Introduction
Edible flowers add aroma, flavor and visual appeal to many dishes. In addition to all the qualities that can be added to gastronomy, they are also a potential source of compounds with biological activity such as antioxidants, which have gained importance in the prevention of degenerative diseases (Lara-Cortés, Osorio-Díaz, Jiménez-Aparicio, & Bautista-Baños, 2013). However, even with all the qualities they possess, edible flowers have not yet received the same attention as fresh fruit and vegetables. To date, no guidelines have been established regarding their storage, due to the limited information on factors that limit their quality. In general, edible flowers should be used within two to five days of being cut; therefore, they require suitable postharvest technology in order to extend their shelf life.
The dahlia (Dahlia spp.) is a flower known for its consumption as food and its nutritional and functional properties. Lara-Cortés et al. (2014) found that purple dahlia flowers, in particular, are an important source of compounds with biological activity, such as antioxidants, and therefore could be a functional food. However, despite its qualities, its use in food is limited since it is highly perishable; once cut, its shelf life is less than three days due to the presence of spoilage microorganisms which place limits on its preservation.
In this regard, it is known that one of the requirements that flowers must meet for consumption is to be free of pathogenic microorganisms that can cause health problems, added to which some microorganisms, without being pathogenic, can also alter the sensory characteristics of flowers. Therefore, control alternatives, such as antimicrobial compounds derived from essential oils found in spices, have been investigated. One such example is cinnamon oil, which contains cinnamaldehyde. Lara-Cortés et al. (2015) have shown that cinnamaldehyde has considerable in vitro bactericidal action against gram-negative bacteria isolated from edible dahlias.
On the other hand, modified atmosphere (MA) packaging has been widely used to maintain quality, extend shelf life and reduce microbial growth in lettuce, broccoli, spinach and many other products (Luo, 2007). Likewise, this technology also extends the shelf life of horticultural products due to the oxygen (O2) concentration and increased carbon dioxide (CO2) in the atmosphere of the packaged product (Wills, Mcglasson, Graham, & Joyce, 2004). Moreover, polymeric materials that are used for packaging fresh products have numerous benefits such as water loss control, mechanical damage protection and reduced contamination of the product during handling (del Nobile et al., 2009). Another important aspect to consider is temperature control since it mainly affects various physiological factors and the quality of the packaged product, such as respiration rate, water loss, color conservation, turgor, etc. (Ščetar, Kurek, & Galić, 2010).
The aim of this study was to evaluate the antimicrobial effect of cinnamaldehyde application on edible dahlias stored under different temperatures (8 and 25 °C) and in two types of packaging materials (polyethylene terephthalate and low-density polyethylene), in terms of safety and quality conservation aspects.
Materials and methods
Plant material
Purple dahlia var. Pompom flowers were provided by the Mexican Dahlia Association (Xochitla, Ecological Park, Tepotzotlán, Mexico, 19° 43’ 4” NL and 99° 12’ 1” WL). During the growing period, irrigation, fertilization and pest and disease control practices suitable for dahlia were followed (Jiménez-Mariña, 2015). Flowers were selected on the basis of being free of mechanical damage, insects and signs of deterioration.
Experiment and treatments
Dahlia flower samples of 50 ± 5 g were placed in polyethylene terephthalate (PET [330 μm thick]) containers with snap-on lids and in low-density polyethylene (LDPE [thickness 220 μm thick]) resealable bags. The antimicrobial compound cinnamaldehyde (CIN) (Sygma, Aldrich) at a concentration of 0.25 % was pipetted onto a filter paper (2 × 3 cm2) and placed inside each packaging material during the storage period. Containers were stored at 8 and 25 °C. Control samples were processed similarly, with exception of the volatile CIN compound which was replaced with water. The treatment design corresponded to the combinations of two temperature levels (8 and 25 °C), two types of packaging materials (PET and LDPE) and the absence and presence of the application of 0.25 % CIN, giving a total of eight treatments: T1 = PET + 8 °C + 0.25% CIN, T2 = PET + 8 °C + water, T3 = LDPE + 8 °C + 0.25 % CIN, T4 = LDPE + 8 °C + water, T5 = PET + 25 °C + 0.25 % CIN, T6 = PET + 25 °C + water, T7 = LDPE + 25 °C + 0.35 % CIN, and T8 = LDPE +25 °C + water. Except for respiration rate and ethylene production, the variables for treatments T1-T4 (8 °C) and T5-T8 (25 °C) were evaluated at eight and three days of storage, respectively.
Microbiological analysis
Microbiological stability was evaluated by determining mesophilic aerobic bacteria (MAB), yeasts and molds (Y/M) and psychrophilic (PSY) bacteria. The 10-g samples of three flowers were homogenized separately for 2 min with 90 mL of sterile peptone solution. Serial dilutions (1 mL) of each mixture were plated on Petri dishes containing potato dextrose agar for Y/M and tryptic soy agar for MAB and PSY, respectively. Once the medium gelled, it was incubated at 35 ± 2 °C for 48 h for MAB, and at 8 °C for seven days for PSY and three to five days for Y/M. Each microbiological evaluation was performed in triplicate and the values were averaged. The results were expressed in log cfu⋅g-1.
Physiological response
Weight loss
A Sartorius balance was used to determine the weight loss of the flowers, which were evaluated at eight and three days for samples stored at 8 and 25 °C, respectively. For the evaluation, three flowers per treatment were taken and their initial and final weight (at the end of the storage period) was recorded. The results were averaged and expressed in percentage of weight loss.
CO2 and ethylene production
Respiration rate and ethylene production were determined according to Watada and Massie (1986) and Troncoso-Rojas, Sánchez-Estrada, Ruelas, García, and Tiznado-Hernández (2005). Air samples (1 mL) were taken from each package and injected into a Varian 3400 cx gas chromatograph equipped with a HayeSep N column (2 m x 3.17 mm inside diameter, Supelco, Inc.) coupled to a thermal conductivity detector (for CO2) and a flame ionization detector (for ethylene). The parameters were: 100 °C injection temperature, 170 °C thermal conductivity detector, and 120 °C flame ionization detector. Nitrogen was used as a carrier gas with a flow rate of 25 mL⋅min-1. The evaluations were carried out daily in three flowers per treatment for three and eight days at 25 and 8 °C, respectively. Data obtained from each day were averaged. Respiration rate and ethylene production were expressed in mL CO2∙kg-1∙h-1 and µL ethylene⋅kg-1∙h-1, respectively.
Quality evaluation of the treated flowers
Color analysis
Color (CIE L*, a*, b*) was determined with a ColorQuest® XE colorimetric-spectrophotometer (Hunter Associates Lab, Reston, Virginia, USA), using as reference the typical color of the purple flowers used. The spectrophotometer was calibrated using a standard trap light and a white ceramic plate (L* = 93.50, a* = -0.89 and b* = 1.01) with D65/10° (illuminant geometry ⁄ display). Three packages with flowers were used and three readings of each ligule were taken, using a total of three flowers per treatment. The hue and chroma values were calculated with the following equations: hue = tan-1 (b* ⁄ a*) and chroma = (a*2 + b*2)1/2. The evaluations corresponding to 8 and 25 °C were carried out at three and eight days, respectively. Data were averaged and expressed as color difference (ΔE).
Total phenolic compounds
Total phenolic compounds were determined following the Folin-Ciocalteau method as modified by Dastmalchi, Damien-Dorman, Laakso, and Hiltunen (2007). Extracts were diluted with 40 % ethanol and the dilution factor was taken into account in the calculations. A 0.5-mL aliquot of extract (diluted) was transferred to a test tube, to which 500 µL of Folin-Ciocalteau reagent, 10 mL of a Na2CO3 solution (200 g⋅L-1) and distilled water to a volume of 25 mL were added. After 1 h of reaction at room temperature, absorbance was determined at 725 nm and compared with a calibration curve made with gallic acid as the reference compound at eight different concentrations (0, 2, 4, 6, 8, 10, 12 and 14 mg⋅L-1). Each analysis of total phenolic compounds, based on three flowers per treatment, was performed in triplicate and the solvent was considered as reference. The evaluations corresponding to 8 and 25 °C were carried out at three and eight days, respectively. The results were averaged and expressed in mg of gallic acid (GA) per g of ligule (mg GA⋅g ligule-1).
Antioxidant capacity
Antioxidant capacity was evaluated using 2, 2-diphenyl1-picrylhydrazyl (DPPH) reagent, according to the method followed by Brand-Williams, Cuvelier, and Berset (1995), with the following modifications: DPPH along with the samples were dissolved in 100 % ethanol, which was also used as reference. The samples of three flowers per treatment were prepared in triplicate and the absorbance was read at a wavelength of 515nm. The evaluations corresponding to 8 and 25 °C were performed at three and eight days, respectively. Data were averaged and expressed as inhibition percent of radical DPPH (% IDPPH.).
Statistical analysis
An analysis of variance was performed based on a factorial treatment design in a completely randomized arrangement. Treatment means were compared using the Tukey test (P ≤ 0.05) to consider significant differences among treatments. Data were presented as the mean ± standard deviation and were processed using the JMP statistical software, version 4.04 (Lara-Cortés et al., 2014).
Results and discussion
Microbiological analysis
Results of the microbiological analysis of the different evaluated treatments are shown in Table 1. The number of MAB, Y/M, and PSY increased in all treatments compared to the initial microbial count. There were significant differences among treatments (P ≤ 0.05) in the three microbial groups studied. The highest coefficient of variation was obtained by Y/M (19.3 %), followed by PSY (14.2 %) and MAB (11.8 %), respectively. The interaction between storage temperature, antimicrobial application and packaging material influenced the development of the microbial groups.
Table 1. MAB, Y/M, and PSY contents in edible dahlia flowers with the use of packages (PET and LDPE) and stored at 8 and 25 °C with/without cinnamaldehyde for eight and three days, respectively. The results are expressed in log CFU∙g ligule-1.
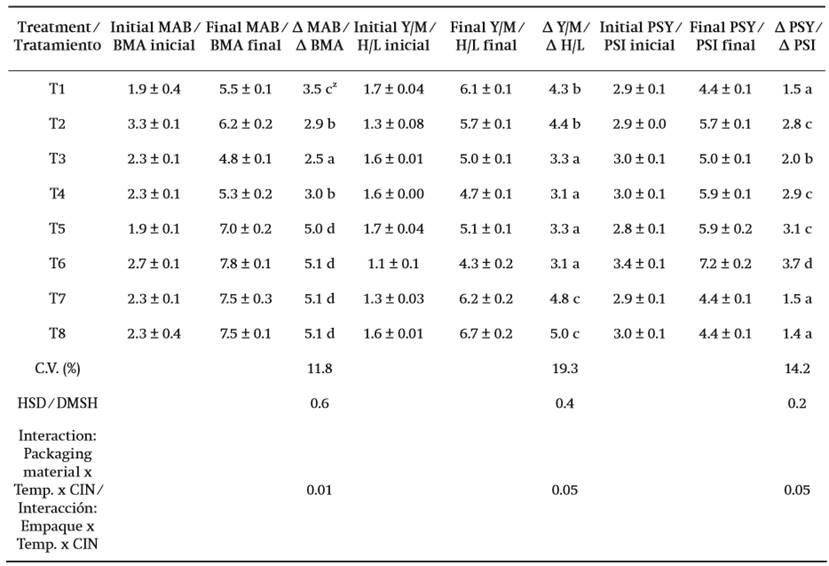
T1 = PET + 8 °C + 0.25 % CIN, T2 = PET + 8 °C + water, T3 = LDP + 8 °C + 0.25 % CIN, T4 = LDPE + 8 °C + water, T5 = PET + 25 °C + 0.25 % CIN, T6 = PET + 25 °C + water, T7 = LDPE + 25 °C + 0.25 % CIN, T8 = LDPE + 25 °C + water
Data of the initial and final counts are presented as the mean ± deviation standard of three measurements.
ZMeans with the same letter within columns are not statistically different (Tukey, P ≤ 0.05).
C.V. = coefficient of variation. HSD = honest significant difference
In MAB a lower increase in microbial count was observed in flowers stored at 8 °C. At this temperature, the presence of microorganisms was lower in flowers packaged in LDPE with CIN, whereas at 25 °C, in addition to the development of the microbial count, there was obvious deterioration and fungal growth on the surface of the ligules. Concerning the Y/M and PSY microbial groups, a defined pattern among treatments was not determined; however, regardless of CIN application, the microbial count of Y/M was lower compared to the other treatments (P ≤ 0.05) with LDPE and PET at 8 and 25 °C, respectively. On the other hand, for PSY, flowers packaged in PET + CIN at 8 °C and in LDPE at 25 °C also had a lower microbial count (P ≤ 0.05).
It can be inferred that the results were possibly due to the initial microbial count of the product, which was high and thus explains why the CIN was not very effective in controlling microbial development during storage. Added to this, it has a documented history of volatilization (Olivares-Cruz & López-Malo, 2013). In this regard, Matos-Chamorro, Quispe-Condori, Quito-Vidal, and Beltrán-Cárdenas (2010) mention that some antimicrobial substances, such as essential oils or derivatives thereof, may present difficulties in their use as preservatives due to their impregnation in the foodstuff or rapid volatilization.
On the other hand, microbiological safety is one of the most important factors to consider in preserving any plant product (Bico, Raposo, Morais, & Morais, 2009). As set out in Appendix B of the Official Mexican Standard NOM-093-SSA1-1994 (1994), raw salads should not exceed MAB microbiological limits of 5.1 log CFU⋅g-1, which are sometimes higher or equal to the results obtained in most of the treatments evaluated in this investigation.
Similarly, high microbial counts also affect the nutritional and sensory characteristics of the product. This is because within the microorganisms evaluated (MAB, Y/M and PSI) we could find some microorganisms that although they are not pathogens themselves they alter and accelerate the senescence process in stored flowers, as has been shown by Piagentini, Pirovani, and Güemes (2004) and Rawat (2015).
Physiological response of treated flowers
Weight loss
In the results, significant differences among quality variables (P ≤ 0.05) were observed (Table 2). The coefficient of variation for the percentage of weight loss was 15.7 %. The treatments in which the flowers had higher weight loss (P ≤ 0.05) were observed in those stored in PET. As mentioned in the materials and methods section, the thickness of each material was 220 and 330 µm for LDPE and PET, respectively; however, sealing the LDPE bags with heat ensured the tightness of the package. In this regard, Liping, Turner, and Luo (2012) evaluated weight loss in carnation flowers and observed that the tightness of the packaging material used had an influence on this variable.
Table 2. Evaluation of percentage of weight loss, color, total phenolic compound concentration and percentage of antioxidant capacity in edible dahlia flowers packaged in PET and LDPE and stored at 8 and 25 ° C, with/ without cinnamaldehyde for eight and three days, respectively.
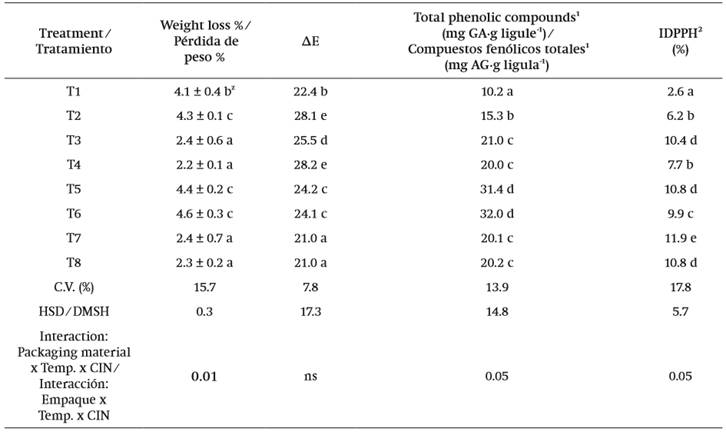
T1 = PET + 8 °C + 0.25 % CIN, T2 = PET + 8 °C + water, T3 = LDP + 8 °C + 0.25 % CIN, T4 = LDPE + 8 °C + water, T5 = PET + 25 °C + 0.25 % CIN, T6 = PET + 25 °C + water, T7 = LDPE + 25 °C + 0.25 % CIN, T8 = LDPE + 25 °C + water
Average Initial content = 25 mg GA∙g ligule-1, 2Average initial content = 15 %. Data are presented as the mean ± deviation standard of three measurements.
ZMeans with the same letter within columns are not statistically different (Tukey, P ≤ 0.05).
C.V. = coefficient of variation. HSD = honest significant difference, ns = not significant, ΔE = final color.
Weight loss is the main cause of deterioration in minimally processed vegetables since it affects the overall appearance of the product (Torales, Chaves, & Rodríguez, 2010). This is explained by the composition of such products, approximately 80-90 % of which is water. In dahlia flowers 88 to 92 % of its composition is water (Lara-Cortés et al. 2014). Among the characteristics that were affected by the weight loss in the dahlia ligules, little turgor and firmness were observed.
CO2 and ethylene production
CO2 production is shown in Figure 1a. Low CO2 production was observed in treatments stored at 8 °C, regardless of the packaging material and CIN application. However, the best treatments were those where the dahlias were stored in PET with or without the antimicrobial agent, because with this packaging material and at 8 °C the production of CO2 was minimal during the eight days of storage. By contrast, at 25 °C the respiratory rate increased significantly in a range of 385 to 520 mL CO2∙kg-1∙h-1. In general, at 25 °C, the difference was mainly observed in those flowers packed with the antimicrobial CIN.
Mapeli et al. (2011) reported that storage temperature affected the respiratory rate of star orchid (Epidendrum ibaguense) flowers, since in their experiments the CO2 rate increased when flowers were stored at 30 ºC, which coincides with the results shown in this study. On the other hand, temperature is an important factor that controls the enzymatic, respiratory and metabolic activities of horticultural products. Wiley (1997) mentions that proper temperature control during storage of fruit and vegetables can inactivate or slow some physiological effects such as respiration.
Figure 1b shows the evolution of ethylene production. Reduced ethylene production was observed when flowers were packaged in PET, regardless of storage temperature and CIN application. In PET, as storage time progressed, ethylene concentrations of the dahlias decreased until the values were almost not detected. In LDPE, ethylene concentrations were higher in comparison to PET; however, it was mostly observed that that the concentration would increase during the storage period.
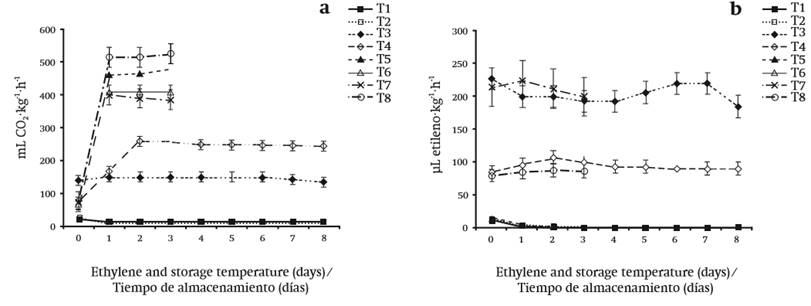
Figure 1. Production of a) CO2 and b) ethylene in edible dahlia flowers in packages (PET and LDPE) and stored at 8 and 25 °C with/without cinnamaldehyde for eight and three days, respectively. T1 = PET + 8 °C + 0.25 % CIN, T2 = PET + 8 °C + water, T3 = LDP + 8 °C + 0.25 % CIN, T4 = LDPE + 8 °C + water, T5 = PET + 25 °C + 0.25 % CIN, T6 = PET + 25 °C + water, T7 = LDPE + 25 °C + 0.25 % CIN, T8 = LDPE + 25 °C + water.
Reid and Wu (1992) demonstrated that there is a close relationship between maximum production of climacteric respiration and ethylene production in carnation flowers, which is considered to be climacteric. This did not occur in dahlia flowers, since ethylene production did not coincide with the highest respiratory rate. In this case, ethylene concentrations in the flower decreased as storage time increased, even when deterioration signs were evident in the flower, which indicates that dahlias are non-climacteric.
Quality assessment of the treated flowers
Color analysis
It was observed that the treatments with the highest color change values were those where dahlias were stored at 25 °C and water was used instead of cinnamaldehyde (Table 2). For this variable, the coefficient of variation was 7.8 %. According to CIE Lab coordinates, the treatment that showed the least color change (P ≤ 0.05) with respect to time zero was T1, which also had the lowest brightness value (L = 12.4) (data not shown), a parameter which indicates the darkness of the evaluated sample. Considering these results, it can be inferred that the packaging material, temperature, and antimicrobial compound had an effect on color deterioration because this attribute was less affected when PET + 8 °C + CIN was used. The color change during flower storage may be related to the degradation of compounds, such as total polyphenols to quinones, which give a dark or brownish color to the damaged area of the plant sample.
There are numerous changes in pigment content during development and maturation of plant products. Some of these continue after harvest and may be desirable or undesirable. In the case of edible dahlia flowers these color changes are part of the senescence process, as well as the decrease in shelf life, and therefore are considered undesirable. Color and its uniformity are two of the main characteristics that determine the quality of a plant product (Mercado-Silva & Aquino-Bolaños, 2005). Although this condition is not generalized to all plant products, the color in edible flowers is one of the most important attributes in selecting and using it as food.
Total phenolic compounds and antioxidant capacity
In this study, significant differences among treatments (P ≤ 0.05) were observed. The factors temperature, packaging material and the CIN antimicrobial had an influence on the content of phenolic compounds and percentage of DPPH. The respective coefficients of variation were 13.9 and 17.8 %. Results indicated that the flowers in PET + 25 °C, with or without CIN, had approximately three times more phenolic compound content than the treatment in PET + 8 °C + CIN (Table 2). On the other hand, in relation to the radical DPPH, the values corresponding to the treatment with PET + 8 °C + CIN were lower (2.6 %) compared to all other treatments (P ≤ 0.05). In this case, the maximum DPPH percentages (11.9 %) were obtained in LDP + 25 °C + CIN. Thus, in this study, the phenolic content did not directly correlate the antioxidant capacity with the use of the radical DPPH method, being unspecific to perform this correlation.
The development and evolution of phenolic compounds affects the quality of products and is partly regulated by polyphenol oxidase activity. This enzyme is involved in the degradation of polyphenols to quinones, which produce the dark or brownish coloration in damaged areas (by oxidation, cutting, etc.) of the plant product. Likewise, Friedman et al. (2007), in studies on Tropaeolum and Begonia flowers, reported that as in dahlia flowers the use of packages in combination with temperatures helped to maintain antioxidant concentrations, including total phenolic compounds. The main difference with this research was the application of the antimicrobial compound; however, in general, no adverse effects on the preservation of dahlias because of its application were observed.
Conclusions
The interaction of the factors packaging material, storage temperature and presence of cinnamaldehyde influenced the preservation quality of edible dahlias. At 8 °C, significant influence on extending the shelf life of edible dahlias was observed. Except for water loss, the PET and LDPE packages generally provided an appropriate level of preservation for dahlias during storage at 8 and 25 °C. The antimicrobial cinnamaldehyde did not control the development of spoilage microorganisms in the flowers, probably due to the high initial microbial count.











 texto en
texto en 


