INTRODUCTION
The biological synthesis of minerals for the formation of shells in bivalve mollusks is a process directed, first, by the genetic information of the organisms (Clark et al., 2020) that triggers phenotypic expression (color, shape, structures, etc.) particular to each species. However, external factors such as food and environmental conditions -including anthropogenic activities that take place in the vicinity of the habitat (Grizzle et al., 2017; Stewart et al., 2021)- cause the shape of the shells as well as their growth to undergo variations that can be detected, even in populations of the same species that are close to each other. Due to the bio-mineralization of the shells, it is possible to infer the geo-climatic conditions to which they were exposed in their lives (Jacob et al., 2008), which is why they represent excellent archives of biological-environmental information.
Shell measurements, commonly reported in field studies on bivalves (height, length, and width) are evaluation tools that, in conjunction with other analytical techniques, offer practical and immediate evidence of possible variations in the growth and shell shape of these invertebrates (Caill-Milly et al., 2014; Dar et al., 2018). Additionally, the elongation, convexity, and compaction of the valves in these mollusks are functional indicators that modify their proportion as a reflection of the adaptation to the conditions of a certain locality (Modestin, 2017), which, finally, are related to factors such as waves and currents (Telesca et al., 2018), food availability (Nakano et al., 2010), and anthropogenic activity (Stewart et al., 2021). On the other hand, morphometric relationships between shell dimensions and body weight in bivalves are used to interpret their development, physiological condition (Zhang et al., 2023), and relative growth, among one or several populations of the same species (Karakulak et al., 2006).
The mangrove oysters C. corteziensis (Hertlein, 1951) and S. palmula (Carpenter, 1857) are bivalves of economic importance and aquaculture potential (Cáceres-Martínez et al., 2012), with geographic coincidence in the Gulf of California (Lodeiros et al., 2020). Both ostreids cohabit attached to the roots of mangroves, rocks exposed to low tides, and shells found in coastal lagoons. However, these ecosystems are influenced by anthropogenic activities, among which mining, aquaculture, and agriculture stand out as sources to the entrance of heavy metals (HM) (Páez-Osuna et al., 2017). The effect that HM have on the bio-mineralization of the shell of some bivalve species has been highlighted, causing its thinning and easy fracture or breakage (Dar et al., 2018; Stewart et al., 2021), in places where there is an increasing contribution of these elements derived from intense anthropogenic activity, as is happening in the southeast of the Gulf of California (Páez-Osuna et al., 2017).
Although there is information related to growth, survival, condition index, and environmental conditions in which these oyster species live (Cabrera-Peña et al., 2001; Castillo-Durán et al., 2010), as well as genetic variability (Pérez-Enríquez et al., 2008; Mazón-Suástegui et al., 2016), pathology (Cáceres-Martínez et al., 2012; Villanueva-Fonseca et al., 2020), morphometric relationships (Góngora-Gómez et al., 2018), biochemical composition (Hurtado et al., 2012), gonadal development (Chávez-Villalba et al., 2008), reproductive biology in the wild (Rodríguez-Jaramillo et al., 2008; Alvarado-Ruiz, 2018) and cultivated (Góngora-Gómez et al., 2020); but there are no reports that relate the level of HM accumulated in the soft-tissue of oysters, with the variation in the shape of their shell and allometry -jointly- for populations within the Gulf of California.
Therefore, the objective of this work was to determine the biometric indicators and relative growth of the shell of S. palmula and C. corteziensis in wild populations of four coastal lagoons in the southeastern Gulf of California, to document possible differences based on the level of HM found in the soft tissue of the two species. It is inferred that, in general, the results will vary -even in the same species- since they depend on the particular conditions (environmental and anthropogenic) of each sampling site.
MATERIALS AND METHODS
Seventy-five specimens of each oyster species (S. palmula and C. corteziensis) were collected from mangrove roots during low tide in September (summer 2019), December (fall 2019), March (winter 2020), and June (spring 2020) in the Altata (AL; 24°36’89.6” N; 107°52’14.2” W), Macapule (ML; 25°24’35.9” N; 108°41’37.1” W), Navachiste (NL; 25°30’31.0” N; 108°50’95.4” W), and El Colorado (ECL; 25°46’14.5” N; 109°24’44.2” W) lagoons, from Sinaloa, Mexico (n = 300 per species and site). These coastal lagoons are located within the Gulf of California (Fig. 1) and are characterized by maintaining a permanent connection with the gulf through one or two mouths. In addition, they are surrounded by four species of mangrove (irregularly distributed), human settlements, agricultural crops, and shrimp farms (Páez-Osuna & Osuna-Martínez, 2015).
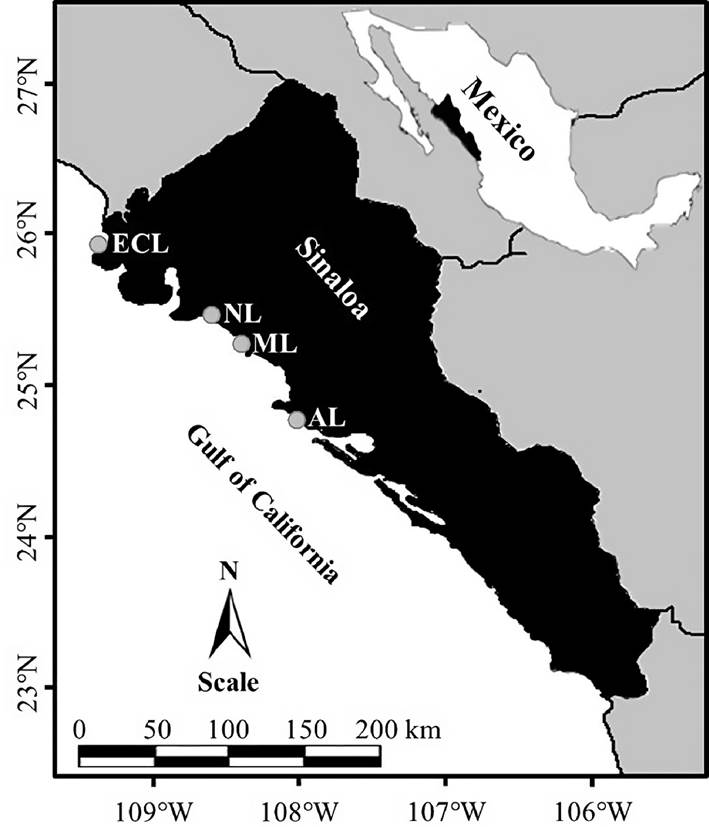
Figure 1 Location of sampling sites -Altata (AL), Macapule (ML), Navachiste (NL), and El Colorado (ECL) coastal lagoons- in the state of Sinaloa, Mexico (SE Gulf of California).
In each sampling, the environmental variables were recorded: water temperature and dissolved oxygen (DO) with an oximeter (YSI, 55/12 FT, Oxymeter, Ohio, USA); to measure salinity, a precision refractometer (ATAGO, S/Mill refractometer) was used; while the pH was obtained with a potentiometer (Hanna, HI 8314 pHmeter, USA) (Góngora-Gómez et al., 2020). The concentrations of organic matter (OM) and inorganic matter (IM) were determined with the gravimetric method described by APHA (1995). The concentration of chlorophyll a (Cl-a) was obtained with the spectrophotometric technique described by Strickland & Parsons (1972) and the equations of Jeffrey & Humphrey (1975).
The oysters were transported in a cooler with seawater (≈ 4 °C) to the laboratory, where they were cleaned with plastic brushes to remove excess sediment and detach adhered shells, epibiont fauna, and mangrove remains (Sepúlveda et al., 2023). A digital Vernier ruler (0.00 mm, Mitutoyo, CD-8” CS) was used to measure the length (SL; maximum distance between the anterior and posterior margins), height (SH; maximum distance from the umbo to the ventral margin), and shell width (SW; maximum distance between the thickest parts of the valves). Additionally, the oysters were dried with absorbent paper before weighing them to obtain the total body weight (BW), using a precision scale (0.001 g, OHAUS, Scout Pro SP 2001) (Rodríguez-Quiroz et al., 2016). The shell measurements of each oyster were used to obtain the biometric indices: elongation (SH/SL), roundness or compaction (SW/SL), and convexity (SW/SH) (Selin, 2007; Modestin, 2017).
To determine the allometry of S. palmula and C. corteziensis, the measurements of SL, SH, and SW were considered. Outliers were removed from all data sets (Durbin-Watson test) and residuals were analyzed for normal distribution using quantile-quantile plot (RStudio, R Core Team 2018). The morphometric relationships between the different dimensions of the shell (SL/SH, SW/SL, and SW/SH, n = 300 per species and site), for each coastal lagoon, were obtained with the linear equation Y = bX+a; where Y and X = shell dimensions (SL, SH, and SW, mm); where a = intercept and b = slope. In these associations with the same unit of measurement, when the exponent b = 1, the morphometric relationships indicate isometric growth.
The concentrations of HM (arsenic, As; cadmium, Cd; copper, Cu; iron, Fe; lead, Pb; zinc, Zn) in the soft tissue of the oysters S. palmula and C. corteziensis were analyzed using atomic absorption spectrophotometry and the accuracy of the analytical method was evaluated with the standard reference material DOLT-5® (National Research Council Canada) mentioned in Sepúlveda et al. (2023).
The mean, standard deviation, minimum, and maximum values of shell dimensions and BW were reported by oyster species and sampling site. All data sets passed the assumptions of normality (Kolmogorov-Smirnov) and homoscedasticity (Bartlett). An analysis of variance and a Tukey test was performed to detect and highlight statistical differences between the oyster shell dimensions and the biometric indexes of each site (α = 0.05). The goodness of fit of the data was analyzed with the Pearson correlation coefficient (r) (Sokal & Rohlf 1995). Finally, a principal component analysis (PCA) was applied to determine the relationship between the oysters’ biometric and allometry variables and environmental variables by species and lagoon, with the level of HM found in the soft tissue. The STATISTICA 7 program (StatSoft, Tulsa, OK, USA) was used.
RESULTS
The environmental variables in the sampling sites presented a fluctuation pattern, according to the annual seasons. The lowest values of water temperature, DO, salinity, pH, OM, IM, and Cl-a were found at the sites ECL (20.9 °C, autumn), ML (4.2 mg L-1, spring), AL (25.0 ‰, winter), ML (7.3, summer), ML (6.4 mg L-1, autumn), AL (19.0 mg L-1, autumn) and AL (1.6 mg m-3, autumn), respectively; while the highest values in AL (32.7 °C, spring), NL (8.7 mg L-1, winter), ECL (41.0 ‰, spring), NL (8.0, spring), AL (16.2 mg L-1, autumn), ECL (41.1 mg L-1, summer) and NL (9.3 mg m-3, winter), respectively (Table 1). Only salinity (F = 3.94, p = 0.03) and pH (F = 4.18, p = 0.03) showed significant differences (p < 0.05) concerning the sampling sites, with intervals of 30.5 ± 5.6 ‰ (AL) to 37.5 ± 2.9 ‰ (ECL) and from 7.5 ± 0.2 (ML) to 7.9 ± 0.1 (NL), respectively.
Table 1 Environmental variables in the sampling sites. Taken from Sepúlveda et al. (2023).
| Sampling site | Temperature (°C) | DO (mg L-1) | Salinity (‰) | pH | OM (mg L-1) | IM (mg L-1) | Cl-a (mg m-3) |
| Altata lagoon | |||||||
| Mean ± SD | 27.1 ± 5.9 | 5.8 ± 0.8 | 30.5 ± 5.6a | 7.6 ± 0.1a | 11.3 ± 4.1 | 26.4 ± 7.5 | 2.7 ± 1.1 |
| Min-Max | 21.3-32.7 | 4.8-6.7 | 25.0-38.0 | 7.5-7.7 | 6.7-16.2 | 19.0-36.8 | 1.6-3.7 |
| Macapule lagoon | |||||||
| Mean ± SD | 26.4 ± 5.7 | 5.4 ± 0.7 | 31.0 ± 2.7a | 7.5 ± 0.2a | 9.1 ± 2.8 | 35.4 ± 5.4 | 3.9 ± 1.2 |
| Min-Max | 21.1-31.5 | 4.2-5.9 | 29.0-35.0 | 7.3-7.8 | 6.4-12.9 | 27.7-39.6 | 2.6-5.3 |
| Navachiste lagoon | |||||||
| Mean ± SD | 26.9 ± 5.6 | 6.5 ± 1.4 | 35.8 ± 1.5ab | 7.9 ± 0.1b | 9.2 ± 0.7 | 32.9 ± 4.7 | 4.8 ± 3.2 |
| Min-Max | 21.4-31.8 | 5.4-8.7 | 35.0-38.0 | 7.8-8.0 | 8.2-9.8 | 26.1-36.5 | 2.3-9.3 |
| El Colorado lagoon | |||||||
| Mean ± SD | 26.8 ± 6.3 | 6.1 ± 0.5 | 37.5 ± 2.9b | 7.6 ± 0.1a | 9.3 ± 2.1 | 34.6 ± 6.0 | 4.4 ± 1.7 |
| Min-Max | 20.9-32.6 | 5.4-6.7 | 34.0-41.0 | 7.5-7.7 | 7.9-12.4 | 29.0-41.1 | 2.5-6.4 |
DO = dissolved oxygen, OM = organic matter, IM = inorganic matter, Cl-a = chlorophyll a, SD = standard deviation, Min = minimum, Max = maximum. Columns with different superscript letters denote significant differences (p < 0.05) among sampling sites.
The range of shell dimensions of S. palmula and C. corteziensis that were considered in this analysis (n = 1200 per species) was respectively 55.0-88.1 and 61.0-108.9 mm for SH, 32.3-68.0 and 32.1- 84.2 mm for SL and 12.4-40.0 and 21.7-49.7 mm for SW; while, for the BW, it was 13.9-65.0 and 30.1-126.9 g (Table 2). Except for SH, the other shell dimensions and BW presented significant differences (p < 0.05) between the sampling sites for each oyster species.
Table 2 Shell dimensions (mm) and body weight (BW, g) of S. palmula y C. corteziensis in the sampling lagoons (Altata lagoon, AL; Macapule lagoon, ML; Navachiste lagoon, NL; El Colorado lagoon, (ECL); Sinaloa, Mexico.
| Sampling site | SH | SL | SW | BW |
| S. palmula | ||||
| AL | 70.16 ± 4.07 | 51.7 ± 6.07c | 28.5 ± 4.77c | 36.75 ± 10.7b |
| ML | 73.20 ± 3.65 | 47.57 ± 5.25ª | 23.27 ± 4.00a | 35.37 ± 10.7b |
| NL | 72.42 ± 4.72 | 48.30 ± 6.02ª | 26.67 ± 3.12b | 33.55 ± 5.50ª |
| ECL | 74.77 ± 3.70 | 50.25 ± 4.92b | 26.32 ± 4.30b | 38.7 ± 8.35c |
| C. corteziensis | ||||
| AL | 75.11 ± 4.91 | 59.95 ± 7.62c | 37.35 ± 4.72c | 56.57 ± 12.95ª |
| ML | 74.75 ± 4.67 | 54.55 ± 7.12ª | 33.65 ± 3.85b | 59.45 ± 12.82b |
| NL | 73.05 ± 4.51 | 56.52 ± 6.60b | 34.07 ± 4.17b | 55.65 ± 13.07ª |
| ECL | 73.01 ± 3.42 | 53.85 ± 6.22ª | 32.6 ± 3.35ª | 59.65 ± 12.37b |
SH = Shell height, SL = Shell length, SW = Shell width. Columns with different superscript letters denote significant differences (p < 0.05) among sampling sites.
The biometric indexes of the oyster shells in the four coastal lagoons were significantly different (p < 0.05). The greatest elongation of S. palmula and C. corteziensis was found in ML and ECL, respectively, while compaction and convexity were greater for both oyster species in AL (Table 3).
Table 3 Biometric indexes (annual mean ± standard deviation) of oyster shells in the sampling lagoons.
| Sampling site | Elongation | Compactness | Convexity |
| S. palmula | |||
| AL | 1.40 ± 0.13a | 0.58 ± 0.10c | 0.40 ± 0.06c |
| ML | 1.54 ± 0.15d | 0.49 ± 0.09a | 0.32 ± 0.07a |
| NL | 1.51 ± 0.16c | 0.56 ± 0.08c | 0.37 ± 0.05b |
| ECL | 1.45 ± 0.13b | 0.52 ± 0.06b | 0.36 ± 0.05b |
| C. corteziensis | |||
| AL | 1.32 ± 0.10a | 0.64 ± 0.09b | 0.48 ± 0.06d |
| ML | 1.41 ± 0.17b | 0.62 ± 0.08ab | 0.45 ± 0.05b |
| NL | 1.33 ± 0.16a | 0.61 ± 0.09a | 0.46 ± 0.06c |
| ECL | 1.46 ± 0.18c | 0.61 ± 0.07a | 0.42 ± 0.05a |
AL = Altata lagoon, ML = Macapule lagoon, NL = Navachiste lagoon, ECL = El Colorado lagoon. Columns with different superscript letters denote significant differences (p < 0.05) among sampling sites.
At each sampling site, morphometric associations for the two oyster species showed a linear and positive trend. In S. palmula, the determination coefficient (R2) ranged from 0.05 (b SW/SH in NL) to 0.55 (b SL/SH in AL) (Fig. 2), while for C. corteziensis, the lowest value (R2 = 0.08) was obtained in ECL for the b SW/SH ratio, and the highest (R2 = 0.70) was recorded in AL for b SL/SH (Fig. 3). In all sampling sites the morphometric relationships showed negative allometry (b < 1).
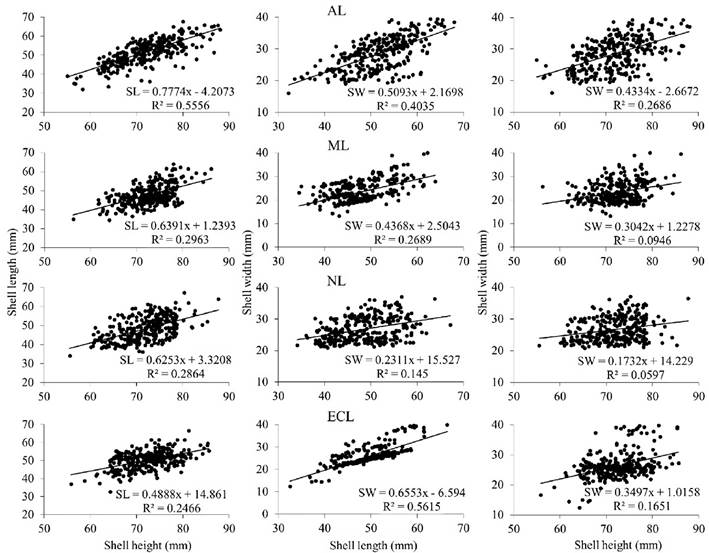
Figure 2 Morphometric relationships (n = 300 oysters per lagoon) among the shell dimensions of S. palmula sampled in four lagoons (AL, Altata lagoon; ML, Macapule lagoon; NL, Navachiste lagoon; ECL, El Colorado lagoon) from the southeast Gulf of California. R2 = coefficient of determination.
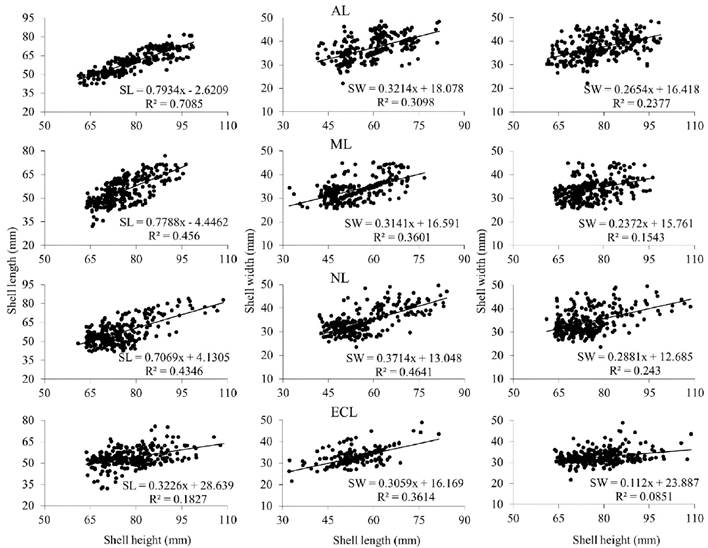
Figure 3 Morphometric relationships (n = 300 oysters per lagoon) among the shell dimensions of C. corteziensis sampled in four lagoons (AL, Altata lagoon; ML, Macapule lagoon; NL, Navachiste lagoon; ECL, El Colorado lagoon) from the southeast Gulf of California. R2 = coefficient of determination.
Table 4 shows the annual average values of the concentration of HM (As, Cd, Cu, Fe, Pb, and Zn) in the soft tissue of the two oyster species (taken from Sepúlveda et al., 2023). Except for Cd in S. palmula and As in C. corteziensis, the concentrations of the other HM showed significant differences (p < 0.05) in the sampling sites for each oyster species. The highest levels of Cu, Pb, and Zn in the tissue of S. palmula and C. corteziensis were found in ECL, while Fe concentrations in both oyster species were higher in ML.
Table 4 Heavy metals (annual mean concentrations, mg/kg, w.w.) in the soft tissue of S. palmula and C. corteziensis from four coastal lagoons in the southeast Gulf of California. Taken from Sepúlveda et al. (2023).
| Site | As | Cd | Cu | Fe | Pb | Zn |
| S. palmula | ||||||
| AL | 4.18±0.05b | 1.74±0.05 | 15.13±0.15ab | 19.53±0.37ª | 1.30±0.01ª | 72.81±0.49ab |
| ML | 3.52±0.06ab | 1.37±0.07 | 23.01±0.16b | 35.36±0.5b | 1.13±0.02ª | 75.34±0.4ab |
| NL | 3.28±0.04ª | 1.89±0.03 | 8.26±0.16ª | 21.01±0.53ª | 1.47±0.02ab | 55.85±0.47ª |
| ECL | 3.6±0.06ab | 1.36±0.08 | 33.99±0.13c | 30.08±0.47ab | 1.74±0.01b | 92.15±0.64b |
| C. corteziensis | ||||||
| AL | 3.71±0.06 | 0.8±0.04ª | 10.51±0.06ab | 38.12±0.32ª | 1.41±0.03b | 60.53±0.32b |
| ML | 3.66±0.06 | 1.05±0.01ª | 12.63±0.07b | 56.44±0.44b | 0.97±0.03ª | 47.43±0.44b |
| NL | 3.48±0.07 | 1.78±0.03b | 3.89±0.05ª | 33.11±0.37ª | 1.27±0.02ab | 30.95±0.32ª |
| ECL | 3.81±0.09 | 0.9±0.02a | 27.73±0.09c | 38.09±0.38a | 1.79±0.02c | 81.53±0.51c |
AL = Altata lagoon, ML = Macapule lagoon, NL = Navachiste lagoon, ECL = El Colorado lagoon. Columns with different superscript letters denote significant differences (p < 0.05) among sampling sites.
OM and IM concentrations showed a correlation with some biometric indicators and allometry of S. palmula in three of the lagoons. OM was associated with compaction and b SL-SH (r = 0.99, p = 0.001 and r = -0.97, p = 0.02, respectively) in ECL, while it only exhibited correlation with b SW-SH (r = 0.95, p = 0.04) in NL. For its part, the IM level was negatively correlated with the elongation values (r = -0.98, p = 0.01), b SW-SL (r = -0.98, p = 0.01), and b SW-SH (r = -0.99, p = 0.001) in ML. Water temperature and salinity showed, respectively, a correlation with compaction (r = 0.98, p = 0.01 and r = -0.96, p = 0.03) in NL and ECL. In the case of C. corteziensis, OM was associated with b SW-SH (r = -0.95, p = 0.04) and convexity (r = 0.99, p = 0.001) in AL and NL, respectively; MI with elongation (r = 0.96, p = 0.03) in AL; DO with convexity and compactness (r = -0.99, p = 0.001 for both) in NL and ECL; and temperature, with convexity (r = 0.98, p = 0.01) in ML.
On the other hand, some HM showed a correlation with the biometric indicators and the allometry of the oysters in the different lagoons. The Pb level in S. palmula showed correlation with SL (r = 0.97, p = 0.02) and SH (r = 0.96, p = 0.03) in ML and ECL, respectively, while Cu was associated with relative growth (b SL-SH, r = 0.96, p = 0.03) in ECL. As and Cd concentrations in C. corteziensis tissue were correlated (r = 0.96, p = 0.03 for both), respectively, with b SW-SL and b SL-SH, in AL and ML. On the other hand, convexity was negatively related to Pb in S. palmula from ML (r = -0.99, p = 0.01) and ECL (r = -0.97, p = 0.02), while compaction was associated with Fe (r = -0.96, p = 0.03) in C. corteziensis from AL.
Of the variances of all the variables analyzed (24) in the four sites, the eigenvalues of three components satisfactorily explain their correlations. The points obtained in the PCA -for the two oysters- in the four lagoons show different groupings of biometric indexes and allometry, in relation to the levels of HM in the soft tissue of S. palmula (Fig. 4) and C. corteziensis (Fig. 5). The dispersion of the points for S. palmula presented an interval of -0.09 to 0.44 in AL, -0.08 to 0.35 in ML, -0.00007 to 0.42 in NL and -0.003 to 0.46 in ECL. In the case of C. corteziensis, the intervals per lagoon were: -0.007 to 0.35, -0.002 to 0.36, -0.01 to 0.43, and -0.01 to 0.42 in AL, ML, NL, and ECL, respectively. All HM were shown, to a different extent, to be associated with the functional indicators of the shell and allometry. Cd accumulated in the soft tissue of oysters was found sequestered to convexity and compaction in S. palmula from AL and ML and to elongation of C. corteziensis from ECL. On the other hand, this metal showed grouping with b SW-SL and b SL-SH of S. palmula in AL and ECL, and with b SL-SH of C. corteziensis from ML. For their part, Cd, Cu, and Zn were sequestered in b SW-SL and b SL-SH of S. palmula and with elongation and compaction in C. corteziensis, the three elements in ECL. As was the only element related to b SW-SL and b SL-SH of S. palmula in ECL.
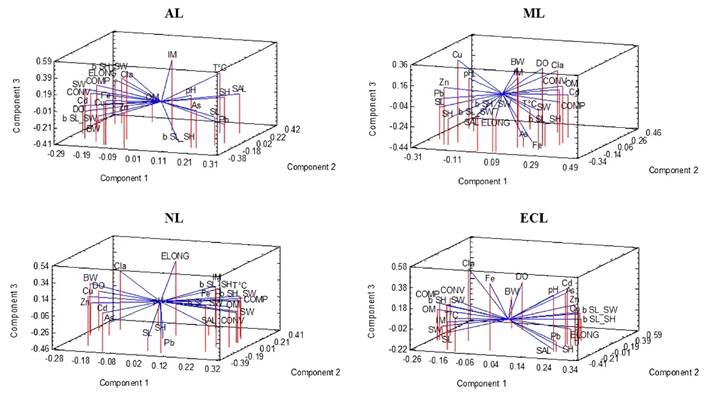
Figure 4 The PCA of S. palmula in the Altata (AL), Macapule (ML), Navachiste (NL), and El Colorado (ECL) lagoon. b SW/SH = shell width/height relationship; b SL/SH = shell length/height relationship; b SW/SL = shell width/length relationship; BW = body weight; Cl-a = chlorophyll a; COMP = shell compactness; CONV = shell convexity; DO = dissolved oxygen; ELONG = shell elongation; IM = inorganic matter; OM organic matter; pH = pH units; SAL = salinity; SH = shell height; SL = shell length; SW = shell width; T °C = temperature; As = arsenic; Cd = cadmium, Cu = copper; Fe = iron; Pb = lead; Zn = zinc.
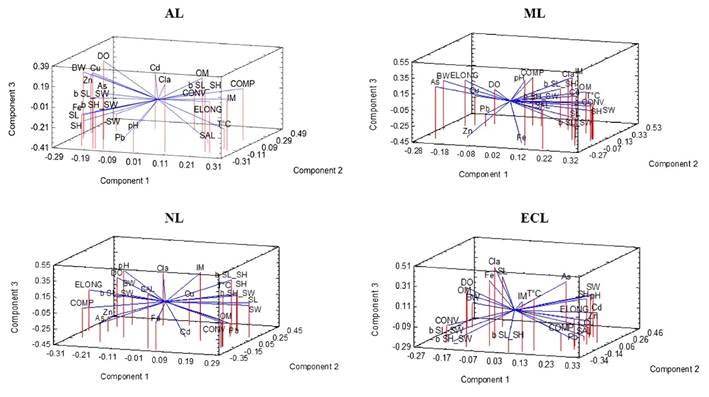
Figure 5 The PCA of C. corteziensis in the Altata (AL), Macapule (ML), Navachiste (NL), and El Colorado (ECL) lagoon. b SW/SH = shell width/height relationship; b SL/SH = shell length/height relationship; b SW/SL = shell width/length relationship; BW = body weight; Cl-a = chlorophyll a; COMP = shell compactness; CONV = shell convexity; DO = dissolved oxygen; ELONG = shell elongation; IM = inorganic matter; OM organic matter; pH = pH units; SAL = salinity; SH = shell height; SL = shell length; SW = shell width; T °C = temperature; As = arsenic; Cd = cadmium, Cu = copper; Fe = iron; Pb = lead; Zn = zinc.
DISCUSSION
The biometric and morphological analysis of the shell of bivalve mollusks is part of the knowledge necessary to know about the interference of the environment in their development. Despite the significant differences (p < 0.05) obtained in salinity and pH between the sampling sites, the average values of all environmental variables -by annual season- in the four coastal lagoons were found within the ideal range considered for the growth of both oyster species (Chávez-Villalba, 2014), and are consistent with those reported by other studies in the area (Páez-Osuna & Osuna-Martínez, 2015; Góngora-Gómez et al., 2016).
Estuaries and coastal lagoons are transition zones between the ocean and the continent, which are exposed to abrupt changes in water variables caused, mainly, by the effect of the rain-evaporation relation ship with the depth of the body of water, and by the quantity and quality of discharges from rivers, irrigation canals, and industrial drains (Costa et al., 2018; Omarjee et al., 2021; Elegbede et al., 2023). The OM and IM concentrations showed the greatest significant association in the biometric indexes and allometry of the two oyster species in the lagoons. The parameters obtained from the studied lagoons may be due to the residence time of the water and the intensity of the tides (Takasu et al., 2020), concentration and quality of particles (Middelburg & Herman, 2007), use of molecules in the first trophic levels (Hope et al., 2020), and contributions of anthropogenic material (Canuel & Hardison, 2016), among others, specific to each of them. The OM and IM suspended in the water column represent all the microcomponents (phytoplankton, dissolved organic and inorganic particles, and even toxic ones -such as HM- among others) that are filtered, absorbed, assimilated, and/or accumulated by bivalves in the soft tissue and/or shell (Qiao et al., 2022). Although there is a coincidence in the type of climate (Csa climate, subtropical with dry summer, Chen & Chen, 2013) and the short distance between the sampled lagoons (≈ 200 km), the dimensions of the shells (SL, SW) and the growth allometric of each oyster species did not show similarity; which, in part, could be explained by various factors, such as the quantity and quality of the particles generated by the different activities located on the periphery and close to each sampling site. For example, LA receives the discharge of 98,518 ha of intensive agriculture, in addition to urban waste from human settlements (≈ 1,059,617 inhabitants) (Frías-Espericueta et al., 2018); while ML and NL suffer the impact of effluents from agriculture (119,994 ha) and aquaculture (18,735 ha) activities, along with urban waste from approximately 295,353 inhabitants (Páez-Osuna & Osuna-Martínez, 2015). In the case of ECL, agricultural activity (196,549 ha), aquaculture (12,639 ha), municipal waste -generated by nearly 450,000 inhabitants- and fishing and livestock operations in the area, contribute strongly to the levels of organic particles. and suspended inorganic substances (Sepúlveda et al., 2023); among the latter, the HM.
The oysters were selected with similar SH (Table 2), however, SL, SW, and BW recorded significant differences (p < 0.05) for each species between the lagoons; which, in turn, caused different values of elongation, convexity, and compaction (p < 0.05). While S. palmula and C. corteziensis were more elongated in different lagoons (ML and ECL, respectively), both species showed the greatest compaction and convexity in AL. Since both ostreids coexist attached to the mangrove root, exposed to the same environmental factors and those derived from the change of tides (desiccation, waves, currents, etc.), such biometric differences could be mostly attributed to their state of sexual maturation (Chong et al., 2020), genetic aspects and availability of food particles (Ballesta-Artero et al., 2018), that is, Cl-a and seston. Specifically, OM and IM were correlated with some dimensions and biometric indicators of oysters; which may be due to the different contributions of nutrients and anthropogenic compounds that vary according to the urban and industrial activities surrounding each lagoon. Mazzola & Sarà (2001) established that the growth of the mussel Mytillus galloprovincialis and the clam Tapes sp. is influenced by the OM represented by phytoplankton, organic waste from the mollusks themselves, and surplus diets for fish dissolved in the water that they filter as food. Cugier et al. (2022) used a 3D ecosystem model (hydrodynamics, primary production, and individual growth) in Bourgneuf Bay, France, to evaluate the growth of the oyster Crassostrea gigas, concluding that IM concentration prevents food uptake and, therefore, the development of bivalves.
Because HM are important components of IM, oyster development could respond to their accumulated concentration. For example, the Pb level in S. palmula was associated with SL and SH in two lagoons (ML and ECL), while the Pb concentration obtained from the soft tissue of S. palmula was associated with low values of compaction and convexity. The same happened with the level of Fe and compaction in C. corteziensis from AL. HM's effect on the dimensions and development of bivalve shells has been reported. For example, Stewart et al. (2021) concluded that the continuous contribution of Zn, Cu, and Pb, from the mining industry of the Isle of Man (North Irish Sea), affects the development of the shell of the queen clam Pecten maximus, causing its weakness and thinning; therefore, such HM are considered a threat to the aquaculture industry on the islands of Great Britain. On the other hand, Beeby et al. (2002) demonstrated experimentally that an increase in the uptake of Pb included in the diet of the garden snail Helix aspera causes a reduction in the mass of its shell because less calcium (Ca) and magnesium (Mg) are deposited in it, due to the extra energy expenditure that this snail must make to excrete excess Pb. The above would explain the partial effect of HM on the shell dimensions of both oyster species in the four lagoons studied. However, to conclude with certainty about the previous point, it is advisable -in parallel with the analysis of HM levels in the soft tissue of oysters- to know their concentrations in the water.
While all morphometric relationships showed a linear and positive trend, the regression equations presented a consistent pattern of negative allometric type (b < 1). The above is coincident with adult oysters (SH > 70 mm); which, in addition, go through stages of sexual maturation and reproduction in an annual cycle, as pointed out by Mena-Alcántar et al. (2017) and Alvarado-Ruíz (2018) for C. corteziensis (SH > 57.1 mm) and S. palmula (SH > 42 mm), respectively. In this work, factors such as environmental variables (El-Sayed et al., 2011), food availability (Lee et al., 2018), and metabolic energy expenditure in the reproductive process (Mann et al., 2014), among others, altered the proportionality of their allometry, generating moderate values of b < 0.80 for the two oyster species. Despite this, different morphometric interactions better described the relative growth for each species (R2 SW/SL = 0.56 for S. palmula in LEC; R2 SH/SL = 0.70 for C. corteziensis in AL) in different locations. The above could be explained by 1) the inter-specific phenotype of their shells, and 2) the intra-specific effect of the anthropogenic activity of each lagoon. For the first, it is documented that S. palmula has a semi-circular and cupped shape, while the SH predominates in C. corteziensis, making it more elongated (Lodeiros et al., 2020). Regarding their specific form, there is evidence that some HM derived from various industries -such as Fe and manganese (Mn)- can cause, respectively, changes in both the coloration and the degree of thinning in the shell of various mollusks (Krupnova et al., 2017). The above would partially explain the differences in the biometry and isometry of the shells in both oysters at the sampling sites.
Some elements -such as Cu, Fe, and Zn- are essential for mollusks at levels that do not exceed their metabolic demand (Singh et al., 2011). Concentrations higher than their physiological requirements are regulated through excretion (Regoli et al., 1991) or even incorporated into their shell (Dar et al., 2018). The results obtained associate some of the HM with allometric growth and biometric indicators of the shell of the two oyster species in different lagoons (Cu, in S. palmula from AL with b SL-SH; As and Cd, in C. corteziensis with b SW-SL and b SL-SH in AL and ML, respectively; Cd, in S. palmula from AL and ML, respectively with convexity and COMP; Cd, Cu, and Zn with elongation for C. corteziensis from ECL; As with S. palmula in ECL), without showing any specific trend in relation to the species or place. The above places the environmental conditions of each lagoon -including their anthropogenic activities- as a possible main cause of the differences in the shell shape and allometry of each ostreid. However, it is not possible to satisfactorily conclude that the load of these HM in the soft tissue of both species, has had any direct effect on their allometry and biometric indexes. It is recommended to analyze the HM not only in the water and sediment but also in the shell of the oysters to know their traceability in the different eco-biological compartments.











 nueva página del texto (beta)
nueva página del texto (beta)


