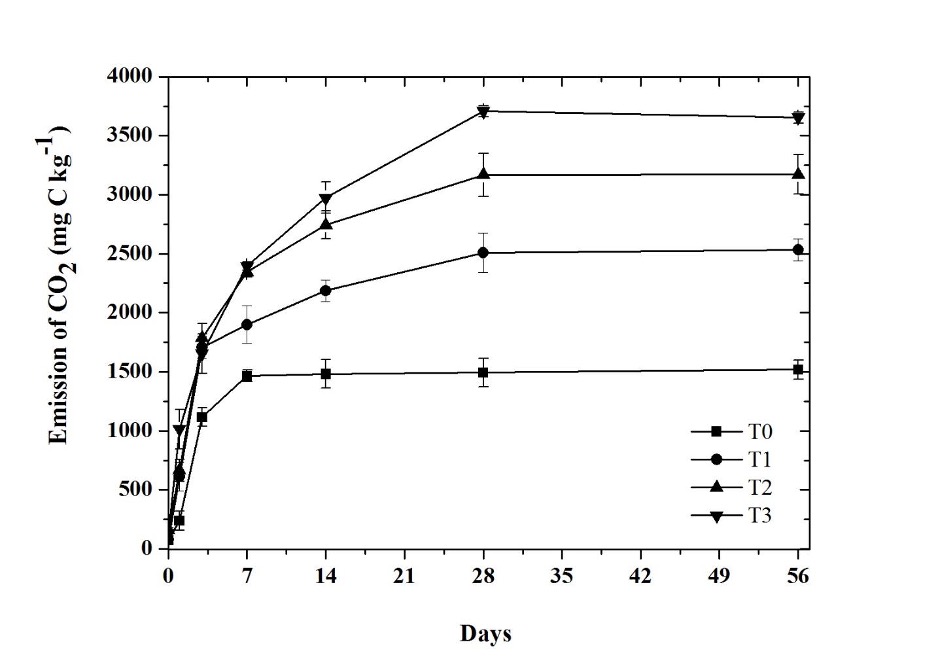
Source: Author’s own elaboration.
Figure 1 CO2 emissions in treatments: control without amendment (T0), 100 mg NH4 + kg-1 (T1), 200 mg NH4 + kg-1 (T2), and biosolid (T3). The bars at each point represent the standard deviation (n = 3).
The volatilization of NH3 increased proportionally with the increase in the application rate of biosolids. The greatest volatilization occurred in the first 14 days of incubation (Figure 2). The total amount of NH3 volatilized after 56 days was 12.75 mg kg-1, 34.83 mg kg-1, 38.85 mg kg-1, and 64.68 mg kg-1 for treatments T0, T1, T2, and T3, respectively. These values were 2.73 (T1), 3.05 (T2), and 5.07 (T3) times more than the volatilized NH3 in T0. The T0 and T3 treatments showed significant differences (p ≤ 0.05) in the volatilization of NH3, due to the fact that they are different matrices (soil and biosolid). Therefore, it was established that the volatilized NH3 comes from the NH4 + content of the biosolid added to the soil, with NH3 volatilization percentages of 34.83%, 19.42%, and 7.52% for the T1, T2, and T3 treatments, respectively.
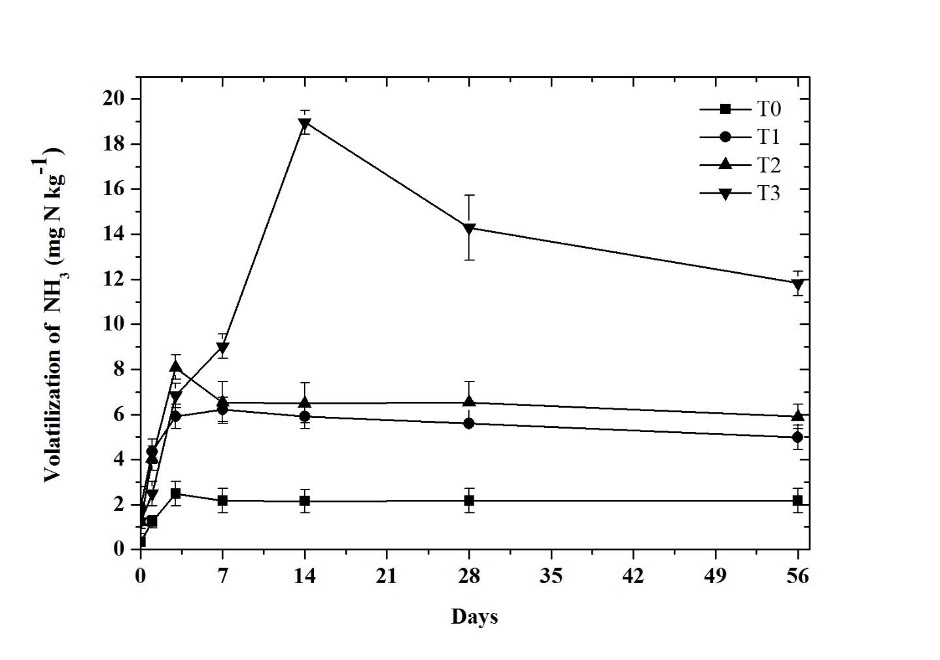
Source: Author’s own elaboration.
Figure 2 Volatilization of NH3 in treatments: control without amendment (T0), 100 mg NH4 + kg-1 (T1), 200 mg NH4 + kg-1 (T2), and biosolid (T3). The bars at each point represent the standard deviation (n = 3).
In each one of the biosolid treatments (T1, T2, and T3), the concentration of NH4 + increased in the first days of incubation, then decreased, and increased again on day 56 (Figure 3a). The concentration of NH4 + on dry soil from the control treatment (T0) remained < 30 mg kg-1 during the incubation period. The average concentration of NH4 + increased with the application rate of biosolid, although there was no significant difference (p ≤ 0.05) between T1 and T2 (75.84 mg kg-1 and 84.05 mg kg-1). However, there were significant differences (p ≤ 0.05) in the T0 and T3 treatments (20.36 mg kg-1 and 163.85 mg kg-1).
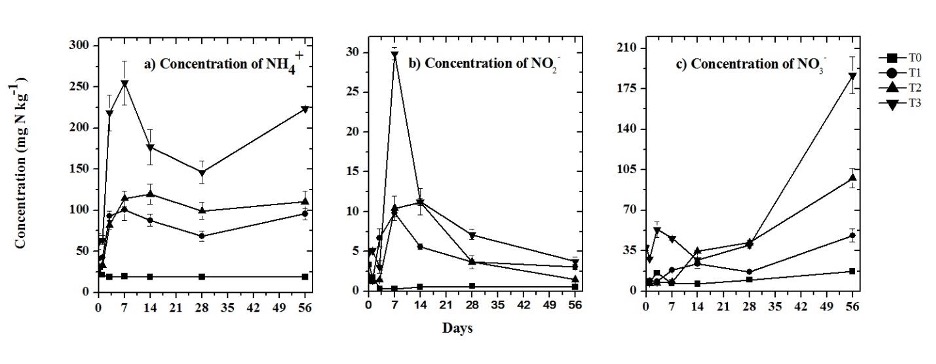
Source: Author’s own elaboration.
Figure 3 Concentration of inorganic nitrogen a) NH4 +, b) NO2 -, c) NO3 - in the treatments: control without amendment (T0), 100 mg NH4 + kg-1 (T1), 200 mg NH4 + kg-1 (T2), and biosolid (T3). The bars at each point represent the standard deviation (n = 3).
The concentration of NO2 - in T0 remained < 3.5 mg kg-1 from the beginning to the end of the incubation period. With respect to biosolids treatments (T1 and T2), the concentration of NO2 - decreased in the first three days. On day 7, there were significant differences (p ≤ 0.05) in the concentration of NO2 - for the T1, T2, and T3 treatments, with maximum concentrations of 9.83 mg kg-1, 11.22 mg kg-1, and 29.87 mg kg-1, respectively. At the end of the incubation period, the concentrations of NO2 - decreased to 3.04 mg kg-1, 1.48 mg kg-1, and 3.77 mg kg-1 for the same treatments (Figure 3b).
The concentration of NO3 - for the treatments remained constant (≤ 18 mg kg-1) in the first days of incubation, except in T3, with a gradual increase from day 28 to reach concentrations of NO3 - of 17.18 mg kg-1, 48.28 mg kg-1, 97.76 mg kg-1, and 186.68 mg kg-1 for T0, T1, T2, and T3, at the end of the incubation period. The average concentration of NO3 - in the T1 and T2 treatments increased with higher added doses of biosolids (Figure 3c). The average amount of N available in the biosolid for the three treatments (T1, T2 and T3) was 3.27 g kg-1, corresponding to 47.65% of its TN content.
About MB-C, by day 7 of incubation, MB-C increased 8.7 and 9.4 times from its initial values in T1 and T2 treatments, while for T0 and T3 treatments it increased 3.6 and 3.2 times their value on day 14 of incubation, reaching maximum MB-C values of 500.47 µg g-1, 1158.77 µg g-1, 1429.65 µg g-1, and 1587.4 µg g-1 for T0, T1, T2, and T3, respectively (Figure 4a). No significant difference was found (p ≤ 0.05) between the T1, T2, and T3 treatments; however, the treatments were significantly different from the T0 treatment. The percentage of organic C of the biosolid added to the treatments incorporated in the MB-C was 6.57% and 3.95% for the T1 and T2 treatments, and 2.03% for the T3 treatment.
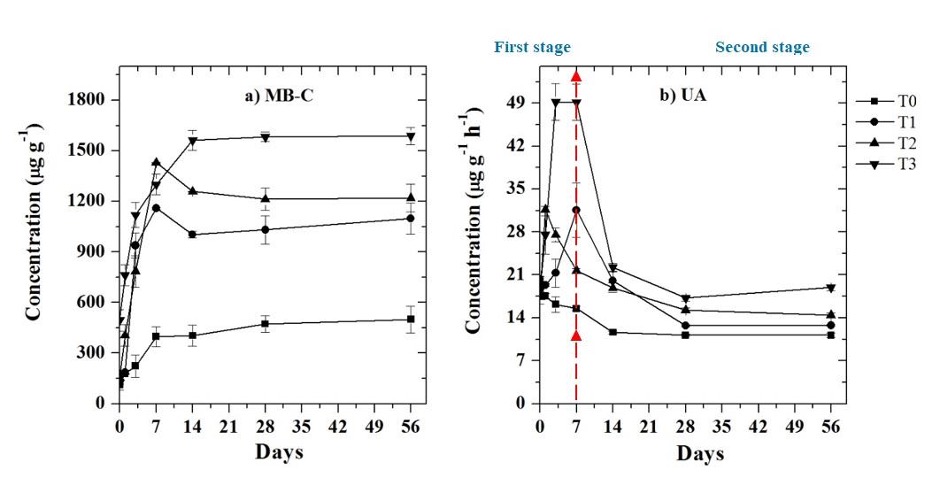
Source: Author’s own elaboration.
Figure 4 Biological properties a) Microbial biomass carbon (MB-C) and b) Urease activity (UA) in the different treatments: control without amendment (T0), 100 mg NH4 + kg-1 (T1), 200 mg NH4 + kg-1 (T2), and biosolid (T3). The bars at each point represent the standard deviation (n = 3).
The average UA in the T0 and T3 treatments were significantly different (p ≤ 0.05) from each other; nevertheless, they did not differ from the T1 and T2 treatments, so it can be assumed that the application rate of biosolids does not affect this parameter. However, UA increased in the first days of incubation for biosolid treatments (T1 on day 7 = 55.20%, T2 on day 1 = 81.40%, T3 on day 3 = 165.80%) compared to the non-biosolid treatment (T0) where this maximum activity occurred on day 0, and then gradually decreased (Figure 4b). With respect to the maximum values reached of UA based on the NH4 + content for T0, T1, T2, and T3 were 19.46 µg g-1 h-1 (day 0), 31.57 µg g-1 h-1 (day 7), 31.68 µg g-1 h-1 (day 1), and 49.21 µg g-1 h-1 (day 3), respectively. After reaching these values, the UA was reduced for all treatments by 45.50% on average with respect to the maximum values reached, the T0 treatment appeared as the one with the greatest decrease in UA.
Discussion
At the end of incubation, the pH values showed a slight decrease in soils amended with biosolid (T1 and T2), being higher according to the dose of biosolid applied. This may be due to the formation of organic acids as an effect of the addition of biosolid to the soil. A similar behavior was reported by Tamaríz et al. (2015), the pH of the unamended soil was neutral with a value of 7.1 and the soil amended with biosolids was slightly acidic with a value of 6.3. Roig et al. (2012) observed a decrease in pH as a result of the application of long-term biosolids to a carbonate-rich soil capable of buffering free acidity in the soil solution. This phenomenon was directly related to the mineralization of organic matter. A similar effect was seen on the soil of this study. On the other hand, it was found that the EC increased according to the dose of biosolid applied, which coincides with the results obtained in other studies (Rojas-Oropeza et al., 2010; Sciubba et al., 2014). However, these values would not imply salinity problems in the soil studied (EC > 4 dS m-1).
With reference to the dynamics of C, the average CO2 emission did not differ significantly (p ≤ 0.05) among treatments. As for the organic C of the biosolid applied to the soil, 10.12% was mineralized during the 56 days of incubation. Higher levels of organic C mineralization of biosolids were reported by Jin et al. (2011), who found mineralization levels of 11%-62% in their treatments after 120 days of application. Rojas-Oropeza et al. (2010) reported that 60% of the biosolids were mineralized in a saline-sodium soil of Lake Texcoco after 90 days of adjusting their CRA to 20% and an incubation temperature of 20 °C. Another study by López-Valdez et al. (2010) reported 16% and 27% biosolid mineralization for a saline-alkaline soil of Lake Texcoco with a pH of 10.3 and an agricultural soil of Otumba in the State of Mexico with a pH of 7.8, respectively. Differences in mineralization percentages may be due to differences in biosolids characteristics (TOC and TN), as well as soil properties both physical-chemical (pH, EC, and texture) and biological (rhizospheric activity, enzymatic activity, microbial biomass and its structure), or even to the incubation conditions of the experiment (incubation period, WHC, and incubation temperature) (Curtin et al., 2014; Harrison-Kirk et al., 2014).
With respect to the volatilization of NH3, the results obtained show an increase as the application rate of biosolid increases as well. However, at the beginning of incubation, the volatilization percentages of NH3 derived from NH4 + of the added biosolids were found to be lower at higher application rates of biosolids. The average volatilized NH3 value was 37.78%. Lower percentages of volatilization of NH3 have been reported in other studies, reaching values of 8% to 17% of the total NH4 + present in the biosolids added in their experiments (Behera et al., 2013; Guerra et al., 2004). Within a period of 90 days of incubation, Rojas-Oropeza et al. (2010) reported values above 63% of NH4 + present in the biosolid added to the soil. The detriment of NH3 by this mechanism may be due to different causes: 1) high soil pH, which increases the concentration of free NH3; 2) environmental conditions such as soil aridity or moisture, high temperatures, soil texture; and 3) low soil cation exchange capacity (de Melo et al., 2018; Rigby & Smith, 2014). In all treatments, the pH was maintained at values of 6.5 to 7.3, which are considered neutral, so the effect of pH for the release of free NH3 is negligible. Therefore, the volatilization percentages of NH3 observed in this study could be attributed to the intrinsic characteristics of the soil, such as texture or moisture, as reported by Peng et al. (2015).
In the same context, the concentration of NH4 + in soil is affected by different abiotic and biotic processes, for example, volatilization of NH3, fixation in soil matrix, immobilization of NH4 +, mineralization of N, and nitrification (López-Valdez et al., 2010; Rigby & Smith, 2014). The concentration of NH4 + increased from the beginning of incubation to day 7 and 14, where it had a subsequent drop, which coincided with an increase in NO3 - on those same days. This indicates that part of NH4 + was oxidized to NO3 - (Heil et al., 2016), which can be attributed to nitrification, where it was higher in T1 and T2 treatments than in T0 and T3. However, by day 56 the concentration of NH4 + increased again as did the concentration of NO3 -. This may be due to the mineralization of a fraction of organic N that is difficult to assimilate within the biosolid, coinciding with the results obtained by Roig et al. (2012).
In the NO3 - concentration dynamics, both the T0 treatment and the T1 and T2 biosolid treatments did not have an increase or decrease in the first 3 days of incubation. Such behavior could indicate that nitrification did not occur during this period. However, from day 7 onwards, the concentration of NO3 - gradually increased until reaching concentrations on day 56 of 2.43, 6.01, 11.86, and 4.93 times the initial value for the T0 (17.18 mg kg-1), T1 (48.29 mg kg-1), T2 (97.76 mg kg-1), and T3 (186.68 mg kg-1) treatments. The processes of fixation and immobilization of N were not evaluated in this study, for which purpose the TN and nitrogen of microbial biomass during the dynamics would need to be determined (Pajares & Bohannan, 2016).
For MB-C, a significant difference was obtained between T0 and the rest of the treatments (p ≤ 0.05), although an increase in this parameter was observed with respect to the application rate of the biosolid. This result coincides with the ones obtained by other authors and can be attributed to the stimulation of soil microbial activity due to the incorporation of nutrients and organic matter in the soil by the addition of biosolid (Celis et al., 2013; Sciubba et al., 2013; Xue et al., 2015). The behavior of MB-C on day 7 for T1 and T2 could be attributed to the incorporation of exogenous microorganisms or easily assimilated substrates of the biosolid into the soil, stimulating of the natural activity of the soil microbiota and even a change in the structure of the microbial community (Azeem et al., 2016; de Melo et al., 2018). It may also be due to a phenomenon known as the "priming effect" (Celis et al., 2011; Rojas-Oropeza et al., 2010). The phenomenon of a stimulation of the mineralization of organic matter that is incorporated into the soil was called the “priming effect” by Bingeman et al. (1953). This means that the priming effect was reflected in the increase in decomposition rate of soil organic matter after adding the biosolids to the soil, linked to an increase in microbial biomass as a consequence of greater availability of energy released by the decomposition of organic matter in the biosolids added. Thus, an increase in both CO2 emission and C-MB from day 7 can be observed in Figure 1 and Figure 4a, respectively.
With reference to UA, an increase was observed for biosolid treatments (T1, T2, and T3) in the first seven days of incubation, followed by an average decrease of 45.50% by the end of the period. These results coincide with those obtained by Adetunji et al. (2017), which showed that UA increases with nitrogen-rich organic fertilization such as biosolids (C/N = 11.36) and decreases with soil tillage. In addition, the higher UA value was not affected by the biosolid dose applied. However, at higher doses (T2) this value was reached by day 1, whereas for treatment with lower doses (T1) it was reached until day 7. This may be associated with the total organic nitrogen content present in the treatments (T0 = 1956.3 mg kg-1, T1 = 2642.0 mg kg-1, T2 = 3327.7 mg kg-1, and T3 = 5891.0 mg kg-1). This statement coincides with the results obtained by Celis et al. (2011), where they associated high levels of urease with a high content of nitrogen compounds in the wastewater sludge evaluated. The behavior of UA in this study can be divided into two stages. In the first stage it increased 100% on average for the T1, T2, and T3 treatments in the first 7 days, as a result of the rapid mineralization of the nitrogen content in the biosolids. In the second stage, an average reduction of 45.50% was present in all treatments until the end of incubation. This could be attributed to a decrease in the synthesis of this enzyme, due to the increased concentration of NH4 + (Chen et al., 2018; Núñez et al., 2012; Redmile-Gordon et al., 2015). It may also be due to rapid mineralization of easily assimilated N. The inhibition by NH4 + of the synthesis of urease and other N-metabolizing systems in various microorganisms are linked to a complex bicyclic cascade controlled by the glutamine formed by the glutamine synthetase activity associated with NH4 + assimilation (Chuckran et al., 2021). McCarty et al. (1992) evaluated the effects of different amounts of inorganic N on urease production in soils amended with glucose, and the author showed that, although microbial metabolism measured by CO2 production was stimulated by the addition of different doses of NH4 + to amended soils, urease production was decreased at a higher dose of N. However, Diacono & Montemurro (2011) mentioned that inorganic N forms (NH4 + y NO3 -) are released over a longer period (> 90 days), which does not coincide with the results obtained in this study for NH4 +. This was produced from the start of the dynamics until reaching its highest values for all treatments between day 7 and 14 (Figure 3), coinciding with the decrease in urease activity (Figure 4b).
Conclusions
The application of biosolids to agricultural soil led to a stimulation of soil microbial activity. The CO2 emissions and MB-C increased after applying the biosolids to the soil, and the increase was proportional to the application rate. Moreover, the addition of biosolids to the soils studied did not affect the pH. On the other hand, the EC was affected and, although the values reached do not represent salinity problems, it is recommended to monitor the EC in soils amended with biosolids in this region. The application of biosolids in the soil improved the quality of C and N content available, which allows for better crop growth. In addition, it was observed that the mineralization of biosolids has two forms of mineralizable organic nitrogen, so the supply of nutrients is favored over time. Easily assimilated nitrogen content stimulates an increase in UA due to the rapid mineralization of this, however, it can also be inhibited over time by the increase of inorganic nitrogen in the soil. Finally, to better establish the existence of a positive or negative effect of the addition of biosolids on the microbial activity of soils, it is necessary to conduct additional studies of both the chemical composition of the biosolids and their mineralization in order to determine such effects and thus establish good practices for their use.
Conflicts of interest
The authors have declared that no competing interests exist.











 nueva página del texto (beta)
nueva página del texto (beta)


