INTRODUCTION
The study of fossil microorganisms has experienced a significant revolution during the last 50 years. The findings of fossil bacteria in a great diversity of sediments in Archean to recent units (Schieber, 2002a), as well as the reports of filamentous structures (Breton et al., 2014; Blanco-Piñón et al., 2014), protoctists, fungi (Ascasso et al., 2005; Martín-González et al., 2009a) and even biomarkers (Dutta et al., 2014) and pyritized fossils (Martín-González et al., 2009b) entrapped in amber, represent an important advance in understanding the different scenarios in which fossilization can take place.
The oldest known fossil microstructures interpreted as microorganisms are from several Archean localities (e.g., the Apex Chert in Australia and the Fig Tree Formation in South Africa). In these sites, septated filaments have been described and compared with Proterozoic and recent cyanobacteria (Schopf et al., 2007); however, the biogenicity of some structures remains under discussion (De Gregorio and Sharp, 2003). Another important microfossil locality is the early Archean (3.4-3.3 Ga) Barberton Greenstone Belt of South Africa (van Zuilen et al., 2007), where microscopic carbonaceous structures have been found in chert and interpreted as fossil microorganisms.
Microbial fossils in sedimentary pyrite are less studied. This mineral is commonly reported replacing remains of macrofossils, such as plants (Brett and Edwards, 1970; Kenrick and Edwards, 1988; Butler et al., 2000; Bajpai et al., 2001; Brock et al., 2006; Grimes et al., 2009) and metazoans (Allison, 1990; Briggs et al., 1996; Glass, 2006; Högström et al., 2009) or even as fill in worm burrows (Schieber, 2002b; Virtasolo et al., 2013). Because of its opacity, the search for fossil microbes in pyrite is more difficult than in chert (Schieber, 2002a) or amber. However, microbial fossils in pyrite have been described by several authors, such as Schopf et al. (1965), Schieber (1989, 2002 a, b, 2003, 2005), Southam et al. (2001), Schieber and Riciputi (2005) and Folk (2005), among many others, indicating that the preservation of microorganisms in pyrite is not as rare as previously thought. However, the preservation of different forms corresponding to different taxonomic groups is notable. This paper provides a general description of six types of biomorphic structures observed with a scanning electron microscope (SEM) in sedimentary pyrite from the Upper Cretaceous Agua Nueva Formation, at Xilitla, San Luis Potosí, Central Mexico (Figure 1), as well as a brief discussion of the taphonomic processes involved in their fossilization.
STRATIGRAPHIC FRAMEWORK: THE AGUA NUEVA FORMATION
The Agua Nueva Formation in the study area (Figure 2) has been previously described and studied by several authors (Maldonado-Koerdell, 1956; Suter, 1990; Blanco-Piñón et al., 2008, 2014; Blanco et al., 2010, 2011; Castañeda-Posadas et al., 2014; Nuñez-Useche et al., 2016) for paleontological and geochemical purposes. In summary, this lithologic unit consists predominantly of alternating fossiliferous, organic matter-rich, laminated, dark gray limestone and non-laminated, organic matter-poor limestone in decimeter-thick beds (10 to 30 cm) with occasional centimetric beds (5 cm) of brown shale that show no apparent internal structures. The section also includes 2 to 4 cm-thick intermittent light olive-colored bentonite layers. Intermittent layers of ca. 5 cm-thick black chert are present in both the laminated and non-laminated limestone. The pyrite analyzed in this work was collected from the first six meters of the section, corresponding to the Cenomanian (Núñez-Useche et al., 2016) based on the presence of the planktonic foraminifera Rotalipora cushmani and Thalmanninella greenhornensis (observation of the authors), as suggested by BouDagher-Fadel (2013). Pyrite occurs as 1 to 2 cm-thick lenses within the well-laminated dark limestone and is parallel to the bedding.
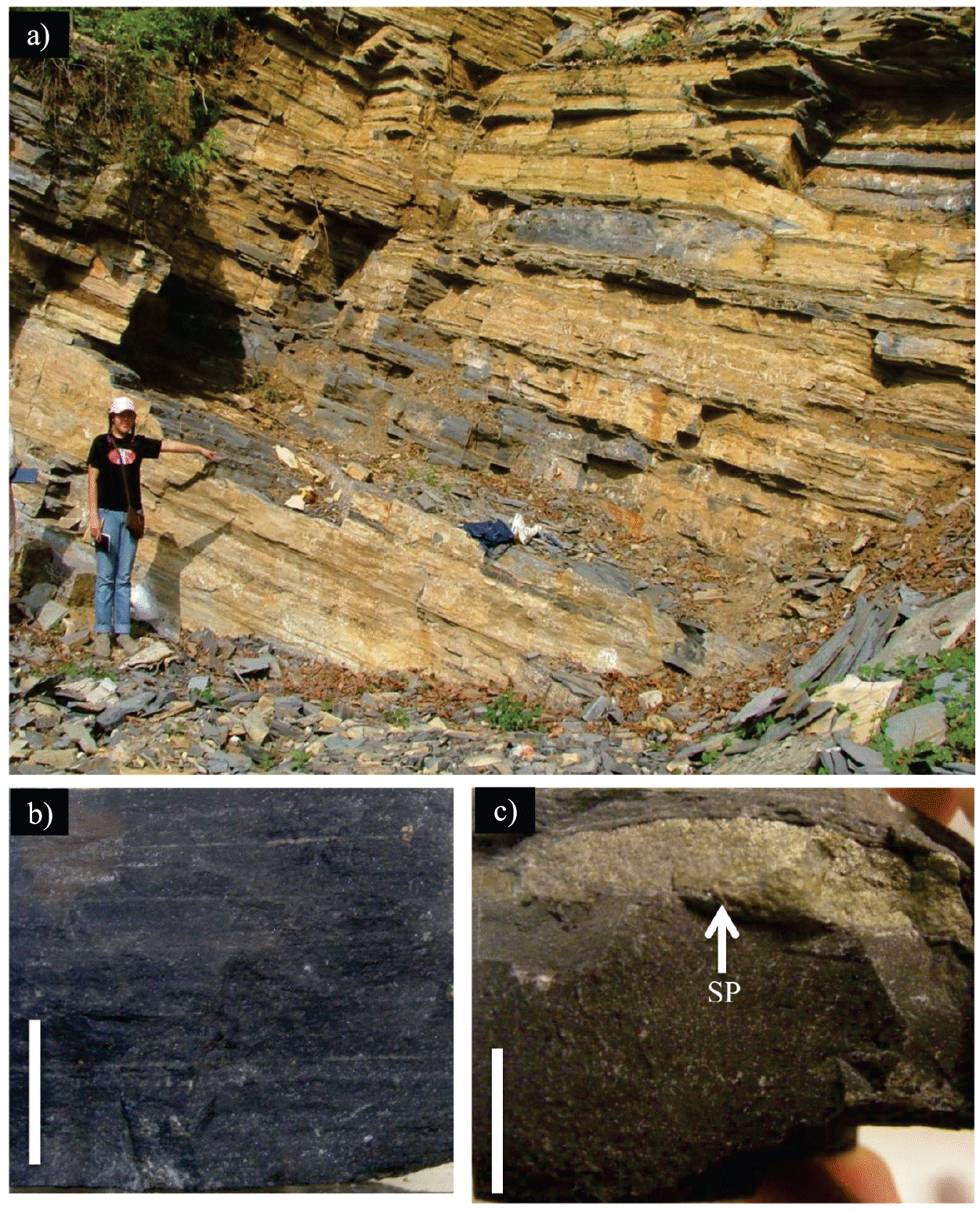
Figure 2 The Agua Nueva Formation in the study area. a) Outcrop, b) laminated limestone, and c) pyrite lenses (SP) indicated by an arrow. Scale bar = 1 cm.
The SEM observations show that the pyrite lenses contain single euhedral crystals that do not exceed 15 µm in size and display a variety of textures, such as cube, pyritohedron and octahedron. Associated with the pyrite crystals, both entire and fragmented framboids were observed (Figure 3a). Complete framboids have diameters ranging from 3 to 14 µm and consist of closely packed equigranular microcrystallites approximately 1 µm in size. Some are sub-spheroidal in outline resembling the polyhedral framboid reported by Butler et al. (2000). Fragmented framboids are irregular in outline and have a maximum length of 7 µm. Núñez-Useche et al. (2016) discussed the origin of the sedimentary pyrite in the study area based on geochemical and textural analysis. They conclude that both syngenetic and diagenetic pyrite are present according to the size and framboid content distribution and that its formation is related to bacterial sulfate-reduction based on negative δ34S data (= -5.2 ‰ to -51.2 ‰) in an oxygen-deficient scenario. The orientation of the centimetric pyrite lenses studied in this work suggests that the pyrite formed during the diagenetic history of the sediment, probably a few centimeters below the sediment-water interface, as suggested by Suits and Wilkin (2009).
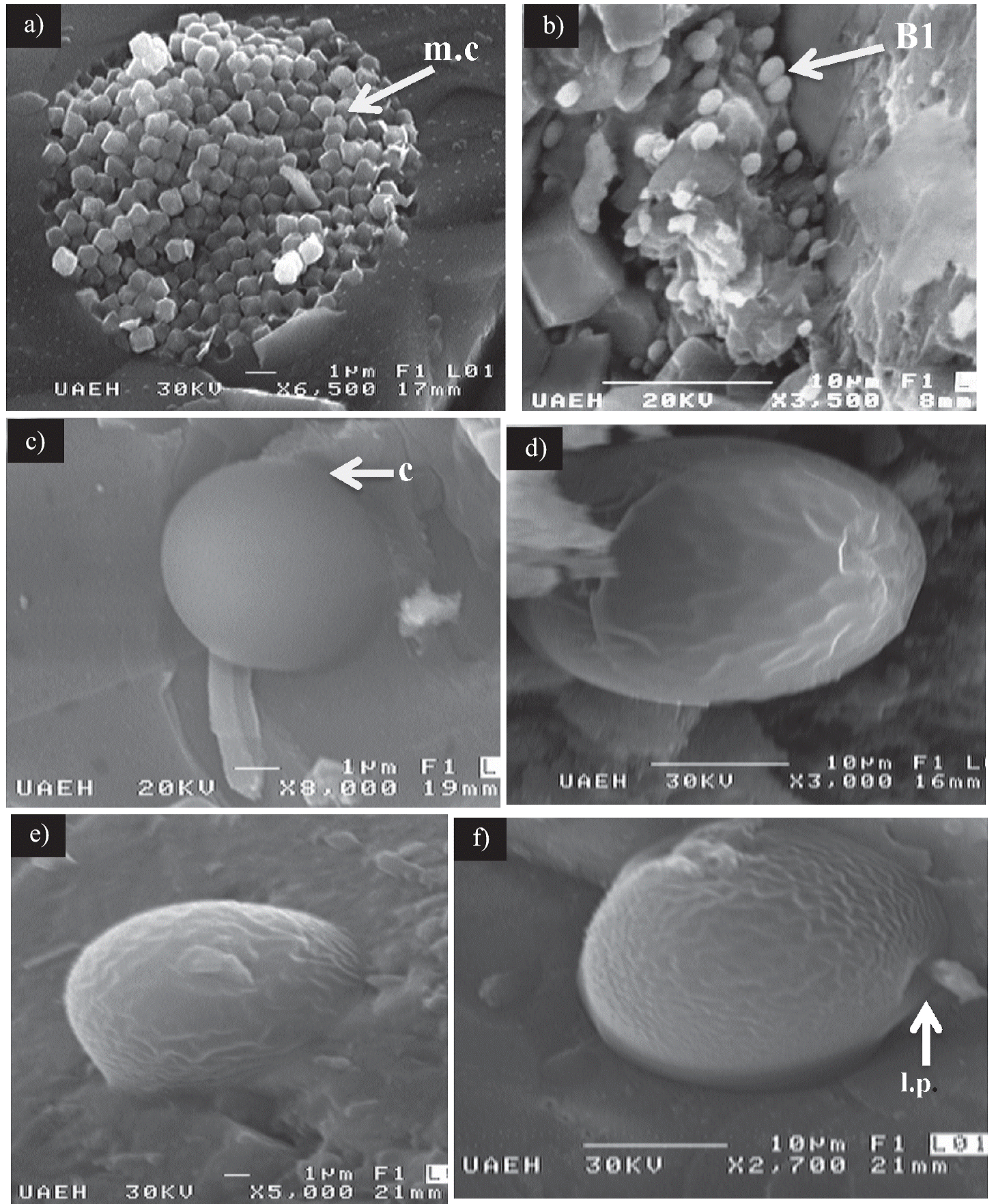
Figure 3 SEM images of framboids and bioform types 1, 2, and 3 from sedimentary pyrite in the Agua Nueva Formation. a) Framboids with microcrystallites (white arrows). b) Three-dimensional coccoid bacteria (B1) showing colonial arrangement (type 1 bioform). c-f) Bioform types 2 and 3 resembling chrysophycean stomatocysts. c) Spherical and smooth element (type 2 bioforms) showing a collar-like structure (c). d) Oval and ornamented structure. e) Stomatocyst embedded in the pyritic matrix. f) Oval and ornamented showing a lateral pore (l.p). d), e) and f) correspond to the type 3 bioform.
The dark gray limestone is characterized by well-preserved macrofauna distributed randomly in the beds. Invertebrates consist of partially dissolved inoceramids and dissolved unidentified ammonite shells. Vertebrates are represented by diverse groups of teleostean fishes as well as shark teeth (Maldonado-Koerdell, 1956; Blanco et al., 2006, Blanco-Piñón et al., 2008, 2014; Núñez-Useche et al., 2016). No fossiliferous material was observed in the shale.
The lithological and paleontological data indicate that the Agua Nueva Formation accumulated in an open marine environment within the Tampico-Misantla Basin (Blanco-Piñón et al., 2014). The primary lamination, pervasive framboidal pyrite, and a high concentration of organic matter (TOC = 1.2 to 8 wt%) in the dark and well-laminated limestone are consistent with persistent recurring oxygen-deficient conditions, whereas the bioturbated and non-laminated limestone (TOC < 1 wt%) represents relatively well-oxygenated episodes (Blanco et al., 2011; Blanco-Piñón et al., 2014). Later, based on a multiproxy study, three general types of facies corresponding to oxic, dysoxic and anoxic conditions were distinguished by Núñez-Useche et al. (2016) for the whole section of the Agua Nueva Formation at Xilitla.
According to Blanco-Piñón et al. (2014) and Núñez-Useche et al. (2016), the Agua Nueva Formation at Xilitla was deposited during the Cenomanian-Early Turonian interval and is coeval with the worldwide development of the Oceanic Anoxic Event 2 (Schlanger and Jenkyns, 1976; Schlanger et al., 1987), as previously suggested by Blanco-Piñón et al. (2008) and Blanco et al. (2011) based on microfacies analysis and preliminary micropaleontological data.
METHODOLOGY
Samples of sedimentary pyrite used in this work were processed according to the method suggested by Schieber (2002a). Pyrite fragments of approximately 1 cm3 were taken from the innermost part of the samples to avoid contamination. The fragments of pyrite were divided into two groups. The first group of samples was leached with HNO3 for 12 seconds and then rinsed with distilled water. The second group was not washed. Later, both groups of samples were coated with gold for three minutes. After coating, the samples were placed into the SEM at the Centro de Investigaciones en Ciencias de la Tierra y Materiales (AACTyM) at the Universidad Autónoma del Estado de Hidalgo (UAEH) for microscopic observation. Finally, some structures described in the pyrite samples were analyzed by energy-dispersive X-ray spectrometry (EDS) to determine their elemental composition.
RESULTS
Biomorphic structures
In this paper, the term “biomorphic structures” is used for those structures or remains associated to microorganisms. These include diverse morphologies and sizes and are preserved in three dimensions.
Type 1 Bioforms
This type of bioform consists of preserved three-dimensional oval to spherical-shaped structures. Their size ranges from 0.6 µm to 3 µm. Some of the coccoids occur as isolated elements, although most occur in groups or patches of cells (Figure 3b). In all of these structures, a smooth texture is observed along the exposed surface.
Type 2 Bioforms
This type of bioform consists of globose elements featuring a very well defined spherical form with diameters varying from 5 µm to 9 µm. They occur as isolated elements and are characterized by their smooth surfaces (Figure 3c). Some of them exhibit a thickening in the distal part of the anterior region resembling the collar observed in the stomatocysts reported by Duff et al. (1995).
Type 3 Bioforms
These bioforms are represented by spherical and oval structures, which are well exposed in the sedimentary pyrite or partially embedded in the pyritic matrix. Specimens show a length that varies from 9 µm to ca. 30 µm and a width between 5.6 µm and 15 µm. Their surface is characterized by a rough texture and in some cases a pore is present. No projection or other types of ornamentation were observed in this type of bioform (Figure 3d-3f).
Type 4 Bioforms
These bioforms consist of a fragmented and irregular-shaped grain with a diameter of approximately 20 µm (Figure 4a). The specimens have been partially altered and only a reduced area of its original surface remains. The surface exhibits a smooth texture; however, the central part of the area shows ornamentation consisting of pits and rounded granules with diameters of approximately 0.5 µm.
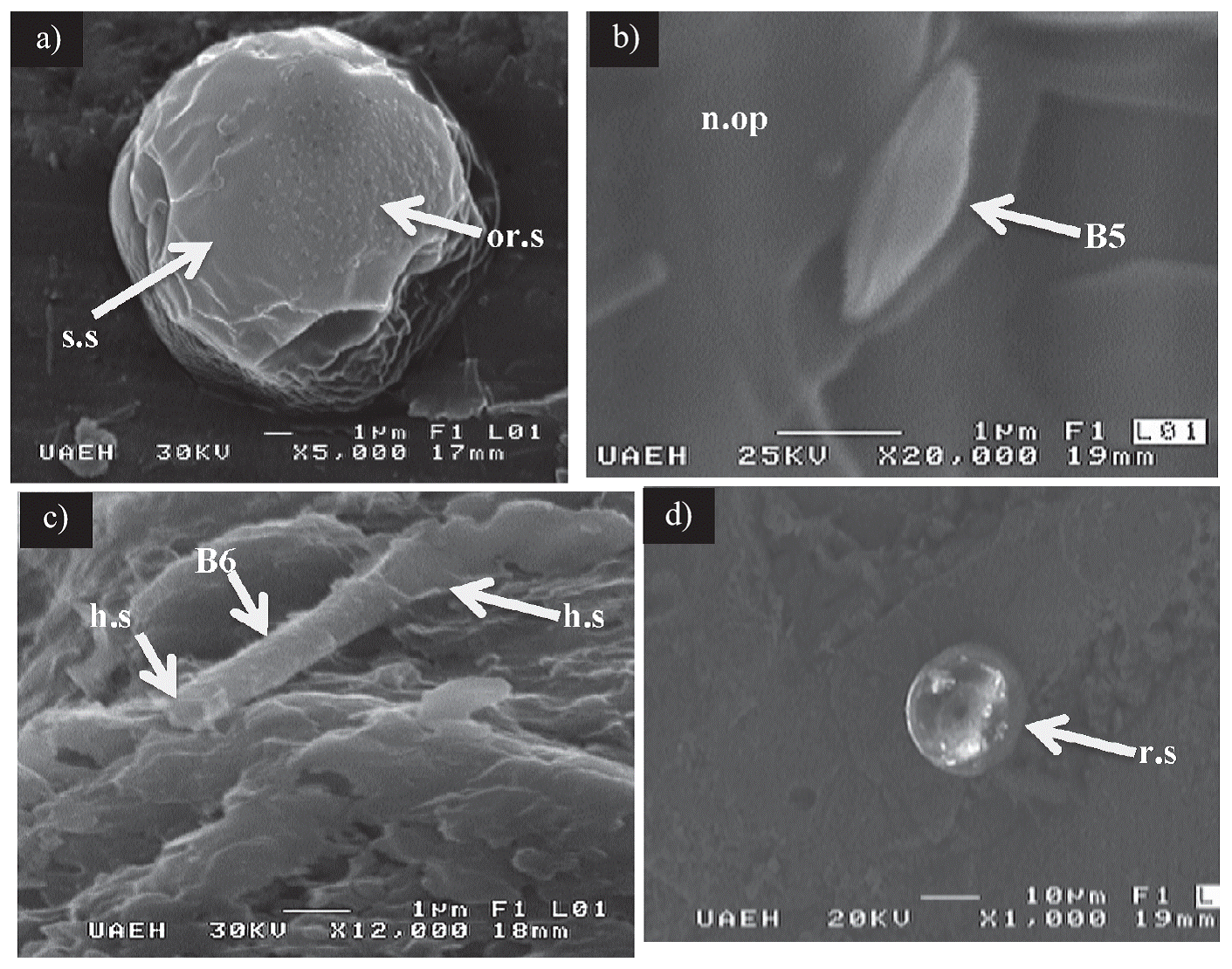
Figure 4 SEM images of bioform types 4, 5, and 6, as well as the reflecting structures. a) Fragments of a pollen-like structure (type 4 bioform) showing smooth (s.s) and ornamented (or. s) surfaces. b) Boat-shaped element (B5) resembling a dried pollen grain (type 5 bioform) exhibiting a central narrow opening (n.op). c) Tubular structures (B6) resembling bacterial sheaths (structure type 6). The hollow nature of these structures (h.s) can be appreciated at the ends. d) Unidentified structures referred to as reflecting spheres (r.s).
Type 5 Bioforms
This type of bioform was preliminarily reported by Blanco et al. (2013) ; however, in this document, an accurate description is provided. The specimens display an elliptic outline resembling a boat-shaped structure with an approximate total length of 2.3 µm and a width of 0.80 µm (Figure 4b). This element features a narrow opening, which runs along the length of the body, reaching a total length of at least 2.0 µm and a width of approximately 0.1 µm. The whole surface of this grain is smooth without any ornamentation.
Type 6 Bioforms
In the analyzed samples, unidentified tubular structures consist of hollow cylindrical elements approximately 5 µm to 20 µm in length with diameters varying from less than 1 µm to 5 µm (Figure 4c). They exhibit a smooth surface without any ornamentation.
Unidentified reflecting spheres
The term ‘unidentified reflecting spheres’ is used in this work to refer to elements whose origin and genesis remain unknown. These structures are represented only by spheres that consist of three-dimensional preserved bodies with an approximate diameter of 10 µm to 15 µm. They are characterized by exposing a surface with a shiny appearance and are referred to in this work as reflecting spheres due to their tendency to reflect visible light. They are partially embedded within the pyrite matrix (Figure 4d). Schieber and Baird (2001) described similar spheres in Devonian black shale from North America.
Energy-dispersive X-ray spectrometry (EDS)
EDS analysis was conducted to determine the elemental composition of the structures present in the pyrite (Figure 5). The analysis reveals the presence of sulfur (S) and iron (Fe) in the biostructures, framboids and the surrounding pyrite matrix. However, the presence of elemental carbon was detected in bioform types 1 and 6, and the values varied between 20% and 40%. This element was absent from the matrix. A higher content of carbon was detected in the enigmatic structures, with values reaching 78.94%.
DISCUSSION
Morphologic diversity and biogenicity
The type 1 bioforms exhibit a general morphology and size that are quite similar to biostructures reported by Schieber (2001, 2002a) in sedimentary pyrite from pre-Mesozoic rocks and interpreted as coccoid bacteria. In the studied samples, these bioforms form groups that include multiple specimens and resemble the colonial organization observed in modern bacteria (Mudryk and Podgórska, 2006). During the degradation of organic matter in both sediment and carcasses, bacteria do not cover the decaying surface homogeneously. Instead, groups of individuals forming patches of bacterial cells, as observed by Bajpai et al. (2001) in pyritized leaf cuticles from the Tertiary in India. This could explain the arrangement of coccoid forms in “micro-colonies” in sedimentary pyrite.
The EDS analysis reveals the presence of S and Fe in the same percentage in the biostructures, framboids and surrounding pyrite matrix (Figure 5). This indicates that the analyzed bioforms were preserved mainly in the form of pyrite, as suggested by Schieber (2002a). However, the same analysis shows the presence of carbon in some structures (e.g., bioform types 1 and 6), whereas carbon is absent from the matrix. It is known that elemental carbon in pyritized cells is not necessarily indicative of the presence of organic cellular remains. Some results reveal that carbon in chert or pyrite is often a relict of calcium carbonate minerals (Morris et al., 1998). However, the pyrite samples studied here do not contain any fragments of the encasing limestone and were free of any calcium carbonate source during the observation. The origin of the elemental carbon in the bioforms remains unknown.
On the other hand, the presence of elemental carbon in microscopic structures has been considered evidence of cellular remains of bacterial colonies (Morris et al., 1998), suggesting a biotic origin. Núñez-Useche et al. (2016) also report elemental carbon in the structures coating some framboids in the Agua Nueva Formation at Xilitla. These kinds of structures have been interpreted to be organic substances (biofilms) related to the framboid formation (McLean et al., 2008; Rickard, 2012).
Elemental carbon is only present within the bioforms. This allows us to suggest that the carbon has a biotic origin and that parts of the cellular walls and/or other structures still preserve remains of the original material as a consequence of the permineralization processes (Fernández-López, 2000). According to Schopf (1975), the bacterial cells are susceptible to rapid decay and are degraded completely after buried. Nevertheless, under specific environmental conditions, such as anoxia/dysoxia, as occurred in Xilitla during the deposition of the laminated beds of the Agua Nueva Formation, the remains can be preserved by authigenic mineralization during early diagenesis, resulting in well-preserved fossil bacteria. After the bacteria die, cellular lysis produces chemical products that can destroy the cellular walls in a few hours or days (Allison, 1990). Therefore, the mineralization of cellular walls must be a consequence of extremely rapid pyrite growth (perhaps tens of days) just before cellular decay, as suggested by Schieber (2005). This process is also consistent with the presence of biomorphic structures preserved in three dimensions without apparent physical distortion, which suggests that pyritization occurred before compaction, as suggested by Schopf et al. (1965).
To date, the coccoid elements from Xilitla have not been assigned to any known bacteria group. However, it is known that, in marine environments, the sulfate-reduction process that allows the formation of sedimentary pyrite constitutes the main pathway for oxygen-deficient decay (Berner, 1984; Allison, 1990). Sedimentary pyrite is a mineral that is biologically induced (Frankel and Bazylinski, 2003) by several groups of sulfate-reducing (or even sulfur-reducing) bacteria during early diagenesis (Berner, 1984; Canfield and Raiswell, 1991). The groups of bacteria participating in the pyrite formation exhibit several morphologies including rods (bacilli), spheres and coccoids. Hence, a taxonomic determination based on morphologic traits is quite complicated.
It has been suggested that negative δ34S values correspond to microbial reduction of marine sulfate (Kohn et al., 1998; Alfonso et al., 2005; Prol-Ledesma et al., 2010). According to Southam et al. (2001), the δ34S values of biogenic sulfides range from -10.2 ‰ to -70 ‰, based on the results of previous work (Chambers and Trudinger, 1979; Hanneke et al., 1997; Passier et al., 1996). The negative δ34S values for sedimentary pyrite (-51.2 ‰) collected from the lowermost level of the Xilitla section (Núñez-Useche et al., 2016) are consistent with pyrite deposited via microbial mediation. According to these data, the most parsimonious assumption is that the type 1 bioforms observed in the pyrite samples in this study may be related to sulfate-reducing bacteria.
The bioform types 2 and 3 consist of smooth spheres and the rugose oval structures, respectively, that exhibit size and morphological characteristics (including the presence of pores and collars) that strongly resemble those of specimens reported for fossil and modern stomatocysts by Duff et al. (1995). Stomatocysts represent silicified remains of golden algae or “chrysophytes”. Both smooth spheres and elliptical rugose specimens present in the Upper Cretaceous marine Agua Nueva Formation at Xilitla correspond to freshwater elements. These forms were identified as external organisms transported from continental areas by ancient rivers that existed during the Cenomanian/Turonian in the Proto-Gulf of Mexico (Castañeda-Posadas et al., 2014).
The bioform types 4 and 5 were interpreted as pollen-like grains. The first element represents a broken pollen grain with a smooth surface and a patch of granulated/perforated ornamentation. The second element has an elliptical outline quite similar to lobe-shaped pollen grains after natural drying processes (Blanco et al., 2013). According to Blanco et al. (2013), these biostructures correspond to the outer wall (sporodermis) of ancient and modern pollen grains. These pollen grains also represent extrabasinal elements and were transported to the deposition area from the continental realm probably by aerial agents or by ancient rivers, similar to the stomatocysts.
In both the stomatocyst- and pollen-like specimens, S and Fe were detected in the EDS analysis, indicating they are also replaced by pyrite. Moreover, some elements are partially embedded in the pyrite matrix, indicating that they are not the result of contaminants during sample handling. Instead, they are elements preserved within the pyrite during the diagenetic process.
The presence of pollen in the Upper Cretaceous sedimentary pyrite from Central Mexico is remarkable because, to date, the only fossil microorganisms preserved in sedimentary pyrite to be identified have been aquatic organisms that lived in the water column and/or sediment. Furthermore, the presence of dried pollen grains represents an important fossil record of the remains of aerial allochthonous structures dispersed by putative organisms, such as land plants, which terminated their life cycle in a continental scenario.
The tubular and hollow elements (type 6 bioforms) reported in this work are quite similar to those reported by Schieber (2007) and interpreted as microbial mats or sheaths of microbes. According to Konhauser (2007), these structures are the outermost surface layer that totally encased filamentous cells. Blanco-Piñón et al. (2014) reported some filamentous structures in sedimentary pyrite from the Upper Cretaceous Agua Nueva Formation in Xilitla, suggesting that the hollow elements reported in this work may correspond to the sheaths of similar filamentous structures.
In the sedimentary pyrite from Xilitla, the reflecting structures show a high content of carbon in the EDS analysis. Similar structures, but with a larger diameter of 0.8 mm, have been reported in previous studies on Devonian black shale from North America (Schieber and Baird, 2001) and have been interpreted as elements formed during early diagenesis. The origin of the reflecting structures in the pyrite from Xilitla remains unknown.
Microbial fossilization and pyrite potential
The fossilization processes for microbes are poorly understood. Pyritization, in particular, depends on many environmental factors (e.g., supersaturation of iron sulfides in the water column, pH, Eh, etc.) in the water column, sediment, and specific microenvironments formed in the cell interior as a consequence of decay and bacterial respiration (Locatelli, 2014). In microorganisms such as bacteria, it seems that pyritization also depends on the ability of the microorganisms to trap sediment and metals from the surrounding environment (Farmer, 1999; Konhauser, 2007). The mineralization rate must be higher than the microbial degradation rate, and mineralization may occur under anoxic and dysoxic conditions in the water column and/or the sediment, as occurred during the deposition of the well-laminated dark limestone in Xilitla (Blanco-Piñón et al., 2014).
It seems that the incorporation of iron sulfides into cellular membranes and walls can enhance microbial preservation (Donald and Southam, 1999; Glikson and Taylor, 2000; Schieber, 2002a; Pósfai and Dunin-Borkowski, 2006). Microbial biomineralization of pyrite is a complex process consisting of the precipitation of iron sulfides on cell membranes, cell walls and exopolymers that provide nucleation sites for crystal growth (Fortin and Ferris, 1998; Konhauser, 2007). These cellular structures posses organic substances (such as polysaccharides) that have abundant anionic compounds that act as binding sites for metallic cations (e.g., iron), promoting the precipitation of pyrite within the cellular wall and sheaths (Konhauser, 1998, 2007; Fortin and Ferris, 1998; Farmer, 1999; Donald and Southam, 1999; Schieber, 2002a) and, consequently, pyritization of the microorganism.
Pollen and spores are highly resistant to decay (Greenwood, 1991), and unlike the bacteria involved in the pyrite formation, they constitute allochthonous elements from both continental land (pollen) and fresh-water (stomatocysts) environments. After aerial or water transport, pollen can remain in suspension in the water column until it sinks to the bottom (Fall, 1987) and can be exposed to reworking (Greenwood, 1991). The pollen wall is composed of cellulose and other cutilarized structures involving complex biopolymers (e.g., sporopollenin) that reinforce the structure and render the pollen resistant to destruction in different environments. According to Rickard et al. (2007) and Locatelli (2014), this structure represents a substantial barrier that prevents external substances (even pyrite precursors) from entering the cell (or pollen) wall. After death and burial and during bacterial decay, the barriers start to decompose, and the pollen wall becomes more permeable to external substances, such as pyrite precursors (iron and sulfur species), allowing the pollen mineralization (Grimes et al., 2002; Locatelli, 2014) and the preservation of anatomical details of the microstructures (Schopf, 1975). These processes can explain the preservation of pollen-like sporodermis structures within the dysoxic beds at Xilitla.
Stomatocysts are morphologically diverse structures that represent refractory remains of golden algae or “chrysophytes”. In this case, the cysts were originally composed of silica, and the EDS analysis indicates that the replacement of the silica by iron sulfides took place in the cyst walls. The replacement of silica by pyrite has been reported by Martín-González et al. (2009b) for pyritized diatoms in amber.
Sedimentary pyrite is a mineral with a high potential for fossil preservation, not only for macroorganisms but also for microbes. It seems that this fossilization type is predisposed to microorganisms with refractory elements (e.g., cellular walls, sheaths and capsules) that are resistant to biodegradation (Farmer, 1999). Unlike hydrothermal pyrite, which has an abiotic origin (Murowchick and Barnes, 1987; Southam et al., 2001), sedimentary pyrite forms in anoxic scenarios via the reaction of detrital iron with dissolved sulfide, which is produced by microbial sulfate reduction (Berner, 1984; Raiswell and Canfield, 1998). Therefore, sedimentary pyrite requires the presence of life for its genesis. Moreover, the detection of microorganisms in sedimentary pyrite leads to the possibility of finding not only fossils of organisms that lived in the aquatic environments but also evidence of organisms, such as the pollen producers, that lived outside the sedimentary environment in which the pyrite formed. This allows obtaining information on continental elements preserved in the marine realm. Therefore, studying the formation of sedimentary pyrite in the early geological history of the Earth (and other terrestrial planets, such as Mars) could produce insights into ancient autochthonous and/or allochthonous microbial activity.
CONCLUSIONS
Sedimentary pyrite is a mineral with a high potential for fossil preservation, not only for macroorganisms but also for microbes. It seems that this fossilization type tends to preserve microorganisms with refractory elements (e.g., cellular walls, sheaths and capsules) that are resistant to biodegradation. In the case of the Xilitla material, this is not the exception: refractory parts, such as cellular walls of bacteriomorphs, pollen, stomatocysts, and sheaths, are well preserved. They exhibit no distortion in their morphology, indicating that the permineralization processes occurred before the compaction of the biological producers. Given that the pyrite forms very soon during early diagenesis, the preservation of microorganisms involved in the pyritization of the calcareous muds in the study area must not be considered rare. It should be expected in localities in which sedimentary pyrite formation occurs, especially for bacterial groups involved in the pyritization process, such as sulfate-reducing bacteria.











 nueva página del texto (beta)
nueva página del texto (beta)




