INTRODUCTION
Heavy metals leached from soil and rocks to aquatic systems are na turally ubiquitous and pose minimal threats to the environment and human health (Páez-Osuna & Osuna-Martínez, 2015). Several anthro pogenic sources, however, such as agriculture, aquaculture, and mi ning increase metal burdens exceeding natural levels. Since bivalves accumulate and exhibit efficient strategies to deal with the potential toxic effect of metals (Arifin & Bendell-Young, 1997), they are used as biomarkers for monitoring metal contamination in aquatic systems (Kanthai et al., 2014).
There are reports from around the world that have used wild and cultured oyster populations from the genus Crassostrea spp. as biomo nitors of trace metals. For instance, the spatial pattern of metal accu mulation within intertidal bivalves was studied in England to measu re the ecological impact of introducing Crassostrea gigas (Thunberg, 1873) in nature mussels (Bray et al., 2015). Sarong et al. (2015) found high levels of Pb, Cd, and Zn in the body tissue of C. gigas harvested from the estuary of Lamnyong River, Indonesia, and Ochoa et al. (2013) concluded that metallic pollutants did not affect oyster cultures in Ebro Delta, Spain. Concentrations of heavy metals (Cu, As, Ni, Pb, and Cd) below the maximum levels for foodstuffs in Brazilian legislation were found in the mussel Perna perna Linnaeus, 1758 and C. gigas in pro duction areas.
Specifically along the central eastern Gulf of California coast, Mexi co, Bergés-Tiznado et al. (2013) evaluated the presence of arsenic compounds in the mangrove oyster Crassostrea corteziensis (Hertlein, 1951). Furthermore, Jara-Marini et al. (2013) compared the bioaccu mulation of trace metals in six bivalve species. Analyzing soft tissue of C. gigas from culture sites along the east coast of the Gulf of Califor nia, Vázquez-Boucard et al. (2014) found Zn, Cd, and Pb concentrations above Mexican tolerance levels due to the presence of pesticides.
The Pacific oyster Crassostrea gigas is the most cultivated shellfish species in the world. Its total standing stock in 2013 was 555,994 t (FAO, 2015). It was introduced in Mexico for commercial purposes in the mid-1970s. The Gulf of California is the most important culture region, having registered a significant increase in the last decade from 407.27 t harvested in 2006, to 3,042 t in 2014 (SAGARPA, 2015). This species is mostly reared in estuarine zones where natural productivity is high, thus ensuring its development. At same time, however, these water bo dies are continuously exposed to pollutants from natural and human activities that spill out along rivers leading to the gulf. Some pollutants include heavy metals (Páez-Osuna et al., 1991). Several studies in the Gulf of California indicate that various anthropogenic activities such as mining (Cadena-Cárdenas et al., 2009), agriculture, aquaculture (Páez-Osuna et al., 2003), and urban development (Ruiz-Luna & de la Lanza-Espino, 1999) are sources of diverse heavy metals.
Compared to other coastal areas of the Gulf of California, such as the east coast (Cadena-Cárdenas et al., 2009) and the northern region (García-Rico et al., 2001), the traditional southeastern oyster-culture areas in the state of Sinaloa are relatively more affected by anthro pogenic activities, mostly agriculture. Its agriculture production of ve getables and grain crops make it a region of national importance. It is surrounded by some 350,000 ha of agriculture, forest, animal farms, and more than 50 shrimp farms (Honorable Ayuntamiento de Guasave, 2016). Therefore, these areas are under permanent pressure from an thropogenic pollutants from various sources located adjacent to the harvesting areas or at a relatively short distance from where oysters are cultured.
Since oysters are sedentary and filter feeders, they are suscepti ble to metal accumulation and, therefore, are ideal sentinel organisms for assessing environmental pollution along tropical and subtropical coasts (Páez-Osuna et al., 1995). Further, this bivalve can be a vector of toxic chemicals for humans because is commonly consumed raw. The information on the levels of heavy metals in oyster species from the southeastern coast of the Gulf of California refers to mangrove (C. corteziensis and C. palmula, Carpenter, 1857), and rock C. Iridescens (Hanley, 1854) wild populations (Páez-Osuna et al., 1991, 1993, 2015). There are few studies on cultured oysters (Osuna-Martínez et al., 2010, 2011; Vázquez-Boucart et al., 2014) from different Sinaloa coastal la goons have compared metal burdens in both rainy and dry seasons. Nevertheless, no information is available on metal concentrations in C. gigas throughout its culture cycle. The presence of high metal levels in cultivated oyster may be an effect of anthropogenic activities, thus indicating a potential risk for human health.
We undertook this investigation to study the status of metal con centrations in cultured Pacific oyster, C. gigas, from a farm located along the southeastern Gulf of California during March-December 2011.
MATERIALS AND METHODS
Study area. La Pitahaya Estuary (Figure 1) is located on the southeas tern coast of the Gulf of California (25°21’N, 108°38’W), in the state of Sinaloa, Mexico, and is a part of the San-Ignacio-Navachiste-Macapule (SINM) lagoon system. The lagoon system is a marine environment during most of the year due to its two outlets that permanently con nect it with the Gulf of California. The lagoon area of SINM has around 22,314 ha, with an estimated population of 91,156 people (Páez-Osuna & Osuna-Martínez, 2015). Mangrove communities surround La Pitaha ya Estuary. The climate of the study area is temperate-subhumid with summer rains (INEGI, 2001). The main activity of the study area is inten sive agriculture (105,000 ha) characterized by the use of irrigation with the application of high levels of fertilizers and pesticides (Hernández-Cornejo et al., 2005; Páez-Osuna & Osuna-Martínez, 2015). Artisanal fishing and well-developed industrial shrimp fisheries are present (Ruiz-Luna & de la Lanza-Espino, 1999). There are 25 shrimp farms equivalent to 6,621 ha and broiler chickens (77,785 chickens/year, Páez-Osuna & Osuna Martínez, 2015).
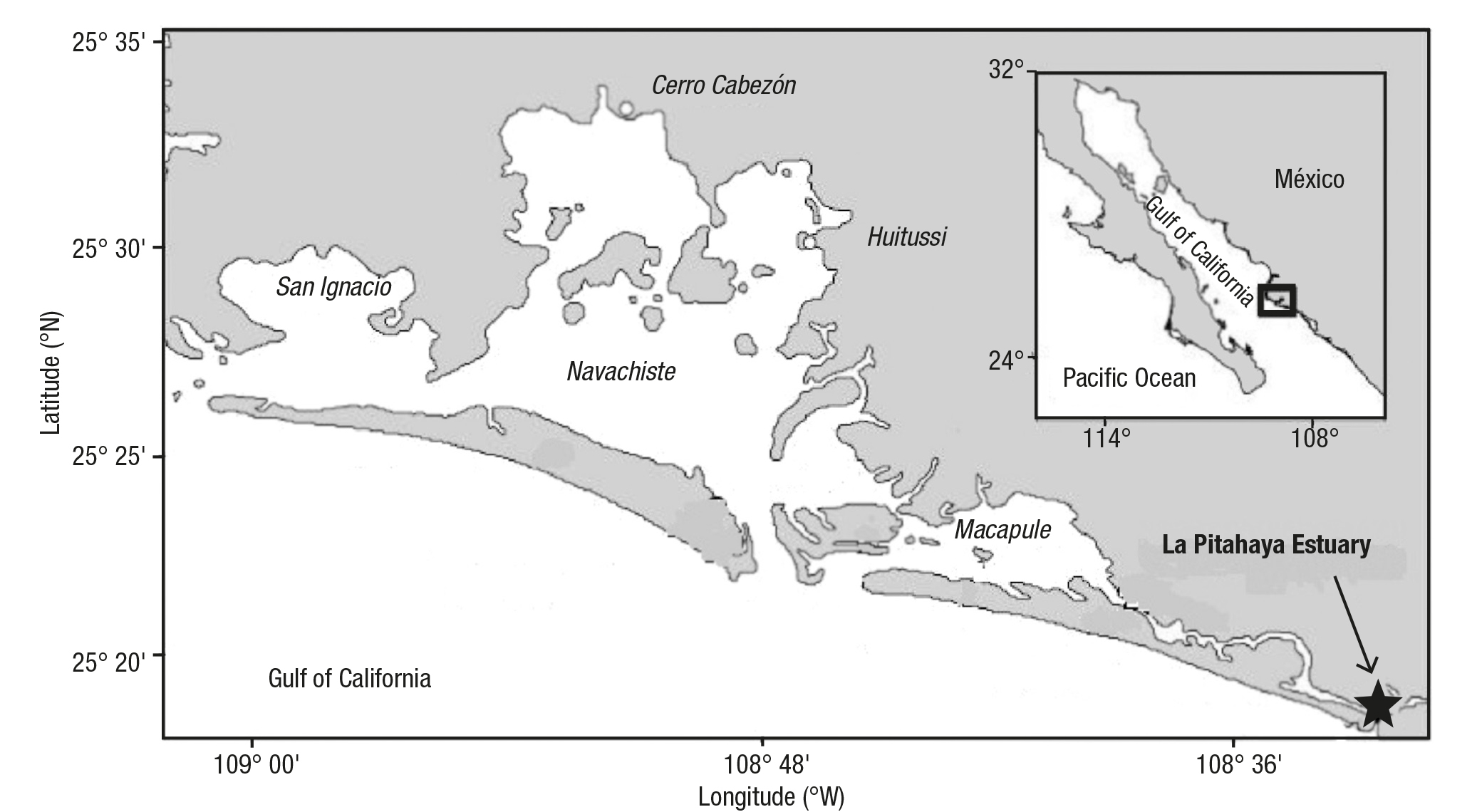
Figure 1 Location of the study area. La Pitahaya estuary (star), part of the San Ignacio-Navachiste-Macapule lagoon system, in Sinaloa, Mexico.
Experimental animals. Seven thousand Japanese oyster juveniles (44.78 ± 7.97 mm shell height, SH; 5.56 ± 2.20 g body weight, BW) were cultured in racks suspended in a long-line system (n = 250 oys ters/rack). Oysters were acclimated as mentioned by Gallo-García et al. (2001) and cultivated according to García-Ulloa et al. (2008).
We recorded oyster BW and water quality parameters monthly (March to December 2011). The BW of 50 oysters was measured in situ with a portable balance (0.00 g). Water temperature and dissolved oxy gen, DO, were determined with an oximeter (YSI 55/12FT, Ohio, USA), salinity with a refractometer (ATAGO, S/Mill), pH by using a pH meter (HANNA, HI 8314, USA), and depth and transparency with a Secchi disk.
Trace metal analysis. Monthly (from March to December 2011), a total of 30 oysters were collected, rinsed with sea water, stored on ice in po lyethylene plastic bags, and transported to the laboratory for cleaning, sacrificing, and shucking. Thus, a pool of 30 oysters was obtained each month. Oysters were chosen of nearly equal length to limit possible size differences given. Bivalves were opened with a knife to remove soft tissues from the shells and thoroughly washed with double distillated water. Samples were subsequently freeze dried, pulverized in a mortar, and homogenized by quartering, so that all fractions of the sample were equal in composition. The flesh weights and dry weights of the samples were recorded using a digital analytical balance (AE Adam, 0.001 g). To avoid metal residues, we used only high-quality reagents (GR grade, Merck Company). All material used were first cleaned with nitric acid (10%) for a 24 h period and rinsed with double distillated water.
To analyze metal burdens, each month an aliquot (1.27-1.46 g, dry weight) of oyster sample was digested with HNO3 using a microwave digester (Paar Physica Multiwave Six Place) at 300 W for 5 min, then at 600 W for 10 min. H2O2 was then slowly added to the vessels, which were kept in a hood until all bubbling ceased. The digestion procedure proved satisfactory. Digestion of Cu, Cr, Cd, Ni, Pb, Zn, and Hg were performed in 50 mL lined digestion vessels, while As was digested in 100 mL vessels, both equipped with safety relief valves. After digestion, extracts were allowed to cool at room temperature for 25 min and then diluted to 10 ml with de-ionized water. Digests were stored in labelled polyethylene vials at 0-5 °C until we performed the metal analysis.
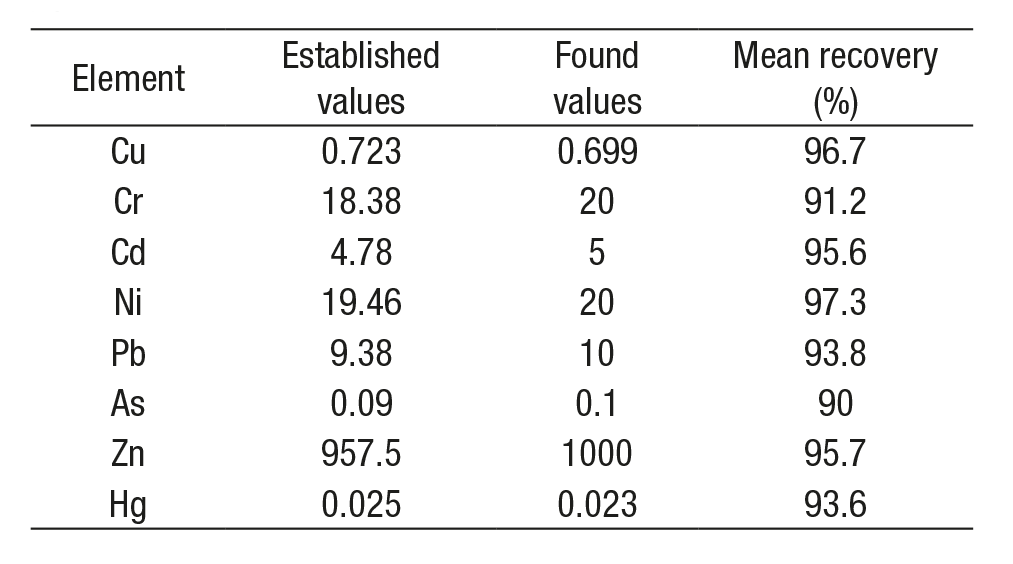
Cu, Cr, Cd, Ni, Pb, and Zn were analyzed by atomic absorption spec trophotometry (AAS) with flame (Perkin-Elmer, AAnalyst100), As by AAS with hydride generation, and Hg by AAS with cold vapor. For quality as surance, the oyster tissue standard reference material sample (1566b for oysters, National Bureau of Standards, NBS), reagent blanks, and duplicate samples were run with each digestion series. Experimental values for all metals (mean recovery) were in good agreement with the NBS certified values (Table 1). Heavy metal concentrations were calculated based on dry weight (µg g-1). For each element (Cu, Cr, Cd, Ni, Pb, As, Zn, and Hg) the detection limits were 0.032, 0.032, 0.008, 0.46, 0.10, 0.002, 0.039, and 0.0003 µg g-1 dry weight, respectively.
Table 1 Analytical results of standard reference oyster material (1566b) in this study (µg g-1, dry wt), developed in La Pitahaya estuary, Sinaloa, Mexico.
Statistical analyses. Descriptive statistics (mean, standard deviation, maximum and minimum limits) were used for metal concentrations each month. The coefficient of variation (CV) of metal burdens was used to test the reliability of data with regard to the effect of parameters and BW. Correlations were made with heavy metal levels, BW, and physic-chemical parameters. Statistical analyses were performed (p< 0.05) using the STATISTICA (StatSoft Inc., Tulsa, OK, USA) software package.
RESULTS
The mean water parameters were 26.91 ± 4.43 °C, 6.27 ± 1.27 mg L-1, 33.35 ± 3.98 ups, 7.49 ± 0.48, 1.86 ± 0.29 m, and 0.78 ± 0.26 m for temperature, OD, salinity, pH, depth, and transparency, respectively.
The concentration of each metal studied in C. gigas varied with the culture time. The highest values were observed as follows: As (0.48 µg g-1) and Pb (3.46 µg g-1) in April; Cd (21.4 µg g-1) in July; Cr (106 µg g-1), Ni (42.6 µg g-1), and Hg (0.041 µg g-1) in August; Cu (85 µg g-1) and Zn (418 µg g-1) in September. The mean metal concentrations (N = 10) were Cu 51.24 ± 25.29; Cr 24.97 ± 32.38, Cd 13.84 ± 4.22, Ni 10.26 ± 12.18, Pb 2.18 ± 1.28, As 0.37 ± 0.08, Zn 267.42 ± 92.29, and Hg 0.02 ± 0.01 µg g-1, dry weight (Table 2). The metals in the oyster samples showed the following rank order of accumulation: Zn>Cu>Cr>Cd>Ni>Pb>As>Hg.
Table 2 Concentration of Cu, Cr, Cd, Ni, Pb, As, Zn, and Hg (dry and fresh weight, µg g-1; mean, standard deviation, maximum and minimum limits) of cultivated oysters from La Pitahaya, Guasave, Sinaloa, Mexico, during 2011.
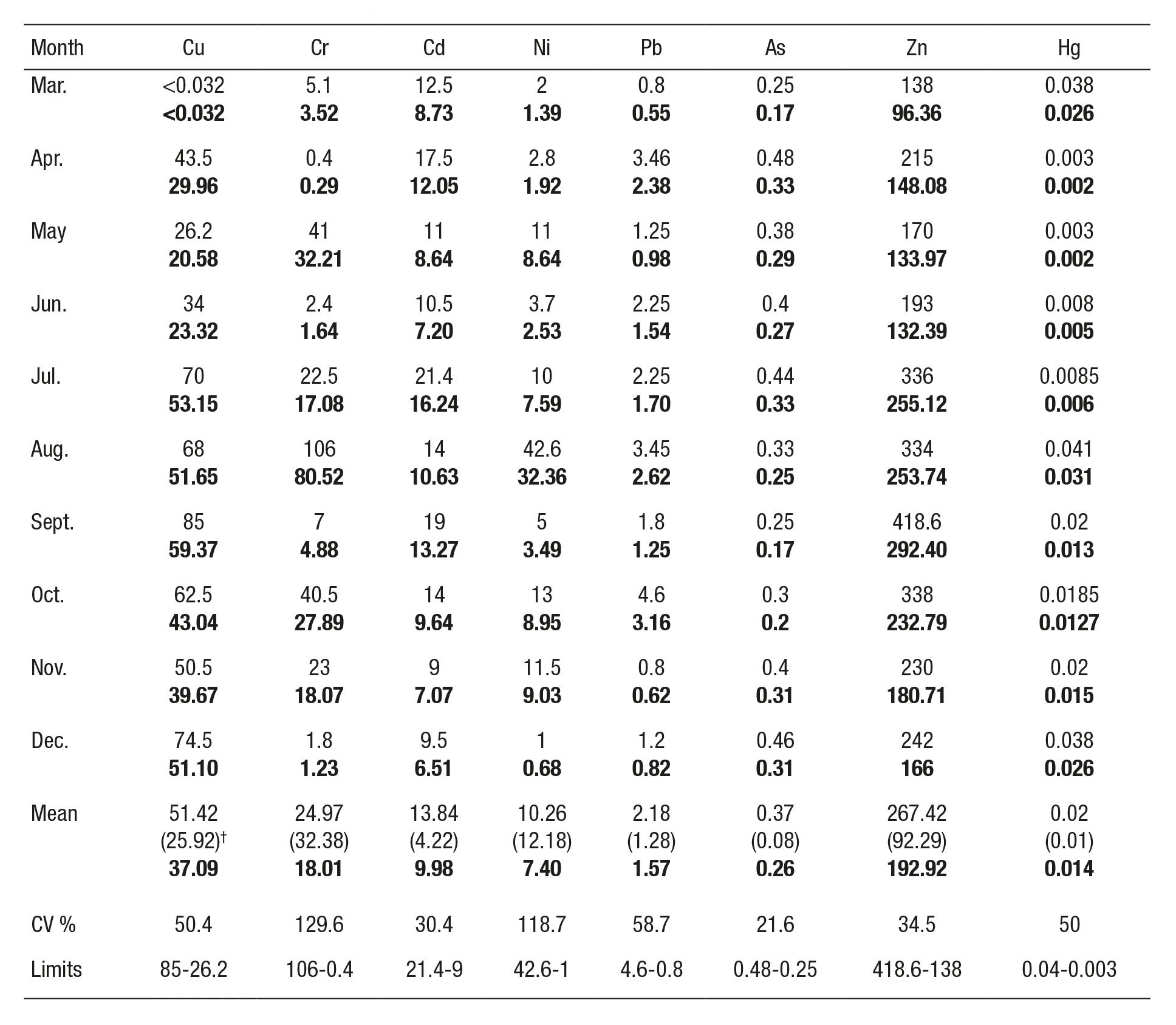
*Bold numbers = mean fresh weight basis (µg g-1). †Standard deviation.
Correlation analyses for metal levels, BW, and parameters (Table 3) showed a positive correlation of Cd, Ni, Pb, Zn, Cu, and Cr with BW and T °C, but a negative correlation of Cr, Ni, Pb, and Zn with pH and DO. Only the BW/T °C and depth/transparency were positively correlated. Strong correlations were obtained for Cr/Ni, Cu/Zn, and Cd/Zn.
Table 3 Spearman rank order correlations (r) for metal levels (Cu, Cr, Cd, Ni, Pb, As, Zn, and Hg), oyster weight, environmental parameters (T °C, depth, pH, salinity, DO and transparency) and between metals, in cultivated C. gigas from La Pitahaya, Sinaloa, Mexico, during March-December 2011.
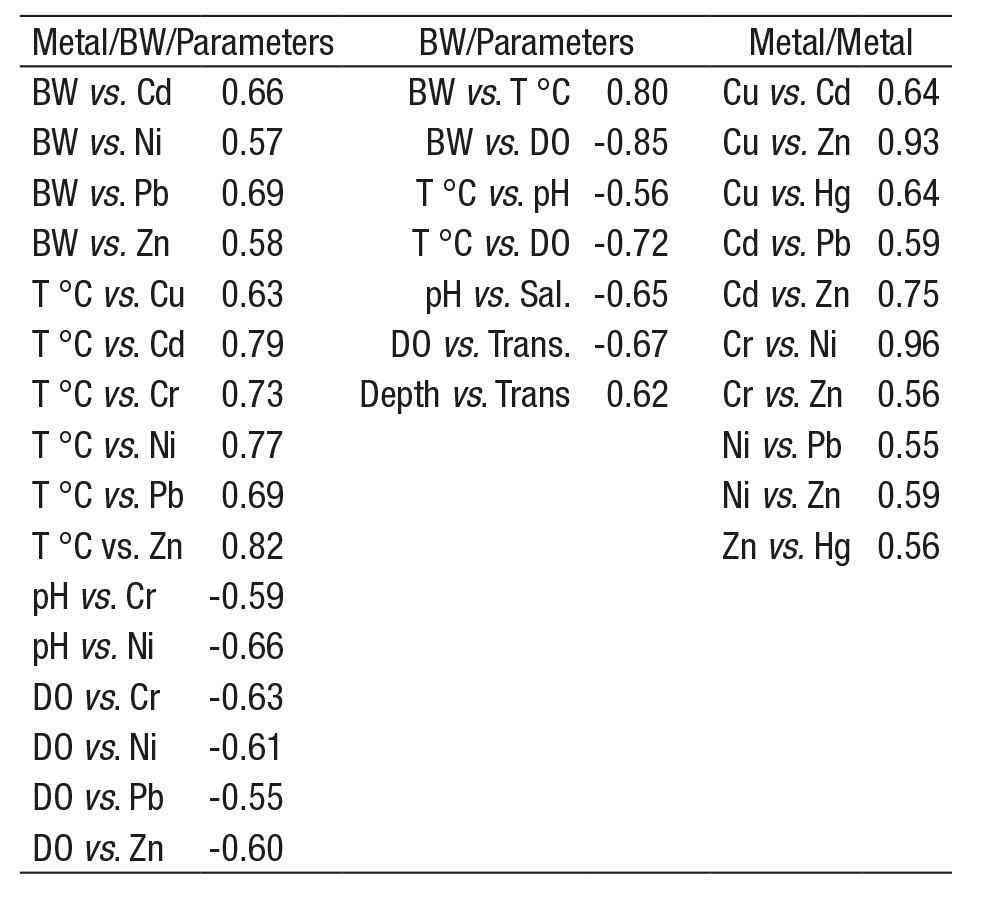
Only significant correlations (p< 0.05) are included.
DISCUSSION
The results of metal analysis showed that the concentrations of these eight elements in C. gigas reared at the La Pitahaya estuary, Sinaloa, varied monthly. Mean values of Cu and Cd burdens were higher when compared to other studies in Mexico but lower when compared to other countries (Table 4). Some trace metals are essential for normal deve lopment of mollusks (Bryan, 1971). Zn, Na, and K are needed for tissue formation and metabolic physiology, while Fe, Cu, and Al are involved in cellular metabolism, protein synthesis, and lipid/carbohydrate meta bolism (Barile, 2008). Levels higher than these requirements, however, may cause physiological damage that could threaten growth perfor mance. In this study, oyster BW showed a constant increase until July. However, with the exception of As and Pb, the remaining metal levels decreased from July to September. Loss of metals in soft tissue could be attributed to a combined effect of water salinity dilution during the rainy season (July to September) and spawning (Robinson et al., 2005; Le et al., 2015), which affect the metal levels by decreasing the con centrations. Jara-Marini et al. (2013) mention that Cu levels in adult oysters decrease during the post-spawning event and Lango-Reynoso et al. (2010) observed that variations of Cd accumulation in C. virginica (Gmelin, 1791) are a result of different stages of reproduction, among other factors. This suggests that oysters accumulated metals as their BW increased until the rainy season, which induced them to spawn and, consequently, caused the BW and metal levels to decrease. In wild populations, fluctuations in the trace metal levels of cultured oysters depend on several factors, such as the season. For instance, Páez-Osuna et al. (1995) concluded that levels of some metals vary sea sonally with gonad maturation, while Páez-Osuna & Marmolejo-Rivas (1990a, 1990b), and Páez-Osuna et al. (1995) found higher levels of Cu and Zn at the end of the reproductive cycle of several oyster species. These previous findings coincide with our results since the higher metal levels were obtained after the oysters reached their highest BW (July-August). As Páez-Osuna et al. (1995) determined, some metal concen trations (Cu, Cd, Cr, Ni, Zn, and Hg) during the oyster pre-spawning period (June) were low due to dilution of metals in the soft tissue as the BW increased.
Table 4 Metal concentrations (µg g-1d.w.) in wild and cultured oysters from various countries and the Gulf of California, Mexico.
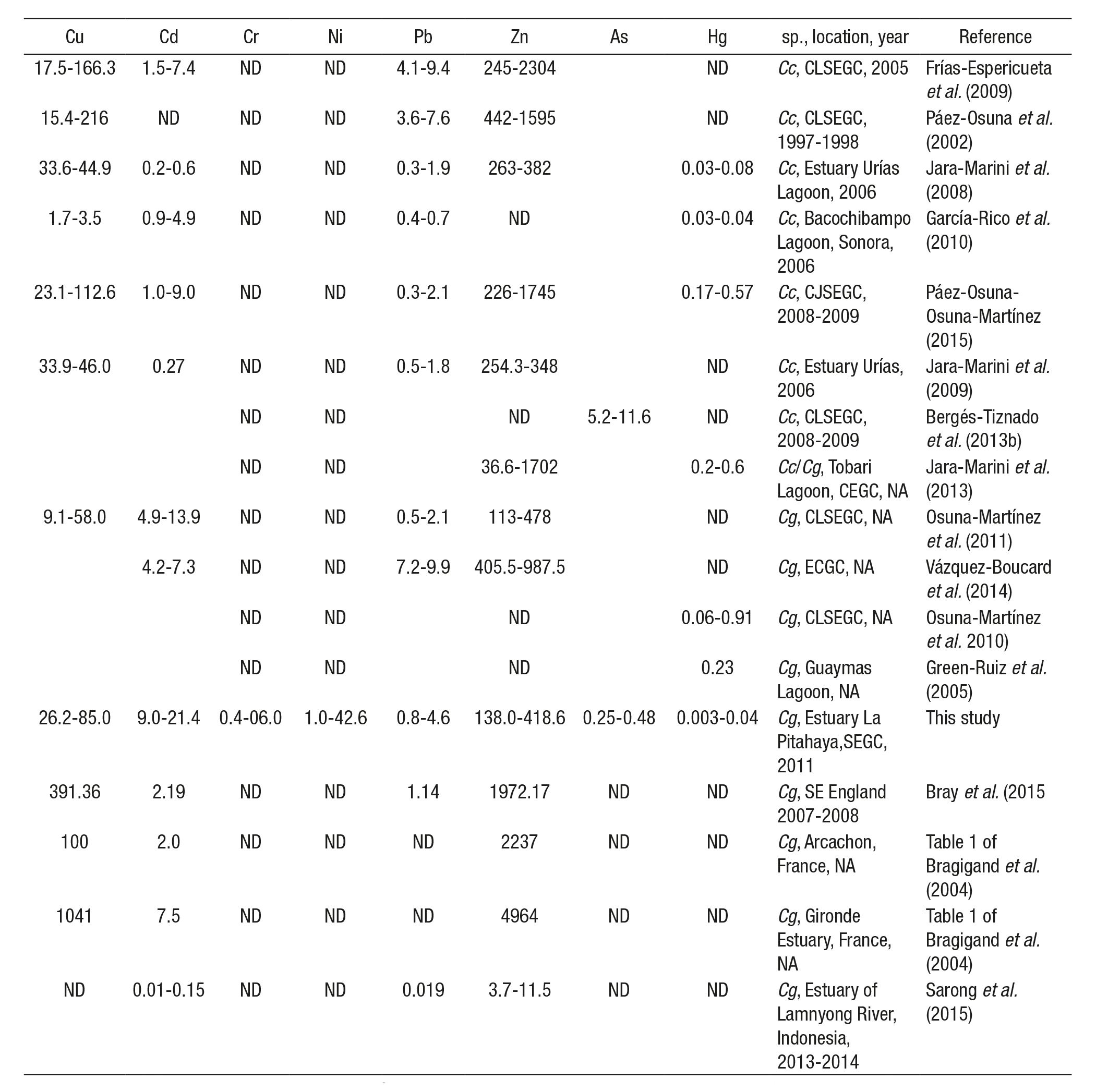
Cc = Crassostrea corteziensis; Cg = Crassostrea gigas; ND = not determined; NA = not available; CLSEGC = coastal lagoons from Southeast Gulf of California; ECGC = East Coast Gulf of California; SEGC = Southeast Gulf of California; CEGC = Central East Gulf of California.
Metal concentrations in C. gigas cultivated at La Pitahaya estuary were initially sampled when oysters had a shell height around 35-45 mm (3-4 months after culture started), coinciding with the specimen size collected by Páez-Osuna et al. (1991) for analyzing trace metals in different bivalve species. In this study, the ranking of metal concentra tions in C. gigas reflects the typical metal accumulation of other Cras sostrea species (Phillips & Muttarasin, 1985; Páez-Osuna & Marmolejo-Rivas, 1990a; Lin & Hsien, 1999), for which Zn posted the highest level and Hg the lowest.
Osuna-Martínez et al. (2010) and Ochoa et al. (2013) highlighted the importance of oyster depuration prior to being analyzed for heavy metal concentration in order to eliminate digested and undigested food and other particles from gut contents with a potentially high-trace me tal burden. This procedure allows us to quantify metal accumulation in body tissue. In our case, C. gigas was not depurated for analytical pur poses since, first, such a procedure is not a common practice among local farmers and vendors; second, this bivalve is consumed raw.
Different national and international metal regulation for seafood with a fresh weight basis establish the following limits: Cu = 32.5 µg g-1 (FAO, 1983), Cr = 13 µg g-1 (FDA, 2003), Cd = 0.5 µg g-1 (NOM, 1993), Ni = 80 µg g-1 (FDA, 2003), Pb = 1 µg g-1 (NOM, 1993), As = 80 µg g-1 (NOM, 1993), Zn = 718 µg g-1 (FDA, 1993), and Hg = 1 µg g-1 (NOM, 1993). The values of Cu, Cd, Cr, and Pb surpassed permissible concen trations. The Cd, Cu, Zn, Pb, and As burdens were higher compared to those reported by García-Rico et al. (2001) for C. gigas cultured on the northwestern Gulf of California coast (0.95, 4.55, 22.75, 0.62, and 0.06 µg g-1, respectively). Also, Cd, Cu, and Pb were higher than the levels obtained by Najiah et al. (2008) for C. iredalei (Faustino, 1932) cultivated in Malaysia (1.60, 38.9, and 0.17 µg g-1, respectively) and by Ochoa et al. (2013) rearing C. gigas in Spain (0.5, 38.83, and 0.26 µg g-1, respectively). Yet the levels of Cu, Ni, and Pb were lower compared to those reported by Cadena-Cárdenas et al. (2009) (181, 12.2, and 5.8 µg g-1, respectively) for several species of clams and mussels sampled along the Gulf of California. Differences can be attributed to the specific environmental conditions at those latitudes, human activities surroun ding the sampled area, and the species studied, among other factors.
García-Rico et al. (2001) and Robinson et al. (2005) indicate that a consistent association between particular groups of metals may reflect their common metabolic route. Cu, Cd, Ni, and Zn posted the highest correlation values among the metals since they are mostly related with anthropogenic activities (Jara-Marini et al., 2013). Some fertilizers such as Agrinutriente Micromax, Agrinutriente Cu and Agrinutriente Zn, and the fungicides based on zinc ethylene-bis-dithiocarbamate (Zineb) and cooper oxychloride (Cupravit) are commonly used in the region (Páez-Osuna et al., 1993) reflecting their source, since such compounds con tain Cu, Ni, and Zn. The SINM lagoon system is connected to the DR 075 and DR 063 Río Sinaloa irrigation districts and receives agrochemicals mainly from agriculture and aquaculture. Escobedo-Urías (2010) es timated that the SINM received 1243.10 t of inorganic nitrogen and 37 t of inorganic phosphorous from anthropogenic activities in 2007. In the same year, Gómez-Arroyo et al. (2013) reported that 6500 t of captan (pentachlorophenol-based fungicide) were applied in 5000 ha of cultures, in northern Sinaloa, among other insecticides (chlorpyri fos, Malathion, carbaryl cypermethrin), herbicides (atrazine, paraquat, mancozeb), and fungicides (cupravit, maneb, benomyl). For example, 121737 t of metam-sodium (dithiocarbamate) and cadusafes (organo phosphorus) were applied at the tomato plantations in Culiacan Valley, Sinaloa, during the 2011-2012 culture cycle. The agrochemicals are eventually leached from the soil and transported to the coastal zones where oyster farms are located, and therefore oysters could be exposed to these metals. As for C. corteziensis in the same lagoon system, Zn and Cu concentrations in C. gigas may be due to the use of Cu- and Zn-based agriculture products (Páez-Osuna & Osuna-Martínez, 2015), as well as the use and application rates of feed additives, liming mate rials, inorganic fertilizers, and antibiotics applied in the shrimp farms. Lyle-Fritch et al. (2006) identified 106 different types of products, and approximately 42 products that are commonly applied at shrimp farms. As, essential element for normal growth and development (Ochoa et al., 2013), Cu is present in aquatic invertebrates and its bioaccumulation can increase with size (Pan & Wang, 2009). The consumption of oysters with high Cu levels can cause irritation, vomiting, and ulcer and kidney damage (ATSDR, 2004). Gorman (1993) indicates that high levels of Cu could even stunt human growth. Cr is an essential micronutrient trace metal that has a similar pattern to Ni and Zn, and, in humans, Cr is an essential part of the glucose tolerance factor (Cheung & Wong, 1992). Its excess, however, may led to diabetes, may lead to diabetes. Cadmium is a nonessential metal for organisms, highly toxic to wildlife, and carci nogenic to humans (Wong et al., 1981) since it tends to accumulate in the liver and kidney (Abbe et al., 2000). Páez-Osuna & Osuna-Martínez (2015) concluded that high Cd levels in the tissue of C. corteziensis from SINM could be related to upwelling events, rather than wastes de rived from anthropogenic activities, which could partially explain the Cd burdens bioaccumulated by C. gigas in this study. In addition, the au thors pointed out that closely related oyster species living in the same site can accumulate significantly different levels of metals. Although Pb is known to be a metabolic poison, low concentrations are often observed in shellfish due to the dietary and dissolved lead available for marine invertebrates (Amiard et al., 1986). Higher levels of Pb in oysters can be due to the river runoff and erosion of this metal from the natu ral bed rock in the region (Soto-Jiménez et al., 2001), as well as hu man activities such as tourism, fisheries, and untreated urban sewage, i.e., potential pollution sources. In this case, the pollution status of the SINM lagoon system, where La Pitahaya Estuary is located, is well do cumented, particularly for heavy metals derived from human activities (Hernández-Cornejo et al., 2005; Escobedo-Urías, 2010; Páez-Osuna & Osuna-Martínez, 2015), which helps to explain the results we obtained.
Based on the metal levels obtained, we conclude that cultivated oysters from La Pitahaya Estuary accumulated Cu, Cd, Cr, and Pb above the permissible limits for human consumption during the 2011 pro duction cycle, thus posing a human-health risk. However, the apparent exposure of metals may not involve a consistent intake since individuals consume oysters occasionally. Due to the intense anthropogenic activi ty and the significant population in the area, the economic importance of the species, and the public-health concerns regarding raw consump tion of oysters, we suggest that studies of heavy-metal concentrations in C. gigas farmed in this region be carried out periodically. Depuration, moving oyster racks to lower metal levels sites, and use of sterilized water to clean oysters before consumption are also possible strategies in lowering metal contents (Okazaki & Panietz, 1981; Katayon et al., 2007; Wang & Wang, 2014).











 nueva página del texto (beta)
nueva página del texto (beta)


